Evaluation of Heavy Metal Tolerance Level of the Antarctic Bacterial Community in Biodegradation of Waste Canola Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Activation of the Microbial Sample
2.2. Bacterial Culture Preparation
2.3. Cell Culture Media
2.4. Effects of Heavy Metals on WCO Biodegradation and Growth of Bacterial Community
2.5. Dose-Response Curve
3. Results
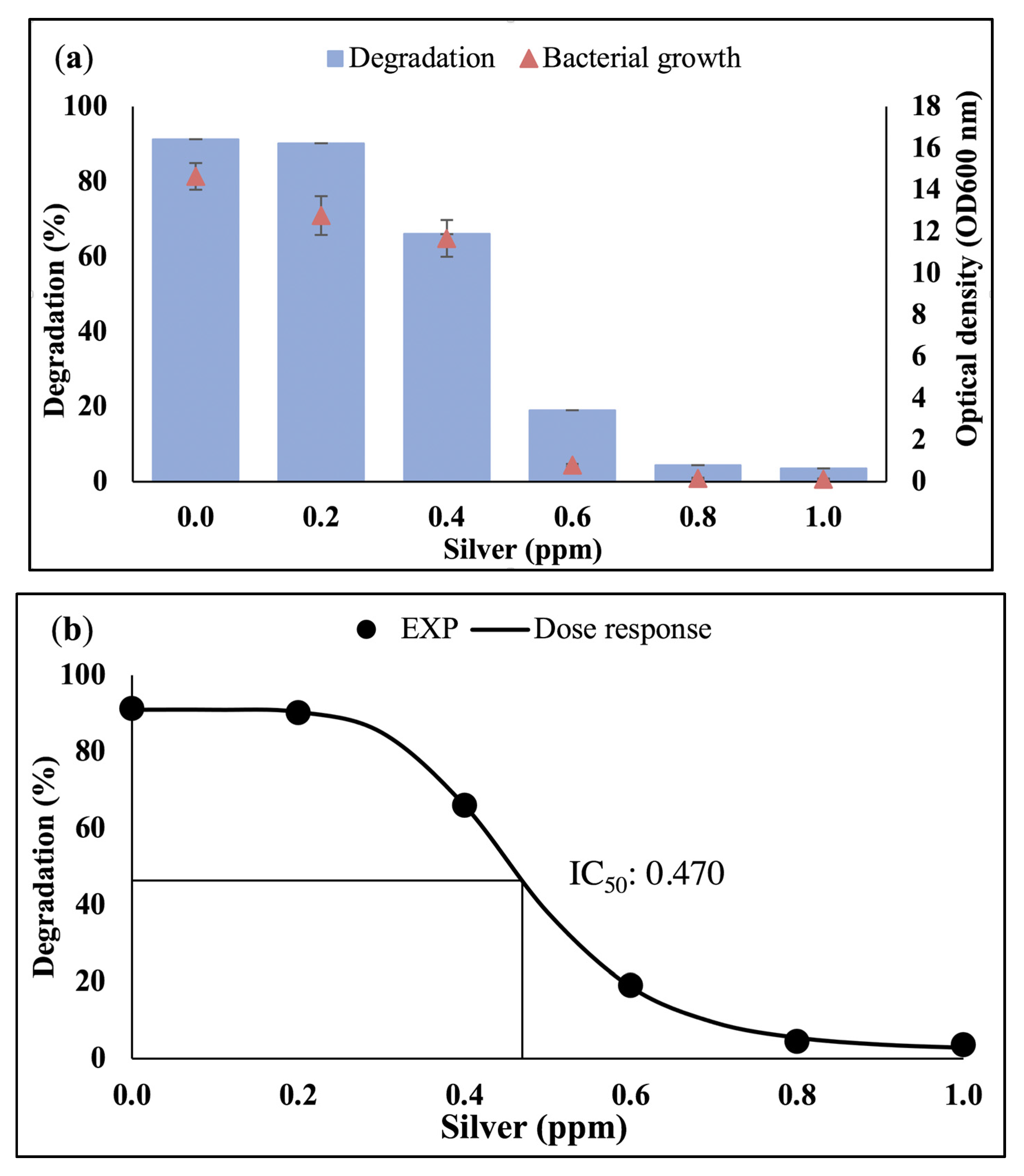
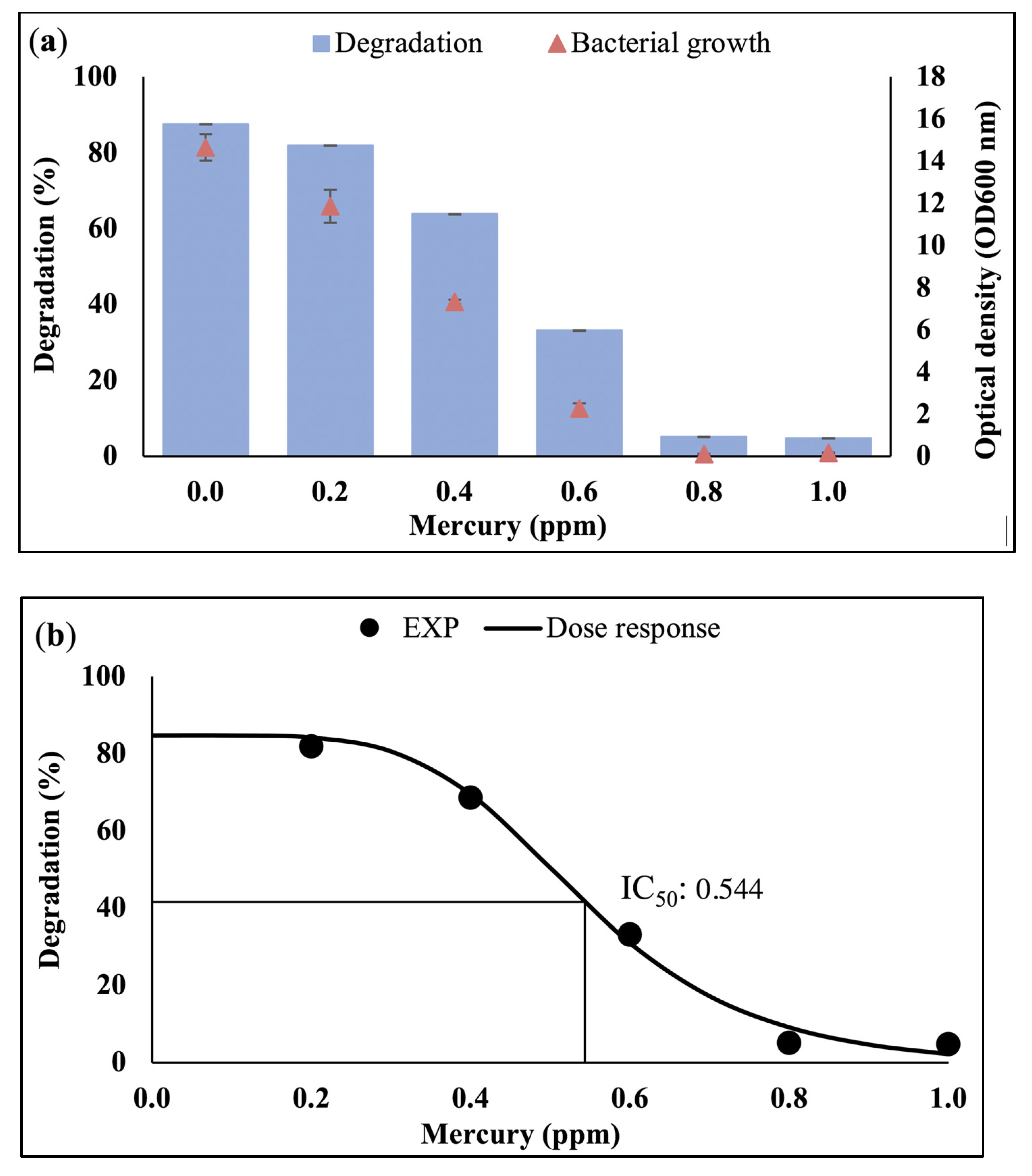
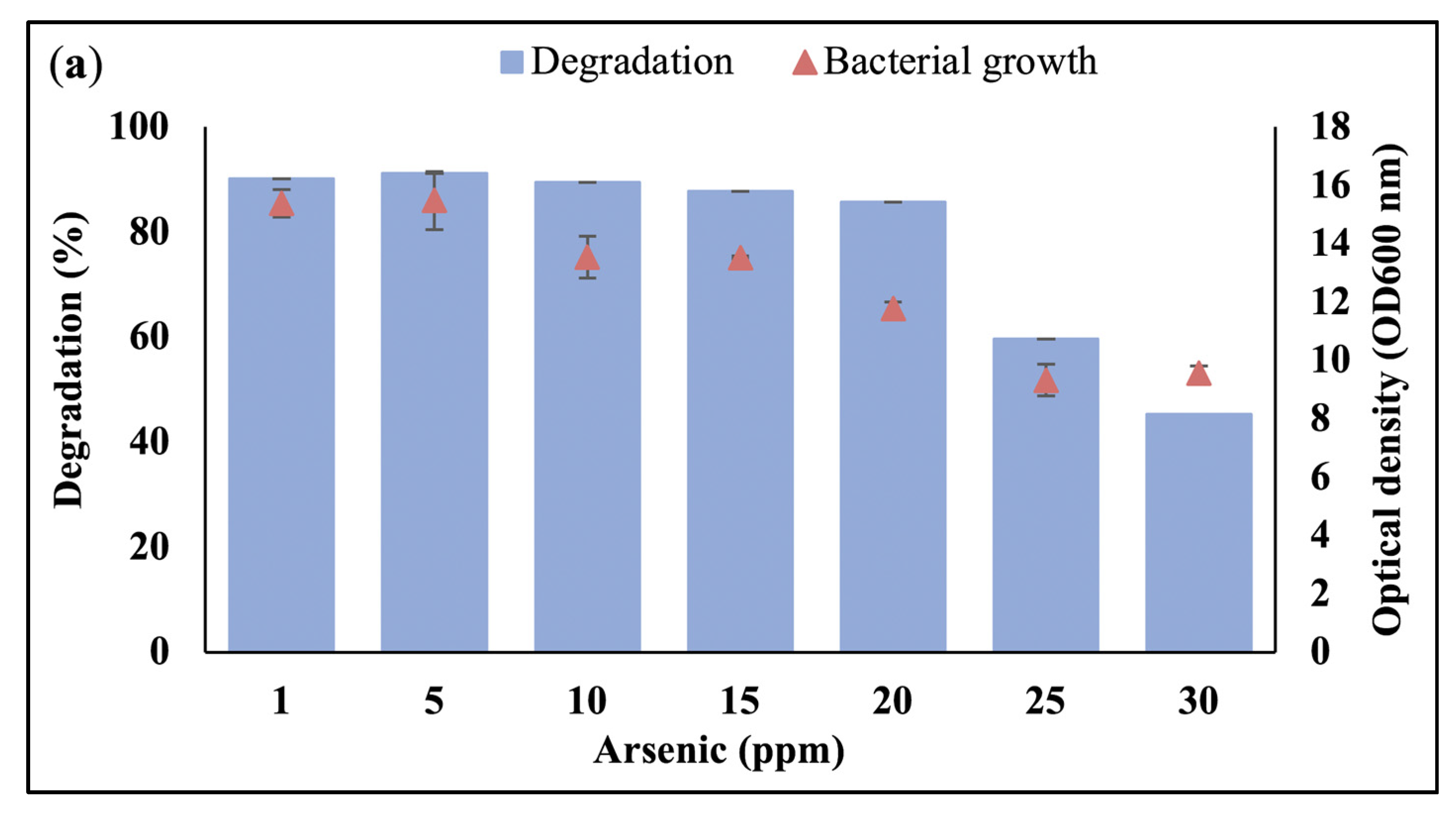
3.1. Effect of Heavy Metals on Bacterial Growth and Waste Canola Oil Biodegradation
3.2. Dose-Response Analysis
3.2.1. Half-Maximal Inhibition Concentration (IC50)
3.2.2. Half-Maximal Effective Concentration (EC50)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffus, J.H. Heavy metals—A meaningless term? Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef] [Green Version]
- Wuana, R.A.; Okiemen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Net. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Igiri, B.E.; Okuduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Odeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Khanafer, M.; Al-Awadhi, H.; Radwan, S. Self-cleaning of very heavily oil-polluted sites proceeds even under heavy-metal stress while involved bacteria exhibit bizarre pleomorphism. Ecotoxicol. Environ. Saf. 2020, 200, 110717. [Google Scholar] [CrossRef] [PubMed]
- Szopinska, M.; Namiesnik, J.; Polkowska, Z. How important is research on pollution levels in Antarctica? Historical approach, difficulties and current trends. Rev. Environ. Contam. Toxicol. 2017, 239, 79–156. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Dang, N.; Kok, Y.; Yap, K.I.; Phang, S. Heavy metal pollution in Antarctica and its potential impacts on algae. Polar Sci. 2019, 20, 75–83. [Google Scholar] [CrossRef]
- Udisti, R.; Dayan, U.; Becagli, S.; Busetto, M.; Frosini, D.; Legrand, M.; Lucarelli, F.; Preunkert, S.; Severi, M.; Traversi, R.; et al. Sea spray aerosol in central Antarctica. Present atmospheric behaviour and implications for paleoclimatic reconstructions. Atmos. Environ. 2012, 52, 109–120. [Google Scholar] [CrossRef]
- Vallelonga, P.; Barbante, C.; Cozzi, G.; Gaspari, V.; Candelone, J.; de Velde, K.V.; Morgan, V.I.; Rosman, K.J.R.; Boutron, C.F.; Cescon, P. Elemental indicators of natural and anthropogenic aerosol inputs to Law Dome, Antarctica. Ann. Glaciol. 2004, 39, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Cool Antarctica. Human Impacts on Antarctica and Threats to the Environment—Mining and Oil. Available online: https://www.coolantarctica.com (accessed on 17 June 2021).
- Kerminen. V.; Teinila, K.; Hillamo, R. Chemistry of sea-salt particles in the summer Antarctic atmosphere. Atmos. Environ. 2000, 34, 2817–2825. [Google Scholar] [CrossRef]
- Dick, A.L.; Peel, D.A. Trace elements in Antarctic air and snowfall. International Glaciological Society. Ann. Glaciol. 1985, 7, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Polyakov, V.; Abakumov, E.; Mavlyudov, B. Black carbon as a source of trace elements and nutrients in ice sheet of King George Island, Antarctica. Geosciences 2020, 10, 465. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Figueira, R.C.L.; Martins, C.C.; Silva, C.R.A.; Franca, E.J.; Bicego, M.C.; Mahiques, M.M.; Montone, R.C. Arsenic and trace element contents in sediment profiles from the Admiralty Bay, King George Island, Antarctica. Mar. Pollut. Bull. 2011, 62, 192–196. [Google Scholar] [CrossRef]
- Romaniuk, K.; Ciok, A.; Decewicz, P.; Witold, U.; Karol, B.; Marta, N.; Julia, P.; Marek, K.Z.; Dariusz, B.; Lukasz, D. Insight into heavy metal resistome of soil psychrotolerant bacteria originating from King George Island (Antarctica). Polar Biol. 2018, 41, 1319–1333. [Google Scholar] [CrossRef] [Green Version]
- Bueno, C.; Kandratavicius, N.; Venturini, N.; Figueira, R.C.L.; Perez, L.; Iglesias, K.; Brugnoli, E. An Evaluation of Trace Metal Concentration in Terrestrial and Aquatic Environments near Artigas Antarctic Scientific Base (King George Island, Maritime Antarctica). Water Air Soil Pollut. 2018, 229, 398. [Google Scholar] [CrossRef]
- Xu, Q.; Chu, Z.; Gao, Y.; Mei, Y.; Yang, Z.; Huang, Y.; Yang, L.; Xie, Z.; Sun, L. Levels, sources and influence mechanisms of heavy metal contamination in topsoils in Mirror Peninsula, East Antarctica. Environ. Pollut. 2020, 257, 113552. [Google Scholar] [CrossRef] [PubMed]
- Abdulrasheed, M.; Roslee, A.F.; Zakaria, N.N.; Lee, G.L.Y.; Convey, P.; Napis, S.; Ahmad, S.A. Effects of heavy metals on diesel metabolism of psychrotolerant strains of Arthrobacter sp. from Antarctica. J. Environ. Biol. 2020, 41, 966–972. [Google Scholar] [CrossRef]
- Ibrahim, S.; Zulkharnain, A.; Zahri, K.N.M.; Lee, G.L.Y.; Convey, P.; Gomez-Fuentes, C.; Sabri, S.; Khalil, K.; Alias, S.; Gonzalez-Rocha, G.; et al. Effect of heavy metals and other xenobiotics on biodegradation of waste canola oil by cold-adapted Rhodococcus sp. strain AQ5-07. Rev. Mex. Ing. Quím. 2020, 19, 1041–1052. [Google Scholar] [CrossRef] [Green Version]
- Abioye, O.P.; Aina, P.F.; Ijah, J.U.J.; Aransiola, A.S. Effects of cadmium and lead on the biodegradation of diesel-contaminated soil. J. Taibah Univ. Sci. 2019, 13, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Khudur, L.S.; Gleeson, D.B.; Ryan, M.H.; Shahsavari, E.; Haleyur, N.; Nugegoda, D.; Ball, A.S. Implications of co-contamination with aged heavy metals and total petroleum hydrocarbons on natural attenuation and ecotoxicity in Australian soils. Environ. Pollut. 2018, 243, 94–102. [Google Scholar] [CrossRef]
- Zukauskaite, A.; Jakubauskaite, V.; Belous, O.; Ambrazaitiene, D.; Stasiskiene, Z. Impact of heavy metals on the oil products biodegradation process. Waste Manag. Res. 2008, 26, 500–507. [Google Scholar] [CrossRef]
- Mustafa, S.; Al-Douseri, A.; Majki, K.; Al-Saleh, E. Potential of crude oil-degrading bacteria to co-resist heavy metals in soil. WIT Trans. Ecol. Environ. 2013, 173, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; You, P.; Hu, Q.; Leng, B.; Wang, J.; Chen, J.; Wan, S.; Wang, B.; Yuan, C.; Zhou, R.; et al. Effects of co-contamination of heavy metals and total petroleum hydrocarbons on soil bacterial community and function network reconstitution. Ecotoxicol. Environ. Saf. 2020, 111083. [Google Scholar] [CrossRef]
- Zahri, K.N.M.; Zulkharnain, A.; Gomez-Fuentes, C.; Sabri, S.; Khalil, K.A.K.; Convey, P.; Ahmad, S.A. The use of response surface methodology as a statistical tool for the optimsation of waste and pure canola oil biodegradation by Antarctic soil bacteria. Life 2021, 11, 456. [Google Scholar] [CrossRef]
- Shahaby, A.F.; Alharthi, A.A.; El Tarras, A.E. Bioremediation of petroleum oil by potential biosurfactant-producing bacteria using gravimetric assay. Inter. J. Microbiol. Appl. Sci. 2015, 4, 390–403. [Google Scholar]
- Brooksbank, A.M.; Latchford, J.W.; Mudge, S.M. Degradation and modification of fats, oils and grease by commercial microbial supplements. World J. Microbiol. Biotechnol. 2014, 23, 977–985. [Google Scholar] [CrossRef]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Development of an efficient bacterial consortium for the potential remediation of hydrocarbons from contaminated sites. Front. Microbiol. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [Green Version]
- Ojuderie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [Green Version]
- Kapahi, M.; Sachdeva, S. Bioremediation options for heavy metal pollution. J. Health Pollut. 2018, 9, 191203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jude, O.U.; Eze, C.N.; Kalu, A.U. Impacts of some heavy metals on bacterial utilization of kerosene in liquid medium. Sci. Res. Essays 2020, 15, 26–32. [Google Scholar] [CrossRef]
- Pina, R.G.; Cervantes, C. Microbial interactions with aluminium. BioMetals 1996, 9, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Purwanti, I.F.; Kurniawan, S.B.; Simanjuntak, D.Y. Removal of Aluminium in contaminated soil using locally isolated Vibrio Alginolyticus J. Ecol. Eng. 2019, 20, 135–140. [Google Scholar] [CrossRef]
- Barras, F.; Fontecave, M. Cobalt stress in Escherichia coli and Salmonella enterica: Molecular bases for toxicity and resistance. Metallomics 2011, 3, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Majtan, T.; Frerman, F.E.; Kraus, J.P. Effect of cobalt on Escherichia coli metabolism and metalloporphyrin formation. BioMetals 2011, 24, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaria, N.N.; Roslee, A.F.A.; Gomez-Fuentes, C.; Zulkharnain, A.; Abdulrasheed, M.; Sabri, S.; Ramirez-Moreno, N.; Ahmad, S.A. Kinetic studies of marine psychrotolerant microorganisms capable of degrading diesel in the presence of heavy metal. Rev. Mexi. Ing. Quim. 2020, 10, 1375–1388. [Google Scholar] [CrossRef]
- Bong, C.W.; Obayashi, Y.; Suzuki, S. Effect of exposure of zinc at low concentration to bacterial production in seawater. Mem. Fac. Agr. Ehime Univ. 2011, 56, 42–45. [Google Scholar]
- Aggary, S.E.; Latinwo, G.K.; Dada, E.O.; Owabor, C.N. Bioremediation of crude oil- Contaminated soil in the presence of nickel, zinc and cadmium heavy metals using bacterial and fungal consortia—bioaugmentation strategy. J. Environ. Treat. Tech. 2019, 7, 179–195. [Google Scholar]
- Giachino, A.; Waldron, K.J. Copper tolerance in bacteria requires the activation of multiple accessory pathways. Mol. Microbiol. 2020, 2020, 377–390. [Google Scholar] [CrossRef]
- Colonella, M.A.; Lizarraga, L.; Rossi, L.; Pena, R.D.; Egoburo, D.; Lopez, N.I.; Lustman, L.J.R. Effect of copper on diesel degradation in Pseudomonas extremaustralis. Extremophiles 2019, 23, 91–99. [Google Scholar] [CrossRef]
- Kedziora, A.; Speruda, M.; Krzyzewska, E.; Rybka, J.; Lukowiak, A.; Bugla-Ploskonsa, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. In. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, S.; Poulse, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. App. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, D.S.; El-Baky, R.M.A.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial activity of silver-treated bacteria against other multi-drug resistant pathogens in their environment. Antibiotic 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishnivetskaya, T.A.; Mosher, J.J.; Palumbo, A.V.; Yang, Z.K.; Podar, M.; Broen, S.D.; Brooks, S.C.; Gu, B.; Southworth, G.R.; Drake, M.M.; et al. Mercury and other heavy metals influence bacterial community structure in contaminated Tennessee streams. Appl. Environ. Microbiol. 2010, 77, 302–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Lin, X.; Wang, J.; Jiang, F.; Wei, L.; Chen, G.; Hao, X. Effects of lead and mercury on sulfate-reducing bacterial activity in a biological process for flue gas desulfurization wastewater treatment. Sci. Rep. 2018, 6, 30455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riis, V.; Babel, W.; Pucci, O.H. Influence of heavy metals on the microbial degradation of diesel fuel. Chemosphere 2020, 49, 559–568. [Google Scholar] [CrossRef]
- Irawati, W.; Soraya, Y.; Baskoro, A.H. A study on mercury resistant bacteria isolated from a gold mine in Pongkor Village, Bogor, Indonesia. Hayati J. Biosci. 2012, 19, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Nweke, C.O.; Umah, S.I.; Ohale, V.K. Toxicity of four metals and their mixtures to Pseudomonas fluorescens: An assessment using fixed ration ray design. Ecotoxicol. Environ. Contam. 2018, 13, 1–14. [Google Scholar] [CrossRef]
- Magdaleno, A.; De Cabo, L.; Arreghini, S.; Salinas, S. Assessment of heavy metal contamination and water quality in an urban river from Argentina. Braz. J. Aquat. Sci. Technol. 2014, 18, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Agency for Toxic Substance and Disease Registry. 2008; Chromium Toxicity. Available online: https://www.atsdr.cdc.gov (accessed on 10 June 2021).
- Oliveira, H. Chromium as an Environmental Pollutant: Insight on induced plant toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Kanmani, P.; Aravind, J.; Preston, D. Remediation of chromium contaminants using bacteria. Int. J. Environ. Sci. Technol. 2012, 9, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Baldiris, R.; Acosta-Tapia, N.; Montes, A.; Hernandez, J.; Viva-Reyes, R. Reduction of hexavalent chromium and detection of chromate reductase (ChrR) in Stenotrophomonas maltophilia. Molecules 2018, 23, 406. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Diaz, M.I.; Diaz-Perez, C.; Vargas, E.; Riveros-Rosas, H.; Campos-Garcia, J.; Cervantes, C. Mechanisms of bacterial resistance to chromium compound. BioMetals 2008, 21, 321–332. [Google Scholar] [CrossRef]
- Dhuldhaj, U.P.; Sharma, N.K.; Singh, S. Microbial Removal of Arsenic: An overview. In Bioremediation of Pollutants; Maheshwari, D.K., Dubey, R.C., Eds.; IK International Publishing House Pvt. Ltd.: New Delhi, India, 2012. [Google Scholar]
- Alka, S.; Shahir, S.; Ibrahim, N.; Chai, T.; Bahari, Z.M.; Manan, F.A. The role of plant growth promoting bacteria on arsenic removal: A review of existing perspectives. Environ. Technol. Innov. 2020, 17, 100602. [Google Scholar] [CrossRef]
- Ordonez, E.; Letek, M.; Valbuena, N.; Gil, J.A.; Mateos, L.M. Analysis of genes involved in arsenic resistance in Corynebacterium glutamicum ATCC 13032. Appl. Environ. Microbiol. 2005, 71, 6206–6215. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Kamli, M.R.; Ali, A. Role of arsenic and its resistance in nature. Can. J. Microbiol. 2011, 57, 79–774. [Google Scholar] [CrossRef]
- Titah, N.S.; Abdullah, S.T.S.; Idris, M.; Anuar, N.; Basri, H.; Mukhlisin, M.; Tangahu, B.V.; Purwanti, I.F.; Kurniawan, S.B. Arsenic resistance and biosorption by isolated Rhizobacteria from the roots of Ludwigia octovalvis. Int. J. Microbiol. 2018, 2018, 3101498. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.; Mengmeng, Z.; Taotao, F.; Juanli, W.; Junbo, N. Mechanisms of bacterial degradation of arsenic. Indian J. Microbiol. Res. 2018, 5, 436–441. [Google Scholar] [CrossRef]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Wassie, M. Application of microorganisms in bioremediation—Review. J. Environ. Microbiol. 2017, 1, 2–9. [Google Scholar]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminant: A overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [Green Version]
- Tiquia-Arashiro, S.M. Lead absorption mechanisms in bacteria as strategies for lead bioremediation. App. Microbiol. Biotechnol. 2018, 102, 5437–5444. [Google Scholar] [CrossRef]
- Hynninen, A. Zinc, Cadmium and Lead Resistance Mechanisms in Bacteria and Their Contribution to Biosensing. Ph.D. Thesis, University of Helsinki, Helsinki, Findland, 2010. [Google Scholar]
- Wong, K.; Quilty, B.; Surif, S. Degradation of crude oil in the presence of Lead (Pb) and cadmium (Cd) by a metal-adapted consortium culture. Adv. Environ. Biol. 2013, 7, 577–585. [Google Scholar]
- Defiery, M.E.J.; Reddy, G. Lag phase and biomass determination of Rhodococcus pyridinivorans GM3 for degradation of phenol. J. Phys. 2014, 1003, 012007. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahri, K.N.M.; Gomez-Fuentes, C.; Sabri, S.; Zulkharnain, A.; Khalil, K.A.; Lim, S.; Ahmad, S.A. Evaluation of Heavy Metal Tolerance Level of the Antarctic Bacterial Community in Biodegradation of Waste Canola Oil. Sustainability 2021, 13, 10749. https://doi.org/10.3390/su131910749
Zahri KNM, Gomez-Fuentes C, Sabri S, Zulkharnain A, Khalil KA, Lim S, Ahmad SA. Evaluation of Heavy Metal Tolerance Level of the Antarctic Bacterial Community in Biodegradation of Waste Canola Oil. Sustainability. 2021; 13(19):10749. https://doi.org/10.3390/su131910749
Chicago/Turabian StyleZahri, Khadijah Nabilah Mohd, Claudio Gomez-Fuentes, Suriana Sabri, Azham Zulkharnain, Khalilah Abdul Khalil, Sooa Lim, and Siti Aqlima Ahmad. 2021. "Evaluation of Heavy Metal Tolerance Level of the Antarctic Bacterial Community in Biodegradation of Waste Canola Oil" Sustainability 13, no. 19: 10749. https://doi.org/10.3390/su131910749
APA StyleZahri, K. N. M., Gomez-Fuentes, C., Sabri, S., Zulkharnain, A., Khalil, K. A., Lim, S., & Ahmad, S. A. (2021). Evaluation of Heavy Metal Tolerance Level of the Antarctic Bacterial Community in Biodegradation of Waste Canola Oil. Sustainability, 13(19), 10749. https://doi.org/10.3390/su131910749








