Lignocellulosic Corn Stover Biomass Pre-Treatment by Deep Eutectic Solvents (DES) for Biomethane Production Process by Bioresource Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Deep Eutectic Solvent
2.3. Sample Collection and Preparation
2.4. Deep Eutectic Solvent Pretreatment of Corn Stover
2.5. Biomass Characterization
2.5.1. Determination of Lignin, Organic Carbon, Ash, and Moisture Content
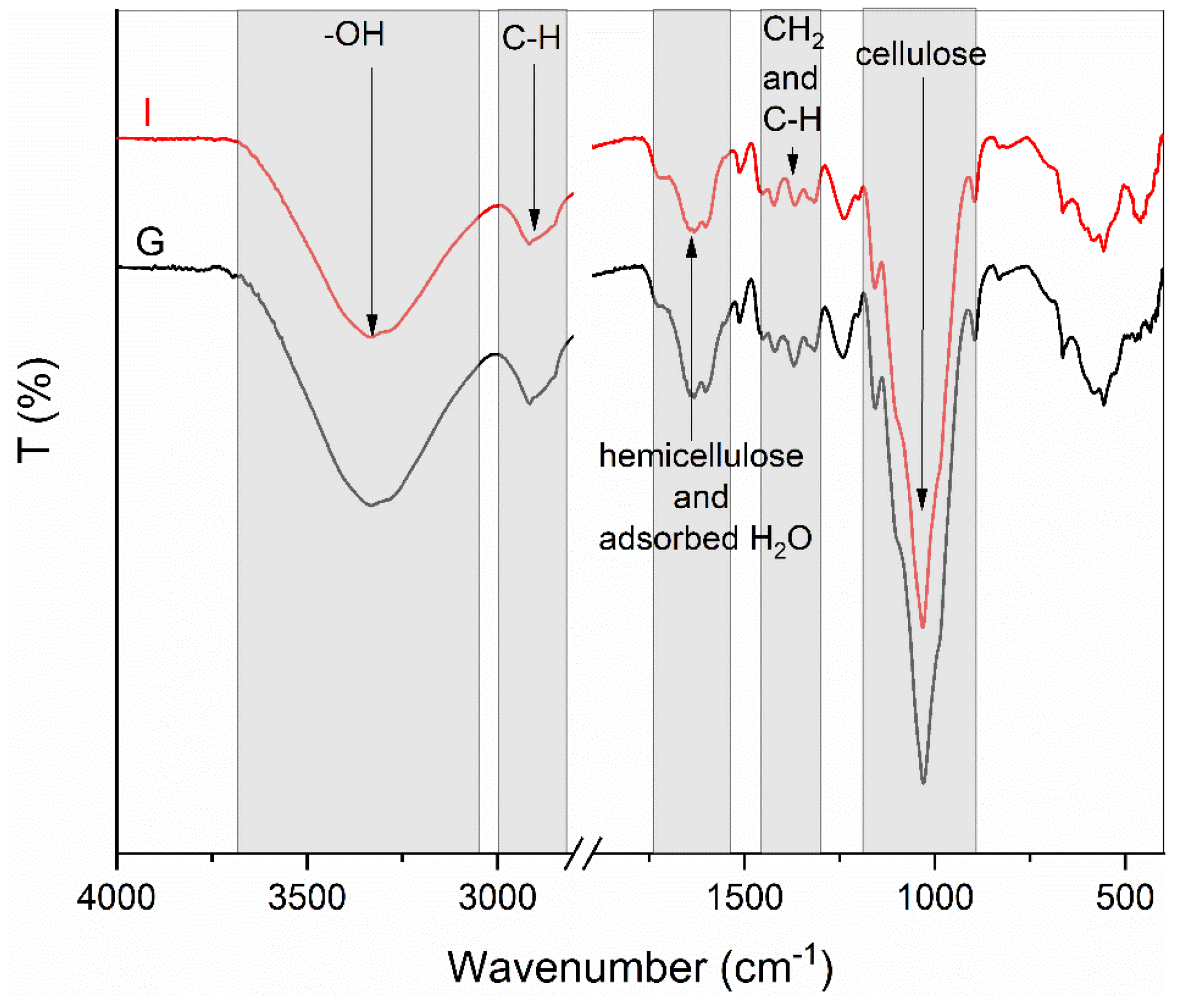
2.5.2. Fourier-Transform Infrared Spectroscopy Analysis
2.5.3. X-ray Powder Diffraction Analysis
2.5.4. Morphology of the Corn Stover Biomass
2.5.5. Elemental Analysis
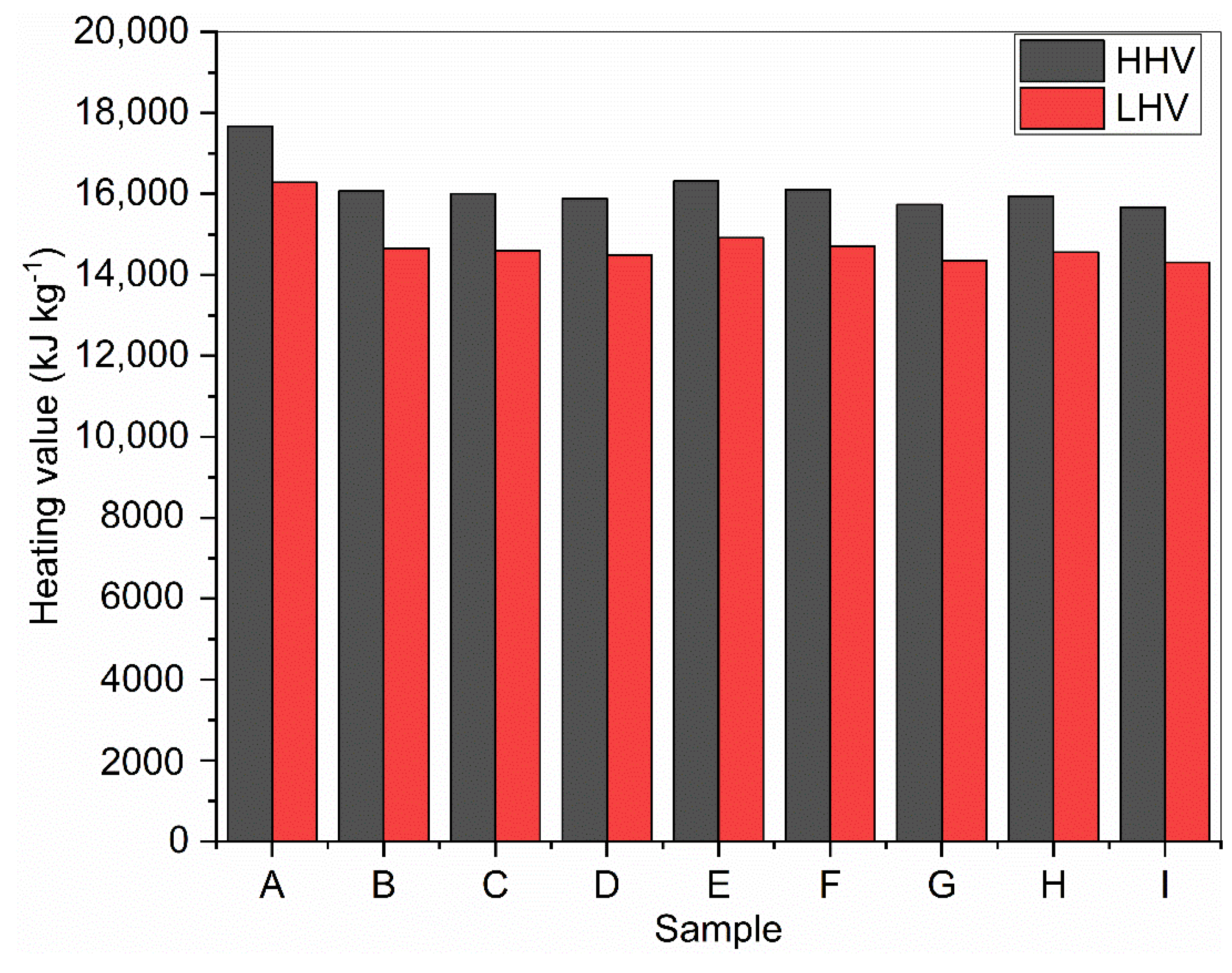
2.6. Calculation of Higher and Lower Heating Values
2.7. Anaerobic Digestion Process
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Deep Eutectic Solvent Pretreatment on Yield Recovery
3.2. Phsicochemical Properties of Raw and Deep Eutectic Solvent Pretreated Samples
3.2.1. Elemental Analysis
3.2.2. Fourier-Transform Infrared Spectroscopy Analysis
3.2.3. X-ray Powder Diffraction Analysis
3.2.4. Morphology
3.3. Determination of Higher and Lower Heating Values
3.4. Daily and Cumulative Biomethane Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bennich, T.; Belyazid, S.; Stjernquist, I.; Diemer, A.; Seifollahi-Aghmiuni, S.; Kalantari, Z. The bio-based economy, 2030 Agenda, and strong sustainability—A regional-scale assessment of sustainability goal interactions. J. Clean. Prod. 2021, 283, 125174. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Gastaldi, M.; Morone, P. RES-T trajectories and an integrated SWOT-AHP analysis for biomethane. Policy implications to support a green revolution in European transport. Energy Policy 2020, 138. [Google Scholar] [CrossRef]
- Hladnik, L.; Vicente, F.A.; Novak, U.; Grilc, M.; Likozar, B. Solubility assessment of lignin monomeric compounds and organosolv lignin in deep eutectic solvents using in situ Fourier-transform infrared spectroscopy. Ind. Crop. Prod. 2021, 164, 113359. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Abaid, A.; Wanderley, R.R.; Knuutila, H.K.; Jakobsen, J.P. Analysis and selection of optimal solvent-based technologies for biogas upgrading. Fuel 2021, 303, 121327. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; le Saché, E.; Price, C.A.H.; Reina, T.R.; Navarrete, B. From biogas upgrading to CO2 utilization and waste recycling: A novel circular economy approach. J. CO2 Util. 2021, 47, 101496. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Huisingh, D.; Morone, P. A circular economy model based on biomethane: What are the opportunities for the municipality of Rome and beyond? Renew. Energy 2021, 163, 1660–1672. [Google Scholar] [CrossRef]
- Rezaee, M.; Gitipour, S.; Sarrafzadeh, M.-H. Different pathways to integrate anaerobic digestion and thermochemical processes: Moving toward the circular economy concept. Environ. Energy Econ. Res. 2020, 4, 57–67. [Google Scholar] [CrossRef]
- Rodríguez, R.W.; Pomponi, F.; Webster, K.; D’Amico, B. The future of the circular economy and the circular economy of the future. Built Environ. Proj. Asset Manag. 2020, 10, 529–546. [Google Scholar] [CrossRef]
- Selvaggi, R.; Valenti, F.; Pecorino, B.; Porto, S.M.C. Assessment of tomato peels suitable for producing biomethane within the context of circular economy: A GIS-based model analysis. Sustainability 2021, 13, 5559. [Google Scholar] [CrossRef]
- Porichha, G.K.; Hu, Y.; Rao, K.T.; Xu, C.C. Crop residue management in India: Stubble burning vs. other utilizations including bioenergy. Energies 2021, 14, 4281. [Google Scholar] [CrossRef]
- Suhartini, S.; Heaven, S.; Banks, C.J. Can anaerobic digestion of sugar beet pulp support the circular economy? A study of biogas and nutrient potential. IOP Conf. Series Earth Environ. Sci. 2018, 131, 012048. [Google Scholar] [CrossRef] [Green Version]
- Baena-Moreno, F.M.; Malico, I.; Marques, I.P. Promoting sustainability: Wastewater treatment plants as a source of biomethane in regions far from a high-pressure grid. A real Portuguese case study. Sustainability 2021, 13, 8933. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Yeganeh, L.P.; Angelidaki, I.; Nizami, A.-S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of lignocelluloses for enhanced biogas production: A review on influencing mechanisms and the importance of microbial diversity. Renew. Sustain. Energy Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Thompson, D.N.; Campbell, T.; Bals, B.; Runge, T.; Teymouri, F.; Ovard, L.P. Chemical preconversion: Application of low-severity pretreatment chemistries for commoditization of lignocellulosic feedstock. Biofuels 2013, 4, 323–340. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.; Cruz-Santos, M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef] [Green Version]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Ouahabi, Y.R.; Bensadok, K.; Ouahabi, A. Optimization of the biomethane production process by anaerobic digestion of wheat straw using chemical pretreatments coupled with ultrasonic disintegration. Sustainability 2021, 13, 7202. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Low-energy biomass pretreatment with deep eutectic solvents for bio-butanol production. Bioresour. Technol. 2017, 243, 464–473. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, C.; Li, Y. Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour. Technol. 2010, 101, 7523–7528. [Google Scholar] [CrossRef]
- Gao, J.; Chen, L.; Yuan, K.; Huang, H.; Yan, Z. Ionic liquid pretreatment to enhance the anaerobic digestion of lignocellulosic biomass. Bioresour. Technol. 2013, 150, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Karp, E.M.; Donohoe, B.S.; O’Brien, M.H.; Ciesielski, P.N.; Mittal, A.; Biddy, M.J.; Beckham, G.T. Alkaline pretreatment of corn stover: Bench-scale fractionation and stream characterization. ACS Sustain. Chem. Eng. 2014, 2, 1481–1491. [Google Scholar] [CrossRef]
- Padrino, B.; Serrano, M.L.; Morales-Delarosa, S.; Campos-Martín, J.M.; Fierro, J.L.G.; Martínez, F.; Melero, J.A.; Puyol, D. resource recovery potential from lignocellulosic feedstock upon lysis with ionic liquids. Front. Bioeng. Biotechnol. 2018, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Grilc, M.; Likozar, B.; Levec, J. Kinetic model of homogeneous lignocellulosic biomass solvolysis in glycerol and imidazolium-based ionic liquids with subsequent heterogeneous hydrodeoxygenation over NiMo/Al2O3 catalyst. Catal. Today 2015, 256, 302–314. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Lee, J.-M. Effect of organic solvent in ionic liquid on biomass pretreatment. ACS Sustain. Chem. Eng. 2013, 1, 894–902. [Google Scholar] [CrossRef]
- Bjelić, A.; Hočevar, B.; Grilc, M.; Novak, U.; Likozar, B. A review of sustainable lignocellulose biorefining applying (natural) deep eutectic solvents (DESs) for separations, catalysis and enzymatic biotransformation processes. Rev. Chem. Eng. 2020, 2019077. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.M.; Hayyan, M.; Hayyan, A.; Basirun, W.J.; Salleh, H.M.; Mirghani, M.E.S. A grand avenue to integrate deep eutectic solvents into biomass processing. Biomass Bioenergy 2020, 137, 105550. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, R.; Rahman, S.; Raynie, D.E. Recent advances of greener pretreatment technologies of lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Y.; Tang, R.; Xue, D.; Sun, Y.; Li, X. Efficient dissolution of lignin in novel ternary deep eutectic solvents and its application in polyurethane. Int. J. Biol. Macromol. 2020, 164, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.; Branco, L.C.; Lapa, N.; Marrucho, I.M. Beneficial and detrimental effects of choline chloride–oxalic acid deep eutectic solvent on biogas production. Waste Manag. 2021, 131, 368–375. [Google Scholar] [CrossRef]
- Procentese, A.; Rehmann, L. Fermentable sugar production from a coffee processing by-product after deep eutectic solvent pretreatment. Bioresour. Technol. Rep. 2018, 4, 174–180. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2004. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Podolski, W.F.; Conrad, V.; Lowenhaupt, D.E.; Winschel, R.A. Materials of construction. In Perry’s Chemical Engineers’ Handbook; Perry, R.H., Green, D.W., Eds.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2008; pp. 24–25. [Google Scholar] [CrossRef]
- Fernández-Cegrí, V.; de la Rubia, M.A.; Raposo, F.; Borja, R. Effect of hydrothermal pretreatment of sunflower oil cake on biomethane potential focusing on fibre composition. Bioresour. Technol. 2012, 123, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Suopajärvi, T.; Ricci, P.; Karvonen, V.; Ottolina, G.; Liimatainen, H. Acidic and alkaline deep eutectic solvents in delignification and nanofibrillation of corn stalk, wheat straw, and rapeseed stem residues. Ind. Crop. Prod. 2020, 145, 111956. [Google Scholar] [CrossRef]
- Zhang, X.; Nghiem, N.P. Pretreatment and fractionation of wheat straw for production of fuel ethanol and value-added co-products in a biorefinery. AIMS Bioeng. 2014, 1, 40–52. [Google Scholar] [CrossRef]
- Mulat, D.G.; Dibdiakova, J.; Horn, S.J. Microbial biogas production from hydrolysis lignin: Insight into lignin structural changes. Biotechnol. Biofuels 2018, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Sasmal, S.; Mohanty, K. Pretreatment of lignocellulosic biomass toward biofuel production. In Biorefining of Biomass to Biofuels; Springer: Cham, Switzerland, 2018; pp. 203–221. [Google Scholar]
- Petravić-Tominac, V.; Nastav, N.; Buljubašić, M.; Šantek, B. Current state of biogas production in Croatia. Energy Sustain. Soc. 2020, 10. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural changes of oak wood main components caused by thermal modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Lao, W.; Li, G.; Zhou, Q.; Qin, T. Quantitative analysis of biomass in three types of wood-plastic composites by FTIR spectroscopy. Bioresources 2014, 9, 6073–6086. [Google Scholar] [CrossRef] [Green Version]
- Duchemin, B. Size, shape, orientation and crystallinity of cellulose Iβ by X-ray powder diffraction using a free spreadsheet program. Cellulose 2017, 24. [Google Scholar] [CrossRef]
- Moyer, P.; Kim, K.; Abdoulmoumine, N.; Chmely, S.; Long, B.K.; Carrier, D.J.; Labbé, N. Structural changes in lignocellulosic biomass during activation with ionic liquids comprising 3-methylimidazolium cations and carboxylate anions. Biotechnol. Biofuels 2018, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ning, Z.; Khalid, H.; Zhang, R.; Liu, G.; Chen, C. Enhancement of methane production from Cotton Stalk using different pretreatment techniques. Sci. Rep. 2018, 8, 3463. [Google Scholar] [CrossRef] [Green Version]
- Demirbas, B. Biomass business and operating. Energy Educ. Sci. Technol. Part A 2010, 26, 37–47. [Google Scholar]
- Sujan, S.; Kashem, M.; Fakhruddin, A. Lignin: A valuable feedstock for biomass pellet. Bangladesh J. Sci. Ind. Res. 2020, 55, 83–88. [Google Scholar] [CrossRef]
- Jekayinfa, S.O.; Omisakin, O.S. The energy potentials of some agricultural wastes as local fuel materials in Nigeria. Agric. Eng. Int. CIGR EJ. 2005, 7, 1–10. [Google Scholar]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The influence of dilute sulfuric acid pretreatment on biogas production form wheat plant. Int. J. Green Energy 2016, 13, 1129–1134. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Horváth, I.S. Improvement of solid-state biogas production from wood by concentrated phosphoric acid pretreatment. Bioresources 2016, 11, 3230–3243. [Google Scholar] [CrossRef]



| Sample | DES/Molar Ratio | Solid-to-Liquid Ratio (w:w) | Pretreatment Temperature (°C) | Pretreatment Time (min) | Yield (%) |
|---|---|---|---|---|---|
| A | AU 1:1 | 1:2 | 80 | 60 | 86.3 |
| B | AU 1:1 | 1:4 | 80 | 60 | 85.6 |
| C | AU 1:1 | 1:2 | 100 | 60 | 83.1 |
| D | AU 1:1 | 1:4 | 100 | 60 | 82.0 |
| E | AU 1:2 | 1:2 | 80 | 60 | 82.8 |
| F | AU 1:2 | 1:4 | 80 | 60 | 83.0 |
| G | AU 1:2 | 1:2 | 100 | 60 | 84.3 |
| Sample | MC 1 (%) | Ash (%) | C/N | TS 2 (%) | VS 3 (%) | AIL 4 (%) | ASL 5 (%) | CHO (%) |
|---|---|---|---|---|---|---|---|---|
| A | 8.1 ± 0.8 | 1.00 ± 0.03 | 46.4 | 92 ± 1 * | 91 ± 1 * | 17.3 ± 0.9 * | 1.00 ± 0.03 * | 73.56 |
| B | 9.0 ± 0.3 | 1.2 ± 0.7 | 46.4 | 91 ± 0.4 | 90 ± 2 | 14.7 ± 0.1 * | 1.3 ± 0.2 * | 75.08 |
| C | 8 ± 2 | 1.1 ± 0.5 | 37.3 | 92 ± 2 * | 91.0 ± 2 | 16.6 ± 0.6 * | 1.5 ± 0.2 * | 74.09 |
| D | 10 ± 1 | 1.4 ± 0.8 | 37.8 | 90 ± 2 | 88 ± 3 * | 16 ± 1 * | 1.60 ± 0.06 | 71.94 |
| E | 8 ± 1 | 3.3 ± 0.9 | 41.9 | 92 ± 2 | 89 ± 3 * | 19 ± 1 * | 1.30 ± 0.06 | 72.44 |
| F | 7 ± 1 | 2.3 ± 0.9 | 45.9 | 93 ± 1 * | 91 ± 3 * | 15 ± 4 * | 1.4 ± 0.1 | 76.86 |
| G | 7.5 ± 0.1 | 0.26 ± 0.05 | 38.5 | 92.5 ± 0.1 | 92.4 * ± 0.1 | 13 ± 3 * | 1.60 ± 0.09 * | 78.10 |
| H | 8.1 ± 0.2 | 1.9 ± 0.2 | 39.7 | 91.9 ± 0.3 | 90.0 ± 0.6 | 14 ± 3 * | 1.5 ± 0.1 * | 76.43 |
| I | 8.1 ± 0.8 | 4.8 ± 0.3 | 49.9 | 92 ± 1 | 87 ± 1 | 17 ± 4 | 1.4 ± 0.2 | 72.85 |
| Sample | AIL Degradation (%) | ASL Degradation (%) |
|---|---|---|
| A | NA | NA |
| B | 13.53 | 7.14 |
| C | 2.35 | NA |
| D | 5.88 | NA |
| E | NA | 7.14 |
| F | 11.77 | NA |
| G | 23.53 | NA |
| H | 17.65 | NA |
| I | A | B | C | D | E | F | G | H | |
|---|---|---|---|---|---|---|---|---|---|
| CrI (%) | 54.9 | 54.1 | 55.9 | 52.9 | 55.1 | 59.3 | 62.1 | 58.4 | 57.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olugbemide, A.D.; Oberlintner, A.; Novak, U.; Likozar, B. Lignocellulosic Corn Stover Biomass Pre-Treatment by Deep Eutectic Solvents (DES) for Biomethane Production Process by Bioresource Anaerobic Digestion. Sustainability 2021, 13, 10504. https://doi.org/10.3390/su131910504
Olugbemide AD, Oberlintner A, Novak U, Likozar B. Lignocellulosic Corn Stover Biomass Pre-Treatment by Deep Eutectic Solvents (DES) for Biomethane Production Process by Bioresource Anaerobic Digestion. Sustainability. 2021; 13(19):10504. https://doi.org/10.3390/su131910504
Chicago/Turabian StyleOlugbemide, Akinola David, Ana Oberlintner, Uroš Novak, and Blaž Likozar. 2021. "Lignocellulosic Corn Stover Biomass Pre-Treatment by Deep Eutectic Solvents (DES) for Biomethane Production Process by Bioresource Anaerobic Digestion" Sustainability 13, no. 19: 10504. https://doi.org/10.3390/su131910504






