Influence of Intraspecific Competition Stress on Soil Fungal Diversity and Composition in Relation to Tree Growth and Soil Fertility in Sub-Tropical Soils under Chinese Fir Monoculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Plantation Establishment
2.2. Soil Sampling and Physiochemical Analysis
2.3. Soil Physiochemical Properties and Canopy Dynamics
2.4. DNA Extraction, PCR Amplification, and Illumina Hiseq 2500 Sequencing
2.5. Sequence Processing and Functional Assignment
2.6. Statistical Analysis
3. Results
3.1. Distribution of Soil Fungal Communities
3.2. Soil Fungal Diversity and Communities Structure
3.3. Potential Drivers of Soil Fungal Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, J. Measurements of the soil microbial community for estimating the success of restoration. Eur. J. Soil Sci. 2003, 54, 801–808. [Google Scholar] [CrossRef]
- Carter, M.; Gregorich, E.; Angers, D.; Beare, M.; Sparling, G.; Wardle, D.; Voroney, R. Interpretation of microbial biomass measurements for soil quality assessment in humid temperate regions. Can. J. Soil Sci. 1999, 79, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Winding, A.; Hund-Rinke, K.; Rutgers, M. The use of microorganisms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 230–248. [Google Scholar] [CrossRef]
- Crecchio, C.; Gelsomino, A.; Ambrosoli, R.; Minati, J.L.; Ruggiero, P. Functional and molecular responses of soil microbial communities under differing soil management practices. Soil Biol. Biochem. 2004, 36, 1873–1883. [Google Scholar] [CrossRef]
- Serna-Chavez, H.M.; Fierer, N.; Van Bodegom, P.M. Global drivers and patterns of microbial abundance in soil. Glob. Ecol. Biogeogr. 2013, 22, 1162–1172. [Google Scholar] [CrossRef]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Farooq, T.H.; Wu, W.; Tigabu, M.; Ma, X.; Zou, X.H.; Rashid, M.H.U.; Gilani, M.M.; Wu, P.F. Growth, biomass production and root development of Chinese fir in relation to initial planting density. Forests 2019, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Farooq, T.H.; Ma, X.; Rashid, M.H.U.; Wu, W.; Xu, J.; Tarin, M.W.K.; He, Z.; Wu, P. Impact of stand density on soil quality in Chinese Fir (Cunninghamia lanceolata) monoculture. Appl. Ecol. Environ. Res. 2019, 17, 3553–3566. [Google Scholar] [CrossRef]
- Farooq, T.H.; Yan, W.; Chen, X.; Shakoor, A.; Rashid, M.H.U.; Gilani, M.M.; He, Z.; Wu, P. Dynamics of canopy development of Cunninghamia lanceolata mid-age plantation in relation to foliar nitrogen and soil quality influenced by stand density. Glob. Ecol. Conserv. 2020, 24, e01209. [Google Scholar] [CrossRef]
- Farooq, T.H.; Chen, X.; Shakoor, A.; Li, Y.; Wang, J.; Rashid, M.H.U.; Kumar, U.; Yan, W. Unraveling the Influence of Land-Use Change on δ13C, δ15N, and Soil Nutritional Status in Coniferous, Broadleaved, and Mixed Forests in Southern China: A Field Investigation. Plants 2021, 10, 1499. [Google Scholar] [CrossRef]
- Wu, P.; Tigabu, M.; Ma, X.; Christer Odén, P.; He, Y.; Yu, X.; He, Z. Variations in biomass, nutrient contents and nutrient use efficiency among Chinese fir provenances. Silvae Genet. 2011, 60, 95. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.H.U.; Asif, M.; Farooq, T.H.; Gautam, N.P.; Nawaz, M.F.; Ahmad, I.; Gilani, M.M.; Wu, P. Cuttings growth response of Dalbergia sissoo (shisham) to soil compaction stress. Appl. Ecol. Environ. Res. 2019, 17, 1049–1059. [Google Scholar] [CrossRef]
- Gilani, M.M.; Irfan, A.; Farooq, T.H.; Wu, P.; Yousaf, M.S.; Khan, M.W.; Talha, Y.; Ma, X. Effects of pre-sowing treatments on seed germination and morphological growth of Acacia nilotica and Faidherbia albida. Sci. For. 2019, 122, 374–382. [Google Scholar] [CrossRef]
- Gilani, M.M.; Tigabu, M.; Liu, B.; Farooq, T.H.; Rashid, M.H.U.; Ramzan, M.; Ma, X. Seed germination and seedling emergence of four tree species of southern China in response to acid rain. J. For. Res. 2020, 32, 471–481. [Google Scholar] [CrossRef]
- Yousaf, M.S.; Farooq, T.H.; Ahmad, I.; Gilani, M.M.; Rashid, M.H.U.; Gautam, N.P.; Islam, W.; Asif, M.; Wu, P. Effect of Drought Stress on the Growth and Morphological Traits of Eucalyptus camaldulensis and Eucalyptus Citriodora. PSM Biol. Res. 2018, 3, 85–91. [Google Scholar]
- Farooq, T.H.; Kumar, U.; Mo, J.; Shakoor, A.; Wang, J.; Rashid, M.H.U.; Tufail, M.A.; Chen, X.; Yan, W. Intercropping of Peanut–Tea Enhances Soil Enzymatic Activity and Soil Nutrient Status at Different Soil Profiles in Subtropical Southern China. Plants 2021, 10, 881. [Google Scholar] [CrossRef]
- Terborgh, J. Enemies maintain hyperdiverse tropical forests. Am. Nat. 2012, 179, 303–314. [Google Scholar] [CrossRef]
- Kos, M.; Veendrick, J.; Bezemer, T.M. Local variation in conspecific plant density influences plant–soil feedback in a natural grassland. Basic Appl. Ecol. 2013, 14, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Pradisty, N.A.; Amir, A.A.; Zimmer, M. Plant species-and stage-specific differences in microbial decay of mangrove leaf litter: The older the better? Oecologia 2021, 195, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Du, H.; Duan, A.; Zhang, J. Effect of stand density and soil layer on soil nutrients of a 37-year-old Cunninghamia lanceolata plantation in Naxi, Sichuan Province, China. Sustainability 2019, 11, 5410. [Google Scholar] [CrossRef] [Green Version]
- Farooq, T.H.; Shakoor, A.; Wu, X.; Li, Y.; Rashid, M.H.U.; Zhang, X.; Gilani, M.M.; Kumar, U.; Chen, X.; Yan, W. Perspectives of plantation forests in the sustainable forest development of China. iForest 2021, 14, 166. [Google Scholar] [CrossRef]
- Farooq, T.H.; Yan, W.; Rashid, M.H.U.; Tigabu, M.; Gilani, M.M.; Zou, X.H.; Wu, P.F. Chinese fir (Cunninghamia lanceolata) a green gold of China with continues decline in its productivity over the successive rotations: A review. Appl. Ecol. Environ. Res. 2019, 17, 11055–11067. [Google Scholar] [CrossRef]
- Wu, P.; Wang, G.; Farooq, T.H.; Li, Q.; Zou, X.; Ma, X. Low phosphorus and competition affect Chinese fir cutting growth and root organic acid content: Does neighboring root activity aggravate P nutrient deficiency? J. Soils Sediments 2017, 17, 2775–2785. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, J.; Lu, C.; Ou, X.; Luo, K.; Li, C.; He, M.; Zhang, H.; Yan, H. Intercropping with turmeric or ginger reduce the continuous cropping obstacles that affect Pogostemon cablin (patchouli). Front. Microbiol. 2020, 11, 2526. [Google Scholar] [CrossRef]
- Tayyab, M.; Islam, W.; Lee, C.G.; Pang, Z.; Khalil, F.; Lin, S.; Lin, W.; Zhang, H. Short-term effects of different organic amendments on soil fungal composition. Sustainability 2019, 11, 198. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Dahiya, D.; Vasanth, D. Characteristics of Microbial Community and Enzyme Activities in Higher Altitude Regions. In Microbiological Advancements for Higher Altitude Agro-Ecosystems & Sustainability; Springer: Singapore, 2020; pp. 201–226. [Google Scholar]
- Dang, P.; Vu, N.H.; Shen, Z.; Liu, J.; Zhao, F.; Zhu, H.; Yu, X.; Zhao, Z. Changes in soil fungal communities and vegetation following afforestation with Pinus tabulaeformis on the Loess Plateau. Ecosphere 2018, 9, e02401. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wang, N.-F.; Liu, H.-Y.; Zhang, Y.-Q.; Yu, L.-Y. Soil pH is a key determinant of soil fungal community composition in the Ny-Ålesund Region, Svalbard (High Arctic). Front. Microbiol. 2016, 7, 227. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Arafat, Y.; Lin, W. Studies on fungal communities and functional guilds shift in tea continuous cropping soils by high-throughput sequencing. Ann. Microbiol. 2020, 70, 7. [Google Scholar] [CrossRef] [Green Version]
- Siles, J.A.; Margesin, R. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient in alpine forest soils: What are the driving factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, S.D.; Palmer, A.S.; Winsley, T.; Lamb, E.; Bissett, A.; Brown, M.V.; van Dorst, J.; Ji, M.; Ferrari, B.C.; Grogan, P. Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol. Biochem. 2014, 78, 10–20. [Google Scholar] [CrossRef]
- Gill, A.S.; Purnell, K.; Palmer, M.I.; Stein, J.; McGuire, K.L. Microbial Composition and Functional Diversity Differ Across Urban Green Infrastructure Types. Front. Microbiol. 2020, 11, 912. [Google Scholar] [CrossRef]
- Ahmed, P.M.; De Figueroa, L.I.; Pajot, H.F. Dual Purpose of ligninolytic-basidiomycetes: Mycoremediation of bioethanol distillation vinasse coupled to sustainable bio-based compounds production. Fungal Biol. Rev. 2020, 34, 25–40. [Google Scholar] [CrossRef]
- Lee, H.; Oh, S.-Y.; Lee, Y.M.; Jang, Y.; Jang, S.; Kim, C.; Lim, Y.W.; Kim, J.-J. Successional variation in the soil microbial community in Odaesan National Park, Korea. Sustainability 2020, 12, 4795. [Google Scholar] [CrossRef]
- Yurkov, A. Yeasts in forest soils. In Yeasts in Natural Ecosystems: Diversity; Springer: Berlin/Heidelberg, Germany, 2017; pp. 87–116. [Google Scholar]
- Štursová, M.; Žifčáková, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose utilization in forest litter and soil: Identification of bacterial and fungal decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Mašínová, T.; Bahnmann, B.D.; Větrovský, T.; Tomšovský, M.; Merunková, K.; Baldrian, P. Drivers of yeast community composition in the litter and soil of a temperate forest. FEMS Microbiol. Ecol. 2017, 93, fiw223. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.; Dikilitas, M.; Abdel-Azeem, A.M.; Ahluwalia, A.S.; Saxena, A.K. Biodiversity, and biotechnological contribution of beneficial soil microbiomes for nutrient cycling, plant growth improvement and nutrient uptake. Biocatal. Agric. Biotechnol. 2021, 33, 102009. [Google Scholar] [CrossRef]
- Kusai, N.A.; Ayob, Z.; Maidin, M.S.T.; Safari, S.; Ali, S.R.A. Characterization of fungi from different ecosystems of tropical peat in Sarawak, Malaysia. Rend. Lincei. Sci. Fis. E Nat. 2018, 29, 469–482. [Google Scholar] [CrossRef]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S.; Lind, E.M.; Seabloom, E.W.; Adler, P.B.; Bakker, J.D. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2015, 18, 85–95. [Google Scholar] [CrossRef]
- Chen, W.; Xu, R.; Wu, Y.; Chen, J.; Zhang, Y.; Hu, T.; Yuan, X.; Zhou, L.; Tan, T.; Fan, J. Plant diversity is coupled with beta not alpha diversity of soil fungal communities following N enrichment in a semi-arid grassland. Soil Biol. Biochem. 2018, 116, 388–398. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tayyab, M.; Abubakar, A.Y.; Yang, Z.; Pang, Z.; Islam, W.; Lin, Z.; Li, S.; Luo, J.; Fan, X. Bacteria with different assemblages in the soil profile drive the diverse nutrient cycles in the sugarcane straw retention ecosystem. Diversity 2019, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wang, X.; Chen, F.; Li, C.; Wu, L. Effects of the successive planting of Eucalyptus urophylla on soil bacterial and fungal community structure, diversity, microbial biomass, and enzyme activity. Land Degrad. Dev. 2019, 30, 636–646. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Bu, R.; Ren, T.; Lei, M.; Liu, B.; Li, X.; Cong, R.; Zhang, Y.; Lu, J. Tillage and straw-returning practices effect on soil dissolved organic matter, aggregate fraction and bacteria community under rice-rice-rapeseed rotation system. Agric. Ecosyst. Environ. 2020, 287, 106681. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, G.; Wu, Z.; Wen, X.; Zhong, H.; Zhong, Z.; Bian, F.; Gai, X. Agroforestry alters the rhizosphere soil bacterial and fungal communities of moso bamboo plantations in subtropical China. Appl. Soil Ecol. 2019, 143, 192–200. [Google Scholar] [CrossRef]
- Gou, X.; Cai, Y.; Wang, C.; Li, B.; Zhang, R.; Zhang, Y.; Tang, X.; Chen, Q.; Shen, J.; Deng, J. Effects of different long-term cropping systems on phoD-harboring bacterial community in red soils. J. Soils Sediments 2021, 21, 376–387. [Google Scholar] [CrossRef]
- Okal, E.J.; Aslam, M.M.; Karanja, J.K.; Nyimbo, W.J. Mini review: Advances in understanding regulation of cellulase enzyme in white-rot basidiomycetes. Microb. Pathog. 2020, 147, 104410. [Google Scholar] [CrossRef]
- Tarin, M.W.K.; Fan, L.; Xie, D.; Tayyab, M.; Rong, J.; Chen, L.; Muneer, M.A.; Zheng, Y. Response of Soil Fungal Diversity and Community Composition to Varying Levels of Bamboo Biochar in Red Soils. Microorganisms 2021, 9, 1385. [Google Scholar] [CrossRef]
- Anthony, M.; Frey, S.; Stinson, K. Fungal community homogenization, shift in dominant trophic guild, and appearance of novel taxa with biotic invasion. Ecosphere 2017, 8, e01951. [Google Scholar] [CrossRef] [Green Version]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Van Nuland, M.E.; Smith, D.P.; Bhatnagar, J.M.; Stefanski, A.; Hobbie, S.E.; Reich, P.B.; Peay, K.G. Warming and disturbance alter soil microbiome diversity and function in a northern forest ecotone. FEMS Microbiol. Ecol. 2020, 96, fiaa108. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dossa, G.G.; Paudel, E.; Zang, H.; Xu, J.; Harrison, R.D. Changes in fungal communities across a forest disturbance gradient. Appl. Environ. Microbiol. 2019, 85, e00080-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, A.; Arif, M.S.; Tufail, M.A.; Shahzad, S.M.; Farooq, T.H.; Ahmed, W.; Mehmood, T.; Farooq, M.R.; Javed, Z.; Shakoor, A. Biochar potential to relegate metal toxicity effects is more soil driven than plant system: A global meta-analysis. J. Clean. Prod. 2021, 316, 128276. [Google Scholar] [CrossRef]
- Saqib, H.M.U.; Ahmad, I.; Rashid, M.H.U.; Farooq, T.H.; Asif, M.; Kashif, M.; Iqbal, A.; Nawaz, M.F. Effect of Compost Application on the Growth of Acacia nilotica. Cercet. Agron. Mold. 2019, 177, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.H.U.; Farooq, T.H.; Iqbal, W.; Asif, M.; Islam, W.; Lin, D.C.; Ahmad, I.; Wu, P.F. Role of indole acetic acid on growth and biomass production of athel tree (Tamarix aphylla) by using different cutting lengths. Appl. Ecol. Environ. Res. 2020, 18, 3805–3816. [Google Scholar] [CrossRef]
- Farooq, T.H.; Shakoor, A.; Rashid, M.H.U.; Zhang, S.; Wu, P.; Yan, W. Annual Growth Progression, Nutrient Transformation, and Carbon Storage in Tissues of Cunninghamia lanceolata Monoculture in Relation to Soil Quality Indicators Influenced by Intraspecific Competition Intensity. J. Soil Sci. Plant. Nutr. 2021, 1–13. [Google Scholar] [CrossRef]
- Rashid, M.H.U.; Tigabu, M.; Chen, H.; Farooq, T.H.; Ma, X.; Wu, P. Calcium-mediated adaptive responses to low phosphorus stress in Chinese fir. Trees 2020, 34, 825–834. [Google Scholar] [CrossRef]
- Wang, J.; Farooq, T.H.; Aslam, A.; Shakoor, A.; Chen, X.; Yan, W. Non-targeted metabolomics reveal the impact of phenanthrene stress on root exudates of ten urban greening tree species. Environ. Res. 2021, 196, 110370. [Google Scholar] [CrossRef]
- Javed, S.A.; Arif, M.S.; Shahzad, S.M.; Ashraf, M.; Kausar, R.; Farooq, T.H.; Hussain, M.I.; Shakoor, A. Can different salt formulations revert the depressing effect of salinity on maize by modulating plant biochemical attributes and activating stress regulators through improved N Supply? Sustainability 2021, 13, 8022. [Google Scholar] [CrossRef]
- Tufail, M.A.; Bejarano, A.; Shakoor, A.; Naeem, A.; Arif, M.S.; Dar, A.A.; Farooq, T.H.; Pertot, I.; Puopolo, G. Can Bacterial Endophytes Be Used as a Promising Bio-Inoculant for the Mitigation of Salinity Stress in Crop Plants?—A Global Meta-Analysis of the Last Decade (2011–2020). Microorganisms 2021, 9, 1861. [Google Scholar] [CrossRef]
- Shakoor, A.; Shakoor, S.; Rehman, A.; Ashraf, F.; Abdullah, M.; Shahzad, S.M.; Farooq, T.H.; Ashraf, M.; Manzoor, M.A.; Altaf, M.; et al. Effect of animal manure, crop type, climate zone, and soil attributes on greenhouse gas emissions from agricultural soils—A global meta-analysis. J. Clean. Prod. 2020, 278, 124019. [Google Scholar] [CrossRef]
- Shakoor, A.; Shahzad, S.M.; Chatterjee, N.; Arif, M.S.; Farooq, T.H.; Altaf, M.M.; Tufail, M.A.; Dar, A.A.; Mehmood, T. Nitrous oxide emission from agricultural soils: Application of animal manure or biochar? A global meta-analysis. J. Environ. Manag. 2021, 285, 112170. [Google Scholar] [CrossRef]
- Shakoor, A.; Shahbaz, M.; Farooq, T.H.; Sahar, N.E.; Shahzad, S.M.; Altaf, M.M.; Ashraf, M. A global meta-analysis of greenhouse gases emission and crop yield under no-tillage as compared to conventional tillage. Sci. Total Environ. 2021, 750, 142299. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, A.; Arif, M.S.; Shahzad, S.M.; Farooq, T.H.; Ashraf, F.; Altaf, M.M.; Ahmed, W.; Tufail, M.A.; Ashraf, M. Does biochar accelerate the mitigation of greenhouse gaseous emissions from agricultural soil?—A global meta-analysis. Environ. Res. 2021, 202, 111789. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ouyang, D.; Lei, J.; Tan, Q.; Xie, L.; Li, Z.; Liu, T.; Xiao, Y.; Farooq, T.H.; et al. Systematical Review of Interactions Between Microplastics and Microorganisms in the Soil Environment. J. Hazard. Mater. 2021, 418, 126288. [Google Scholar] [CrossRef]
- Farooq, T.H.; Xincheng, X.; Shakoor, A.; Rashid, M.H.U.; Bashir, M.F.; Nawaz, M.F.; Kumar, U.; Shahzad, S.M.; Yan, W. Spatial distribution of carbon dynamics and nutrient enrichment capacity in different layers and tree tissues of Castanopsis eyeri natural forest ecosystem. Environ. Sci. Pollut. 2021, 1–13. [Google Scholar] [CrossRef]
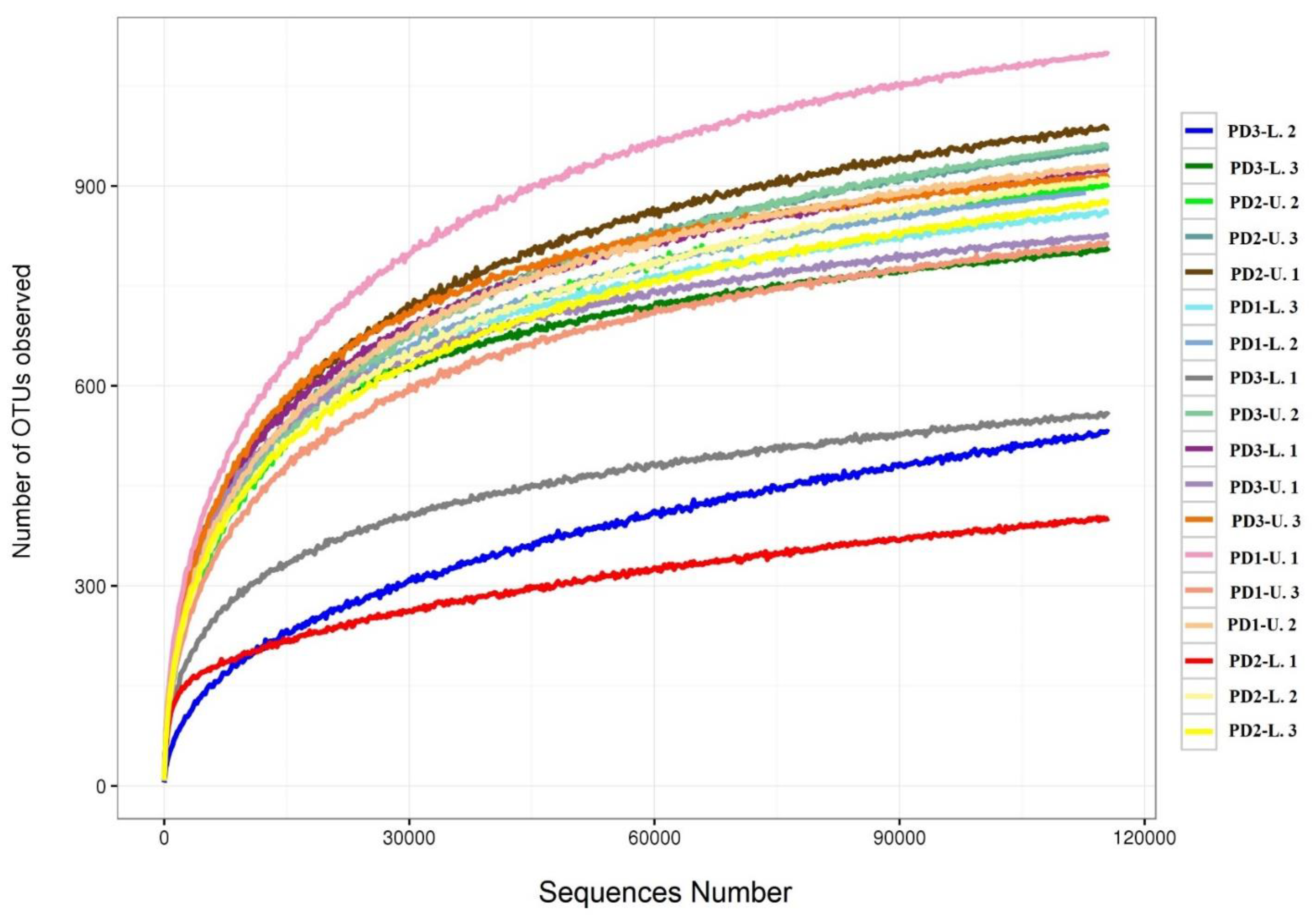


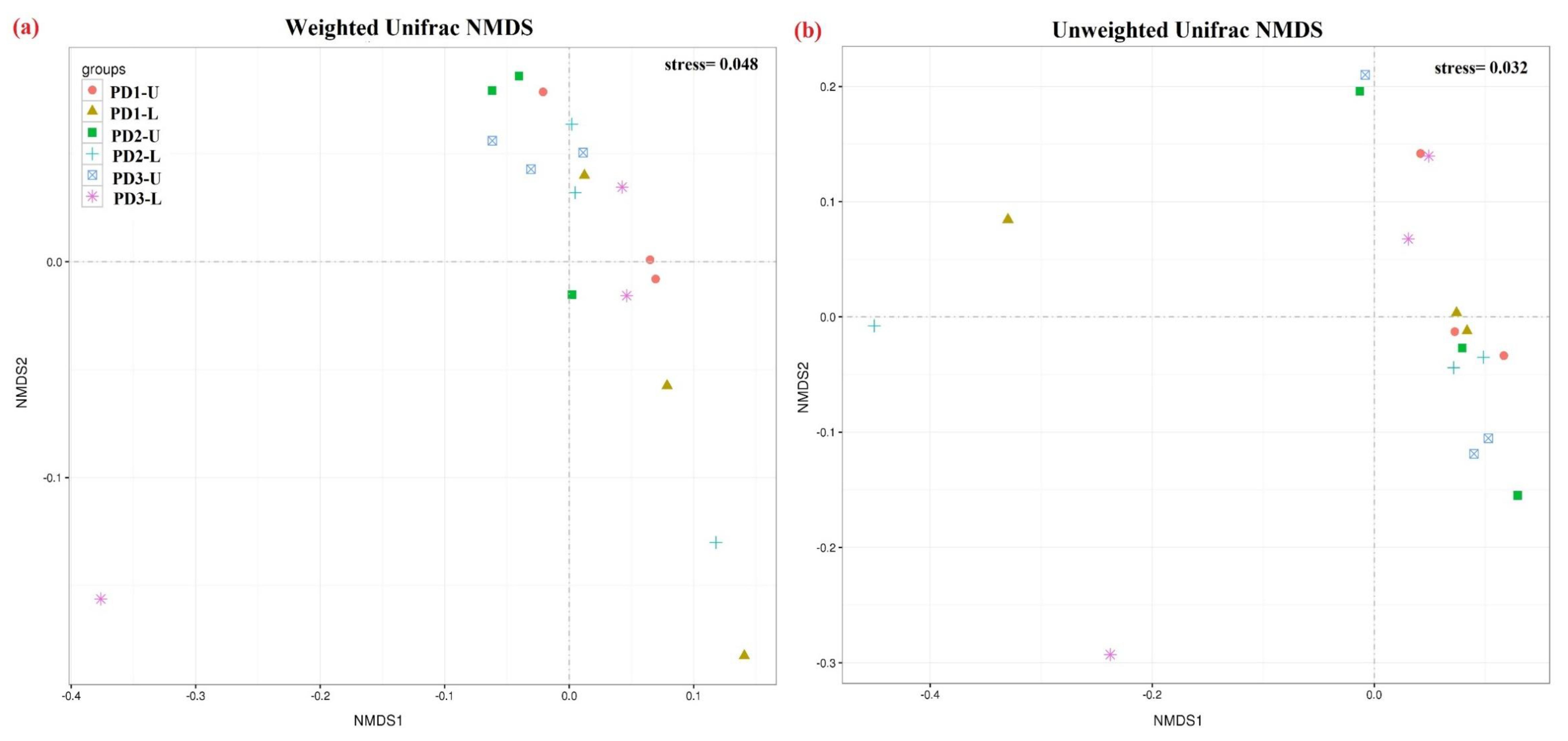



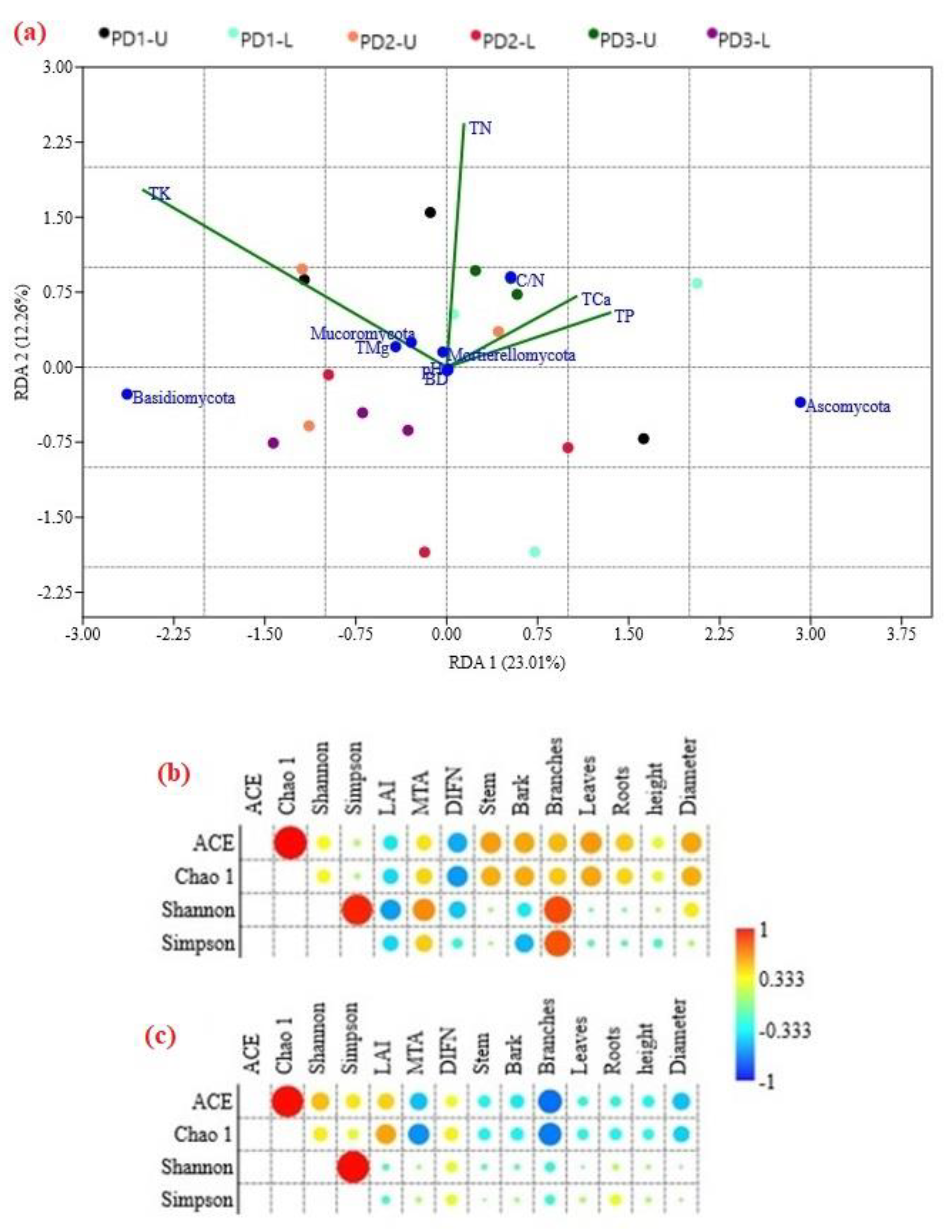
| Analysis | Method/Equipment |
|---|---|
| pH | Potentiometric method (1:2.5 soil:water) |
| Electrical conductivity (EC) | Conductivity meter |
| Bulk density (BD) | The core method of the Nanjing Institute of Soil Science (1978) |
| Total nitrogen (TN) | CN elemental analyzer |
| Total phosphorus (TP) | Molybdenum-antimony colourimetric method |
| Total potassium (TK) | Flame photometry |
| Total calcium (TCa) | CN elemental analyzer |
| Total magnesium (TMg) | CN elemental analyzer |
| C:N ratio | CN elemental analyzer |
| Soil moisture content (SMC) | Calculated based on a wet and dry weight |
| (A) | ||||||
| Stand Density | pH | aBD (g/m3) | EC | SOM (g/kg) | SMC (%) | Soil Type |
| Low (PD1) | 4.21 b | 1.22 ab | 0.02 a | 31.90 b | 11.49 b | SO |
| Intermediate (PD2) | 4.31 a | 1.28 a | 0.02 a | 36.56 a | 9.53 b | SO |
| High (PD3) | 4.27 a | 1.16 b | 0.02 a | 35.47 a | 17.2 b | SO |
| (B) | ||||||
| Stand Density | TN (g/kg) | TP (g/kg) | TK (g/kg) | TMg (g/kg) | TCa (g/kg) | C/N |
| Low (PD1) | 0.99 a | 0.59 a | 22.5 a | 7.02 b | 2.23 a | 15.21 a |
| Intermediate (PD2) | 0.74 c | 0.48 b | 21.2 a | 8.51 a | 2.25 a | 15.80 a |
| High (PD3) | 0.92 b | 0.59 a | 21.8 a | 7.79 ab | 2.14 a | 15.59 a |
| Stand Density | aLAI | LAIe (m2 m−2) | MTA (o) | DIFN | ACF | FNC (g/kg) |
| Low (PD1) | 3.97 c | 2.48 c | 34.8 b | 0.15 a | 0.990 b | 16.92 a |
| Intermediate (PD2) | 4.56 b | 2.85 b | 44.5 ab | 0.11 b | 0.993 ab | 13.51 b |
| High (PD3) | 5.07 a | 3.17 a | 48.7 a | 0.09 b | 0.995 a | 15.93 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, T.H.; Kumar, U.; Shakoor, A.; Albasher, G.; Alkahtani, S.; Rizwana, H.; Tayyab, M.; Dobaria, J.; Hussain, M.I.; Wu, P. Influence of Intraspecific Competition Stress on Soil Fungal Diversity and Composition in Relation to Tree Growth and Soil Fertility in Sub-Tropical Soils under Chinese Fir Monoculture. Sustainability 2021, 13, 10688. https://doi.org/10.3390/su131910688
Farooq TH, Kumar U, Shakoor A, Albasher G, Alkahtani S, Rizwana H, Tayyab M, Dobaria J, Hussain MI, Wu P. Influence of Intraspecific Competition Stress on Soil Fungal Diversity and Composition in Relation to Tree Growth and Soil Fertility in Sub-Tropical Soils under Chinese Fir Monoculture. Sustainability. 2021; 13(19):10688. https://doi.org/10.3390/su131910688
Chicago/Turabian StyleFarooq, Taimoor Hassan, Uttam Kumar, Awais Shakoor, Gadah Albasher, Saad Alkahtani, Humaira Rizwana, Muhammad Tayyab, Jalpa Dobaria, Muhammad Iftikhar Hussain, and Pengfei Wu. 2021. "Influence of Intraspecific Competition Stress on Soil Fungal Diversity and Composition in Relation to Tree Growth and Soil Fertility in Sub-Tropical Soils under Chinese Fir Monoculture" Sustainability 13, no. 19: 10688. https://doi.org/10.3390/su131910688









