Smart and Sustainable Aquaculture Farms
Abstract
:1. Introduction
2. Methodology
3. Aquaculture Farm Concept and Design
3.1. General Concept
3.2. Farm Management
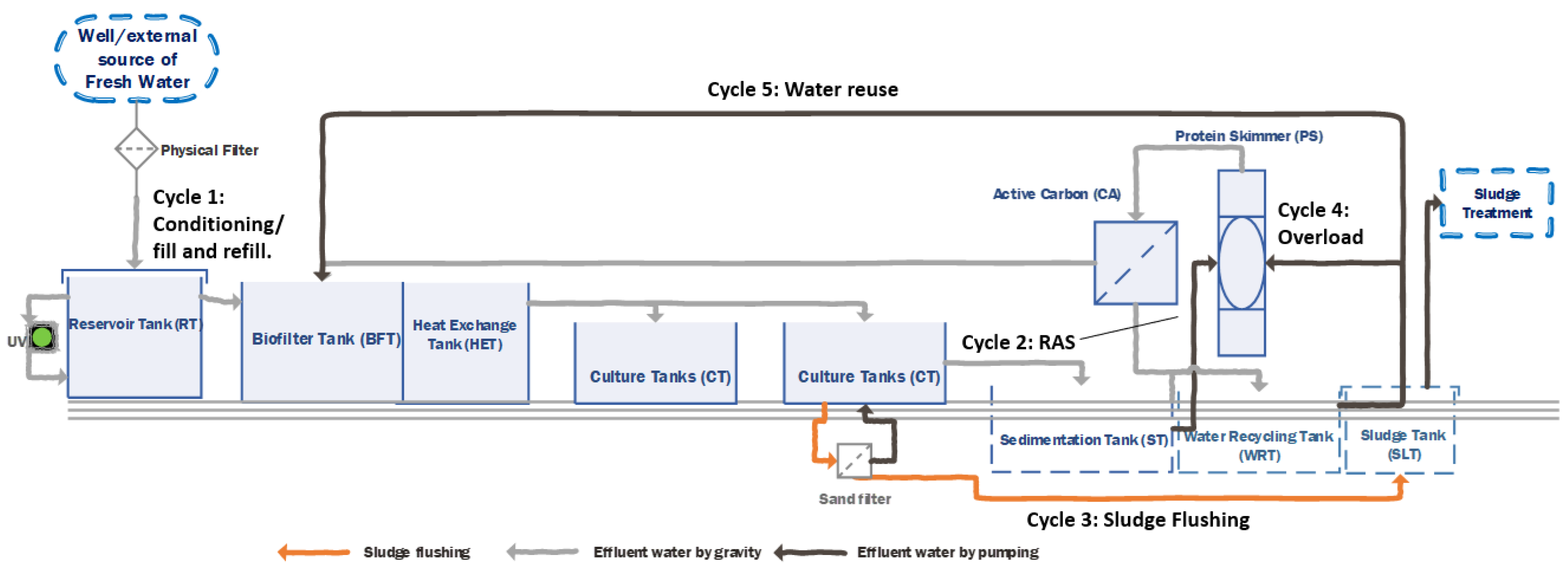
Hydraulic System and Water Flow
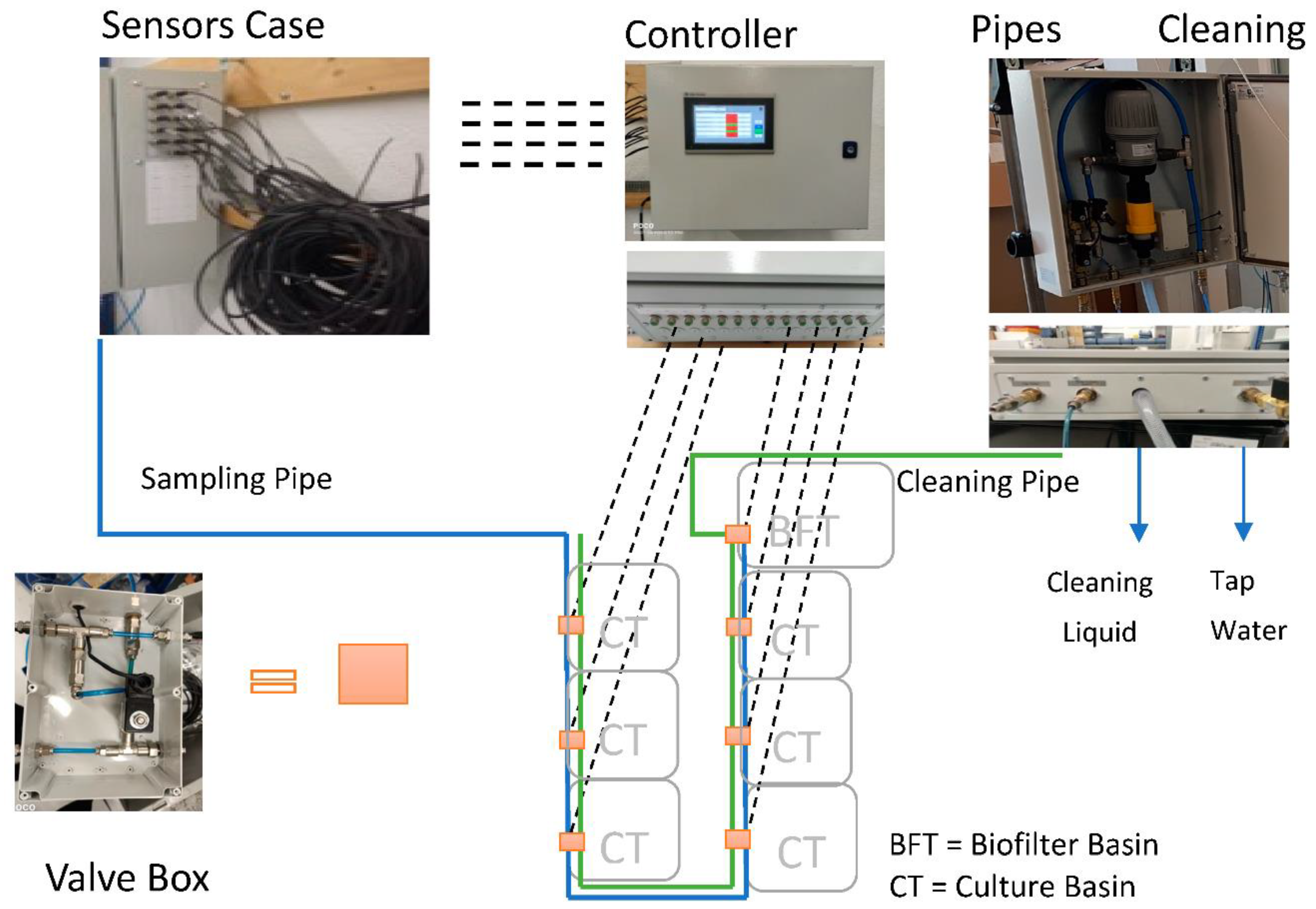
3.3. Monitoring System
Architecture
3.4. Monitoring Process
3.5. Feeding Regime
4. Implementation, Results, and Discussion
4.1. Water Quality
4.2. Shrimp Growth Rate
4.3. Feed and Post-Larval Shrimp (PLs) Used
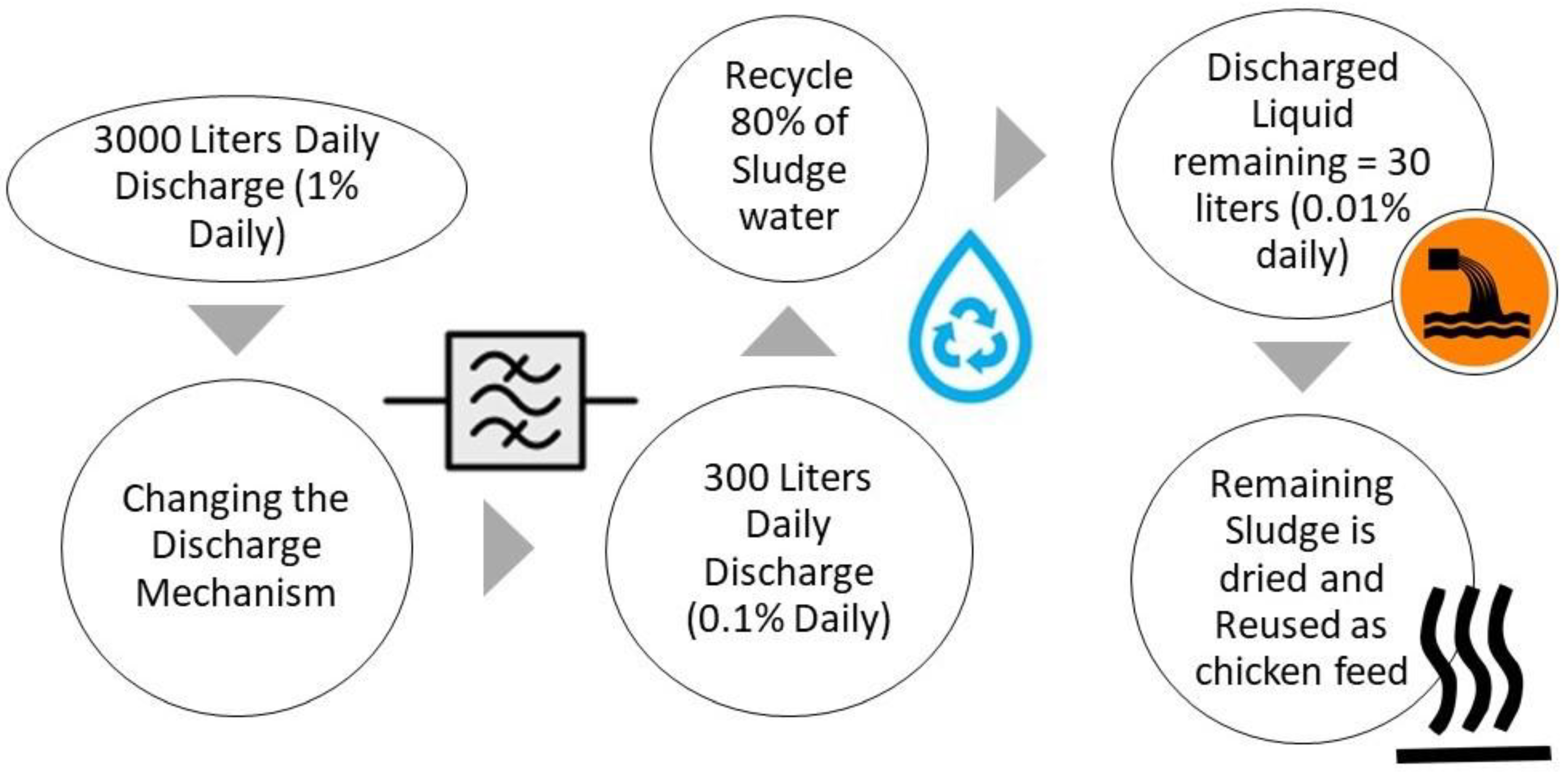
4.4. Energy Consumption and Global Cultivation
4.5. Shrimp Quality
4.6. Initial Costs of Investments and Operational Costs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAO Yearbook. Fishery and Aquaculture Statistics, 2017. Available online: http://www.fao.org/3/ca5495t/ca5495t.pdf (accessed on 27 June 2021).
- Nadarajah, S.; Flaaten, O. Global aquaculture growth and institutional quality. Mar. Policy 2017, 84, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Hilborn, R.; Amoroso, R.O.; Anderson, C.M.; Baum, J.K.; Branch, T.A.; Costello, C.; De Moor, C.L.; Faraj, A.; Hively, D.; Jensen, O.P.; et al. Effective fisheries management instrumental in improving fish stock status. Proc. Natl. Acad. Sci. USA 2020, 117, 2218–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S. Aquaculture, capture fisheries, and wild fish stocks. Resour. Energy Econ. 2010, 32, 65–77. [Google Scholar] [CrossRef]
- FAO. The 2020 edition of The State of World Fisheries and Aquaculture. Nat. Resour. 2020, 35, 4–13. [Google Scholar]
- Veiga, P.; Mendes, M.; Lee-Harwood, B. Reduction Fisheries: SFP Fisheries Sustainability Overview 2018. Sustainability Fisheries Partnership. Available online: https://www.sustainablefish.org/Media/Files/Reduction-Fisheries-Reports/2018-Reduction-Fisheries-Report (accessed on 27 June 2021).
- Gauthier, D.T. Bacterial zoonoses of fishes: A review and appraisal of evidence for linkages between fish and human infections. Vet. J. 2015, 203, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Glebova, I.A.; Larionova, A.A.; Zaitseva, N.A.; Grunina, A.A.; Chvyakin, V.A.; Takhumova, O.V.; Glagoleva, L.E. Organic Aquaculture as a Promising Direction for the Production of Organic Food. Ekoloji 2019, 28, 537–543. [Google Scholar]
- Farheen, U. Automatic Controlling of Fish Feeding System. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 362–366. [Google Scholar] [CrossRef]
- Suantika, G.; Pratiwi, M.I.; Situmorang, M.L.; Djohan, Y.A.; Muhammad, H.; Astuti, D.I. Ammonium Removal by Nitrifying Bacteria Biofilm on Limestone and Bioball Substrate Established in Freshwater Trickling Biofilter. Poultry Fish. Wild. Sci. 2016, 4, 1000157. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Kim, D.H. An optimization scheme for water pump control in smart fish farm with efficient energy consumption. Processes 2018, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Garlock, T.; Asche, F.; Anderson, J.; Bjørndal, T.; Kumar, G.; Lorenzen, K.; Ropicki, A.; Smith, M.D.; Tveterås, R. A Global Blue Revolution: Aquaculture Growth Across Regions, Species, and Countries. Rev. Fish. Sci. Aquac. 2020, 28, 1–10. [Google Scholar] [CrossRef]
- FAO. Aquaculture Newsletter, 2018. Available online: http://www.fao.org/3/i9200en/I9200EN.pdf (accessed on 27 June 2021).
- Avnimelech, Y. Control of microbial activity in aquaculture systems: Active suspension ponds. World Aquac.-Baton Rouge 2003, 34, 19–21. Available online: https://www.was.org/Magazine/Vol/34/4#.X4zuM9BKjIU (accessed on 1 August 2021).
- Muhammad, H.; Situmorang, M.L.; Djohan, Y.A.; Aditiawati, P.; Suantika, G. Biological, Technical, and Financial Feasibilities Study of Zero Water Discharge (ZWD) System Application in Low Salinity White Shrimp (Litopenaeus vannamei Boone) Urban Aquaculture, Study Case: Gresik District, East Java, Indonesia. J. Fish. Livest. Prod. 2016, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Suantika, G.; Situmorang, M.L.; Kurniawan, J.B.; Pratiwi, S.A.; Aditiawati, P.; Astuti, D.I.; Azizah, F.F.N.; Djohan, Y.A.; Zuhri, U.; Simatupang, T.M. Development of a zero water discharge (ZWD)—Recirculating aquaculture system (RAS) hybrid system for super intensive white shrimp (Litopenaeus vannamei) culture under low salinity conditions and its industrial trial in commercial shrimp urban farming in Gresik, East Java, Indonesia. Aquac. Eng. 2018, 82, 12–24. [Google Scholar] [CrossRef]
- Suantika, G.; Situmorang, M.L.; Nurfathurahmi, A.; Taufik, I.; Aditiawati, P.; Yusuf, N.; Aulia, R. Application of Indoor Recirculation Aquaculture System for White Shrimp (Litopenaeus vannamei) Growout Super-Intensive Culture at Low Salinity Condition. J. Aquac. Res. Dev. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Suantika, G.; Turendro, O.R.; Situmorang, M.L. Use of Nitrifying Bacteria for Promoting Giant Freshwater Prawn (Macrobrachium rosenbergii de Man) Nursery Phase in Indoor System. J. Fish. Livest. Prod. 2017, 5, 228. [Google Scholar] [CrossRef] [Green Version]







| Parameter | Optimum Range | Action, If Water Quality Doesn’t Fall in the Optimum Range |
|---|---|---|
| Ammonia (ppm) | <1 | Circulation through RAS |
| Nitrite (ppm) | <1 | Circulation through RAS |
| pH | 7–8.5 | Addition of CaCO3 5–10 ppm (if pH < 7) Addition of Bacillus 2–5 ppm (if pH > 8.5) |
| ∆pH (day-night) | 0–0.5 | Addition of NaHCO3 2–5 ppm |
| Total Vibrio (CFU/mL) | <104 | Addition of Bacillus, 0.5–1.5 ppm with fermented molasses enrichment |
| Alkalinity level (ppm) | 120–150 | Addition of NaHCO3 2–5 ppm |
| Diatom/Green Algae Dominance (%) | 50–90 | Addition of Kaolin Clay powder 2–5 ppm |
| Temperature (°C) | 27–30 | Set up water heater (for increasing the temperature) or chiller (for decreasing the temperature) |
| Salinity (ppt) | 25–30 | Addition of seawater to increase salinity and freshwater. to reduce salinity |
| Dissolved Oxygen/DO (ppm) | 4–9 | Aeration set up |
| MBW (g) | FR (%) | MBW (g) | FR (%) |
|---|---|---|---|
| 1.0–1.9 | 11–8 | 11.0–11.9 | 3.5–3.25 |
| 2.0–2.9 | 8–7 | 12.0–12.9 | 3.25–3.0 |
| 3.0–3.9 | 7–6 | 13.0–13.9 | 3.0–2.75 |
| 4.0–4.9 | 6–5.5 | 14.0–14.9 | 2.75–2.5 |
| 5.0–5.9 | 5.5–5.0 | 15.0–15.9 | 2.5–2.3 |
| 6.0–6.9 | 5.0–4.5 | 16.0–16.9 | 2.3–2.1 |
| 7.0–7.9 | 4.5–4.25 | 17.0–17.9 | 2.1–2.0 |
| 8.0–8.9 | 4.25–4.0 | 18.0–18.9 | 2.0–1.9 |
| 9.0–9.9 | 4.0–3.75 | 19.0–19.9 | 1.9–1.8 |
| 10.0–10.9 | 3.75–3.5 | 20.0–20.9 | 1.8–1.7 |
| The Residual Feed—In Feeding Tray | Scoring | Action | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 0 | 0 | 0 | 0 | 4/4 | Add 5–10% |
| 0 | 0 | 0 | + | 3/4 | Add 0–5% |
| + | 0 | + | 0 | 2/4 | Decrease 0–5% |
| + | + | 0 | + | 1/4 | Decrease 10–15% |
| + | + | + | + | 0/4 | Decrease 20–30% |
| Basin 1 | Basin 2 | Basin 3 | Basin 4 | Basin 5 | Basin 6 | |
|---|---|---|---|---|---|---|
| 1 | 2.29 | 3.56 | 3.77 | 3.76 | 4.64 | 4.32 |
| 2 | 3.91 | 3.73 | 2.55 | 3.71 | 2.68 | 3.66 |
| 3 | 2.68 | 3.54 | 3.89 | 3.85 | 2.87 | 3.44 |
| 4 | 3.36 | 3.93 | 4.08 | 2.97 | 2.54 | 3.65 |
| 5 | 2.95 | 2.81 | 1.13 | 4.31 | 2.95 | 4.38 |
| 6 | 4.72 | 4.87 | 2.78 | 4.02 | 2.52 | 3.11 |
| 7 | 3.35 | 4.99 | 3.43 | 2.93 | 4.51 | 2.71 |
| 8 | 2372 | 4.63 | 3.68 | 3.4 | 4.1 | 3.27 |
| 9 | 2.79 | 4.24 | 2.07 | 3.15 | 3.06 | 3.39 |
| 10 | 3.15 | 2.34 | 3.75 | 3.85 | 3.77 | 3.14 |
| 11 | 3.71 | 2.78 | 3.63 | 4.07 | 5.64 | 5.75 |
| 12 | 3.54 | 3.19 | 3.32 | 4.86 | 5.34 | 3.9 |
| 13 | 3.11 | 4.33 | 3.92 | 3.34 | 4.98 | 3.79 |
| 14 | 3.46 | 2.51 | 3.99 | 4.25 | 4.14 | 3.94 |
| 15 | 2.12 | 3.78 | 3.05 | 3.85 | 5.34 | 5.76 |
| 16 | 3.07 | 3.02 | 4.01 | 1.9 | 4.03 | 4.89 |
| 17 | 3.03 | 5.06 | 4.48 | 5.74 | 4.55 | 4.76 |
| 18 | 3.21 | 5.69 | 4.24 | 2.3 | 3.91 | 6.23 |
| 19 | 2.23 | 2.17 | 3.68 | 4.86 | 3.46 | 3.66 |
| 20 | 2.23 | 3.36 | 4.06 | 2.61 | 4.13 | 3.69 |
| 21 | 8.35 | 4.89 | 3.7 | 3.5 | 3.86 | 5.51 |
| 22 | 5 | 3.17 | 4.18 | 3.4 | 3 | 4.69 |
| 23 | 3.3 | 4.59 | 2.78 | 3.67 | 3.87 | 5.61 |
| 24 | 5.14 | 3.58 | 3.77 | 3.74 | 2.74 | 5.48 |
| 25 | 5.44 | 3.82 | 3.63 | 3.85 | 4.13 | 1.76 |
| 26 | 4.76 | 3.17 | 4.79 | 4.2 | 3.79 | 4.34 |
| 27 | 4.7 | 2.84 | 4.34 | 3.73 | 4.89 | 4.33 |
| 28 | 3.26 | 2.56 | 5.15 | 3.53 | 3.96 | 5.78 |
| 29 | 3.01 | 2.91 | 4.13 | 5.15 | 4.23 | 3.35 |
| 30 | 5.57 | 2.5 | 4.74 | 3.93 | 4.46 | 4.44 |
| Sampling | Av. Weight | Daily Growth | Max Record |
|---|---|---|---|
| Week 4 | 0.81 | 0.03 | 1.15 |
| Week 5 | 1.55 | 0.11 | 2.83 |
| Week 6 | 2.42 | 0.12 | 4.38 |
| Week 7 | 3.68 | 0.18 | 6.32 |
| Week 8 | 4.86 | 0.17 | 8.35 |
| Week 9 | 6.39 | 0.22 | 9.14 |
| Week 10 | 7.96 | 0.22 | 10.84 |
| Week 11 | 9.00 | 0.15 | 11.17 |
| Week 12 | 10.00 | 0.14 | 12.21 |
| Week 13 | 11.94 | 0.27 | 15.64 |
| Feeding Suggestion: Shrimp Age–Size | Feed Type | Form | Size (mm) | Crude Protein (Min) | Crude Fat (Min) | Ash (Max) | Fiber (Max) | Moisture (Max) |
|---|---|---|---|---|---|---|---|---|
| 1–10 days | Starter 1 | Crumbles | 0.6–1.2 | 34% | 7% | 15% | 4% | 11% |
| 11–30 days | Starter 2 | Crumbles | 1.2–2.0 | 34% | 7% | 15% | 4% | 11% |
| 3–5 gr | Starter 3 | Crumbles | 1.4–2.5 | 34% | 7% | 15% | 4% | 11% |
| 5–15 gr | Grower 1 | Pellet | 2.2 × 3.0–5.0 | 34% | 7% | 15% | 4% | 11% |
| >15 gr | Grower 2 | Pellet | 2.2 × 3.0–6.0 | 34% | 7% | 15% | 4% | 11% |
| Total Cost of Investment | USD 26.43 per Kilogram |
|---|---|
| Isolated Hangar (1564 m2) and Administration Building (482 m2) | 76% |
| Plumping and Heating System | 6% |
| Smart System | 6% |
| Culture System | 10% |
| Project Management and Installation | 3% |
| Total Fixed Costs | USD 4.87 per Kilogram |
| Depreciation | 34% |
| Maintenance | 17% |
| Farm Keeper Salary | 4% |
| Electricity | 41% |
| Preparation Labor Costs | 1% |
| Harvesting Labor Cost | 1% |
| Cleaning Labor Costs | 1% |
| Transportation | 1% |
| Total Variable Costs | USD 2.87 per Kilogram |
| Shrimp Feed | 68% |
| Prebiotic and Probiotics | 4% |
| Shrimp Larvae | 25% |
| Shrimp Larvae Transportation | 1% |
| Harvesting Package Costs | 2% |
| Total Cost of Production | USD 7.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassem, T.; Shahrour, I.; El Khattabi, J.; Raslan, A. Smart and Sustainable Aquaculture Farms. Sustainability 2021, 13, 10685. https://doi.org/10.3390/su131910685
Kassem T, Shahrour I, El Khattabi J, Raslan A. Smart and Sustainable Aquaculture Farms. Sustainability. 2021; 13(19):10685. https://doi.org/10.3390/su131910685
Chicago/Turabian StyleKassem, Taher, Isam Shahrour, Jamal El Khattabi, and Ahmad Raslan. 2021. "Smart and Sustainable Aquaculture Farms" Sustainability 13, no. 19: 10685. https://doi.org/10.3390/su131910685
APA StyleKassem, T., Shahrour, I., El Khattabi, J., & Raslan, A. (2021). Smart and Sustainable Aquaculture Farms. Sustainability, 13(19), 10685. https://doi.org/10.3390/su131910685








