Understanding the Dynamics of Human–Wildlife Conflicts in North-Western Pakistan: Implications for Sustainable Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Climate
2.1.2. Flora and Fauna
2.2. Questionnaire Survey
2.3. Analytical Approach
2.4. Model Selection
3. Results
3.1. Livelihood System in the Study Area
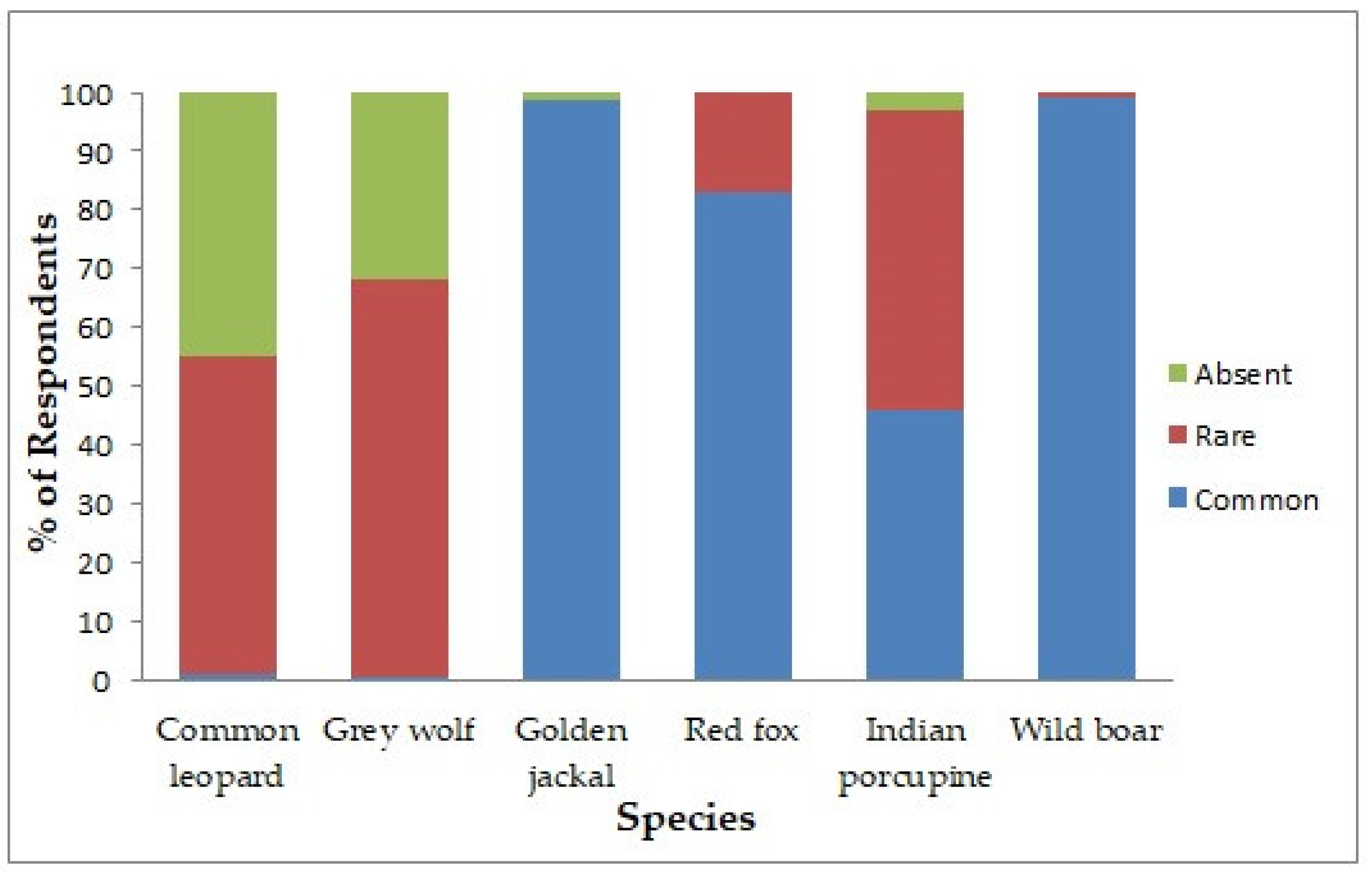
3.2. Sighting Report and Status
3.3. Human–Wildlife Conflicts
3.3.1. Livestock Predation and Economic Loss
3.3.2. Human Attitude toward Wildlife
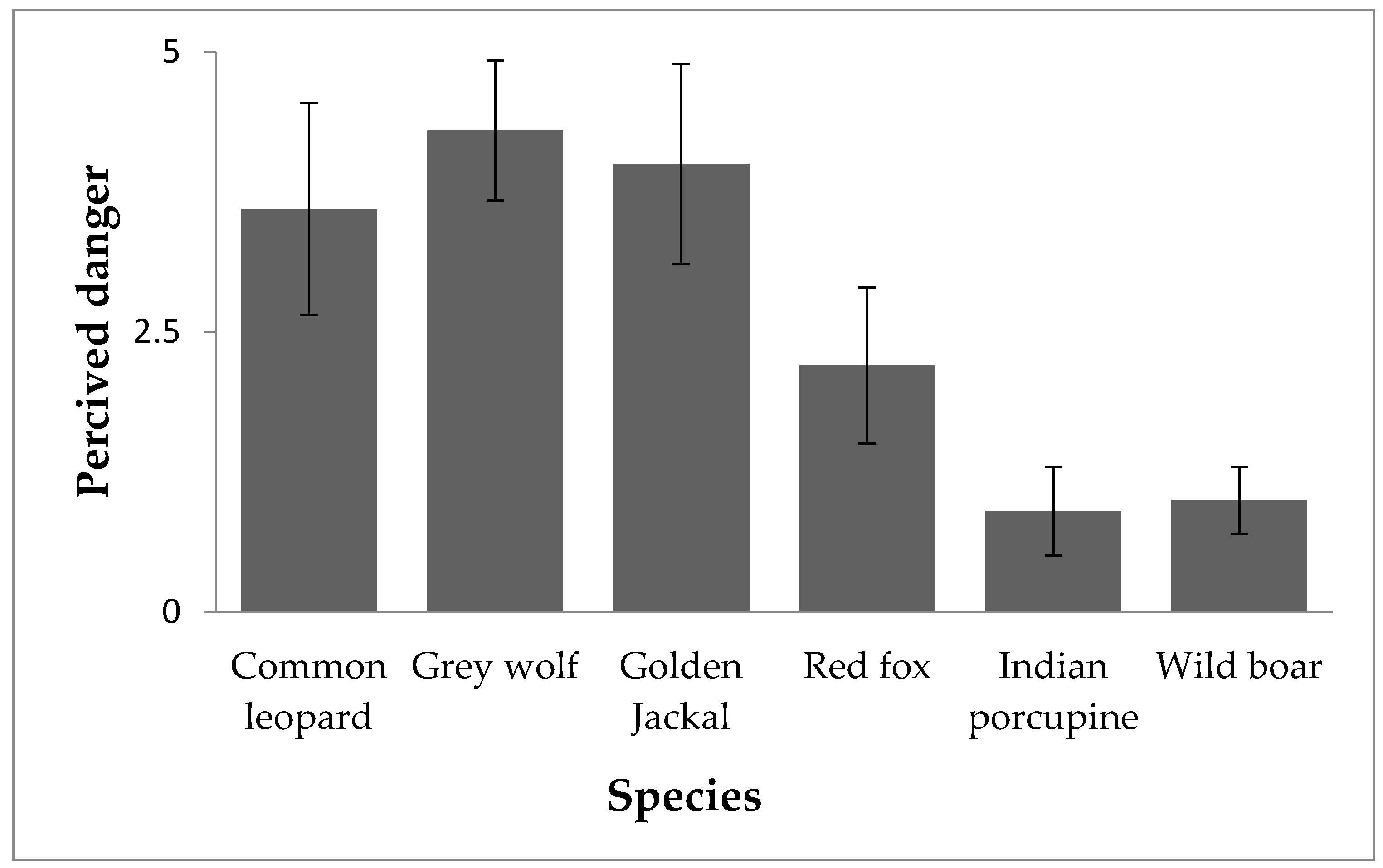
3.3.3. Perceived Danger
3.3.4. Consequences of Human–Wildlife Conflict
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Attia, T.S.N.; Martin, T.N.; Forbuzie, T.P.; Angwafo, T.E.; Chuo, M.D. Human Wildlife Conflict: Causes, Consequences and Management Strategies in Mount Cameroon National Park South West Region, Cameroon. Int. J. For. Anim. Fish. Res. 2018, 2, 34–49. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human Domination of Earth’s Ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- Crutzen, P.J. Geology of mankind. In Paul J. Crutzen: A Pioneer on Atmospheric Chemistry and Climate Change in the Anthropocene; Springer: Berlin/Heidelberg, Germany, 2016; pp. 211–215. [Google Scholar]
- Barua, M.; Bhagwat, S.A.; Jadhav, S. The Hidden Dimensions of Human–Wildlife Conflict: Health Impacts, Opportunity and Transaction Costs. Biol. Conserv. 2013, 157, 309–316. [Google Scholar] [CrossRef]
- Inskip, C.; Zimmermann, A. Human-Felid Conflict: A Review of Patterns and Priorities Worldwide. Oryx 2009, 43, 18–34. [Google Scholar] [CrossRef]
- Woodroffe, R.; Thirgood, S.; Rabinowitz, A. The Impact of Human-Wildlife Conflict on Natural Systems. Conserv. Biol. Ser. 2005, 9, 1. [Google Scholar]
- Mekonen, S. Coexistence between Human and Wildlife: The Nature, Causes and Mitigations of Human Wildlife Conflict around Bale Mountains National Park, Southeast Ethiopia. BMC Ecol. 2020, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Madden, F. Creating Coexistence between Humans and Wildlife: Global Perspectives on Local Efforts to Address Human–Wildlife Conflict. Hum. Dimens. Wildl. 2004, 9, 247–257. [Google Scholar] [CrossRef]
- Newmark, W.D.; Leonard, N.L.; Sariko, H.I.; Gamassa, D.-G.M. Conservation Attitudes of Local People Living Adjacent to Five Protected Areas in Tanzania. Biol. Conserv. 1993, 63, 177–183. [Google Scholar] [CrossRef]
- McCleery, R.A.; Moorman, C.E.; Peterson, M.N. Urban Wildlife Conservation: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 1489975004. [Google Scholar]
- Soulsbury, C.D.; White, P.C.L. Human–Wildlife Interactions in Urban Areas: A Review of Conflicts, Benefits and Opportunities. Wildl. Res. 2015, 42, 541–553. [Google Scholar] [CrossRef]
- Sillero-Zubiri, C.; Sukumar, R.; Treves, A. Living with Wildlife: The Roots of Conflict and the Solutions. In Key Topics in Conservation Biology; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 266–272. [Google Scholar]
- Liu, F.; McShea, W.J.; Garshelis, D.L.; Zhu, X.; Wang, D.; Shao, L. Human-Wildlife Conflicts Influence Attitudes but Not Necessarily Behaviors: Factors Driving the Poaching of Bears in China. Biol. Conserv. 2011, 144, 538–547. [Google Scholar] [CrossRef]
- Marchini, S.; Macdonald, D.W. Predicting Ranchers’ Intention to Kill Jaguars: Case Studies in Amazonia and Pantanal. Biol. Conserv. 2012, 147, 213–221. [Google Scholar] [CrossRef]
- Acha, A.; Temesgen, M. Approaches to Human-Wildlife Conflict Management in and around Chebera-Churchura National Park, Southern Ethiopia. Asian J. Conserv. Biol. 2015, 4, 136–142. [Google Scholar]
- Biru, Y.; Tessema, Z.K.; Urge, M. Perception and Attitude of Pastoralists on Livestock-Wildlife Interactions around Awash National Park, Ethiopia: Implication for Biodiversity Conservation. Ecol. Process. 2017, 6, 13. [Google Scholar] [CrossRef]
- Thirgood, S.; Woodroffe, R.; Rabinowitz, A. The Impact of Human-Wildlife Conflict on Human Lives and Livelihoods. Conserv. Biol. Ser. 2005, 9, 13. [Google Scholar]
- Basak, S.M.; Wierzbowska, I.A.; Gajda, A.; Czarnoleski, M.; Lesiak, M.; Widera, E. Human–Wildlife Conflicts in Krakow City, Southern Poland. Animals 2020, 10, 1014. [Google Scholar] [CrossRef]
- Gemeda, D.O.; Meles, S.K. Impacts of Human-Wildlife Conflict in Developing Countries. J. Appl. Sci. Environ. Manag. 2018, 22, 1233–1238. [Google Scholar] [CrossRef]
- Lamarque, F.; Anderson, J.; Fergusson, R.; Lagrange, M.; Osei-Owusu, Y.; Bakker, L. Human-Wildlife Conflict in Africa: Causes, Consequences and Management Strategies; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009; ISBN 9251063729. [Google Scholar]
- Rangarajan, M.; Desai, A.; Sukumar, R.; Easa, P.S.; Menon, V.; Vincent, S.; Ganguly, S.; Talukdar, B.K.; Singh, B.; Mudappa, D. Gajah. Securing the Future for Elephants in India. 2010. Available online: https://www.environmentandsociety.org/sites/default/files/key_docs/Gajah.pdf (accessed on 26 July 2021).
- Madhusudan, M.D.; Sankaran, P. Seeing the Elephant in the Room: Human-Elephant Conflict and the ETF Report. Econ. Political Wkly. 2010, 45, 29–31. [Google Scholar]
- Dar, N.I.; Minhas, R.A.; Zaman, Q.; Linkie, M. Predicting the Patterns, Perceptions and Causes of Human–Carnivore Conflict in and around Machiara National Park, Pakistan. Biol. Conserv. 2009, 142, 2076–2082. [Google Scholar] [CrossRef]
- Saberwal, V.K.; Gibbs, J.P.; Chellam, R.; Johnsingh, A.J.T. Lion-human Conflict in the Gir Forest, India. Conserv. Biol. 1994, 8, 501–507. [Google Scholar] [CrossRef]
- Silwal, T.; Kolejka, J.; Bhatta, B.P.; Rayamajhi, S.; Sharma, R.P.; Poudel, B.S. When, Where and Whom: Assessing Wildlife Attacks on People in Chitwan National Park, Nepal. Oryx 2017, 51, 370–377. [Google Scholar] [CrossRef]
- Acharya, K.P.; Paudel, P.K.; Neupane, P.R.; Köhl, M. Human-Wildlife Conflicts in Nepal: Patterns of Human Fatalities and Injuries Caused by Large Mammals. PLoS ONE. 2016, 11, e0161717. [Google Scholar] [CrossRef]
- Packer, C.; Ikanda, D.; Kissui, B.; Kushnir, H. Lion Attacks on Humans in Tanzania. Nature 2005, 436, 927–928. [Google Scholar] [CrossRef]
- Lodhi, A. Conservation of Leopards in Ayubia National Park, Pakistan. 2007. Available online: http://wildlifeofpakistan.com/ResearchPapers/LodhiAsadsm.pdf (accessed on 26 July 2021).
- White, L.A.; Gehrt, S.D. Coyote Attacks on Humans in the United States and Canada. Hum. Dimens. Wildl. 2009, 14, 419–432. [Google Scholar] [CrossRef]
- Wang, S.W.; Macdonald, D.W. Livestock Predation by Carnivores in Jigme Singye Wangchuck National Park, Bhutan. Biol. Conserv. 2006, 129, 558–565. [Google Scholar] [CrossRef]
- Din, J.U.; Ali, H.; Ali, A.; Younus, M.; Mehmood, T.; Norma-Rashid, Y.; Nawaz, M.A. Pastoralist-Predator Interaction at the Roof of the World. Ecol. Soc. 2017, 22, 32. [Google Scholar] [CrossRef]
- Chattha, S.A.; Iqbal, S.; Rasheed, Z.; Razzaq, A.; Husain, M.; Abbas, M.N. Human-Leopard Conflict in Machiara National Park (MNP), Azad Jamu and Kashmir (AJ and K), Pakistan. Pak. J. Zool. 2013, 47, 222–228. [Google Scholar]
- Hussain, S. The Status of the Snow Leopard in Pakistan and Its Conflict with Local Farmers. Oryx 2003, 37, 26–33. [Google Scholar] [CrossRef]
- Hussain, S. Protecting the Snow Leopard and Enhancing Farmers’ Livelihoods. Mt. Res. Dev. 2000, 20, 226–231. [Google Scholar] [CrossRef]
- Rehman, E.U.; Din, J.U.; Ahmad, S.; Hameed, S.; Shah, K.A.; Mehmood, T.; Nawaz, M.A. Insight into Occupancy Determinants and Conflict Dynamics of Grey Wolf (Canis lupus) in the Dry Temperate Zone of Hindukush Range. Glob. Ecol. Conserv. 2021, 25, e01402. [Google Scholar] [CrossRef]
- Rehman, A.; Jingdong, L.; Shahzad, B.; Chandio, A.A.; Hussain, I.; Nabi, G.; Iqbal, M.S. Economic Perspectives of Major Field Crops of Pakistan: An Empirical Study. Pacific. Sci. Rev. B Humanit. Soc. Sci. 2015, 1, 145–158. [Google Scholar] [CrossRef]
- Afzal, M.; Naqvi, A.N.; WFP, K.L.; Balochistan, P.I.; Livestock Resources of Pakistan: Present Status and Future Trends. Quarterly Science Vision 9. 2004. Available online: http://www.sciencevision.org.pk/BackIssues/Vol9/22.livestock.pdf (accessed on 6 September 2021).
- Faraz, A.; Younas, M.; Pastrana, C.I.; Waheed, A.; Tauqir, N.A.; Nabeel, M.S. Socio-Economic Constraints on Camel Production in Pakistan’s Extensive Pastoral Farming. Pastoralism 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Khan, M.; Sajjad, M.; Hameed, B.; Khan, M.N.; Jan, A.U. Participation of Women in Agriculture Activities in District Peshawar. Sarhad J. Agric. 2012, 28, 121–127. [Google Scholar]
- Ahmad, S.; Hameed, S.; Ali, H.; Khan, T.U.; Mehmood, T.; Nawaz, M.A. Carnivores’ Diversity and Conflicts with Humans in Musk Deer National Park, Azad Jammu and Kashmir, Pakistan. Eur. J. Wildl. Res. 2016, 62, 565–576. [Google Scholar] [CrossRef]
- Naughton-Treves, L. Predicting Patterns of Crop Damage by Wildlife around Kibale National Park, Uganda. Conserv. Biol. 1998, 12, 156–168. [Google Scholar] [CrossRef]
- Linkie, M.; Dinata, Y.; Nofrianto, A.; Leader-Williams, N. Patterns and Perceptions of Wildlife Crop Raiding in and around Kerinci Seblat National Park, Sumatra. Anim. Conserv. 2007, 10, 127–135. [Google Scholar] [CrossRef]
- Frank, K.T.; Petrie, B.; Choi, J.S.; Leggett, W.C. Trophic Cascades in a Formerly Cod-Dominated Ecosystem. Science 2005, 308, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Nasreen, N.; Niaz, S.; Sajjad Ali Shah, S.; Mitchell III, R.D.; Ayaz, S.; Naeem, H.; Khan, L.; De León, A.P. Tick Burden and Tick Species Prevalence in Small Ruminants of Different Agencies of the Federally Administered Tribal Areas (FATA), Pakistan. Int. J. Acarol. 2019, 45, 374–380. [Google Scholar] [CrossRef]
- Sheikh, K.M.; Molur, S. Status and Red List of Pakistan’s Mammals. In Proceedings of the Based on the Conservation Assessment and Management Plan Workshop; IUCN: Islamabad, Pakistan, 2004. [Google Scholar]
- Kabir, M.; Ghoddousi, A.; Awan, M.S.; Awan, M.N. Assessment of Human–Leopard Conflict in Machiara National Park, Azad Jammu and Kashmir, Pakistan. Eur. J. Wildl. Res. 2014, 60, 291–296. [Google Scholar] [CrossRef]
- Bibi, S.S.; Minhas, R.A.; Awan, M.S.; Ali, U.; Dar, N.I. Study of ethno-carnivore relationship in Dhirkot, Azad Jammu and Kashmir (Pakistan). J. Anim. Plant Sci. 2013, 23, 854–859. [Google Scholar]
- Ali, U.; Minhas, R.A.; Awan, M.S.; Ahmed, K.B.; Qamar, Q.Z.; Dar, N.I. Human-Grey Wolf (Canis lupus Linnaeus, 1758) Conflict in Shounther Valley, District Neelum, Azad Jammu and Kashmir, Pakistan. Pak. J. Zool. 2016, 48, 861–868. [Google Scholar]
- Chughtai, M.S.; Altaf, M.; Manzoor, I.; Safeer, B.; Yasrub, S. Assessment of Human and Wild Boar (Sus scrofa) Conflict from District Bagh, Azad Jammu and Kashmir, Pakistan. J. Wildl. Ecol. 2018, 2, 10–21. [Google Scholar]
- Kabir, M.; Waseem, M.; Ahmad, S.; Goursi, U.H.; Awan, M.N. Livestock Depredation by Leopard, an Alarming Intimidation for Its Conservation in Pir Lasoora National Park Nakial, Azad Jammu and Kashmir. Int. J. Conserv. Sci. 2017, 8, 295–302. [Google Scholar]
- Khan, U.; Ferretti, F.; Shah, S.A.; Lovari, S.A. Large Carnivore among People and Livestock: The Common Leopard. In Problematic Wildlife II; Springer: Cham, Switzerland, 2020; pp. 93–110. [Google Scholar]
- Nabeel Awan, M.; Yaqub, A.; Kamran, M. Survey of Human-Leopard (Panthera Pardus) Conflict in Ayubia National Park, Pakistan. J. Bioresour. Manag. 2020, 7, 39–46. [Google Scholar] [CrossRef]
- Younus, S.; Nazer, S.; Altaf, M.; Manzoor, I.; Safeer, B.; Yasrub, S.; Reviewed, P. Study of Human and Asiatic Jackal (Canis aureus) Conflict from Bagh District, Azad Jammu and Kashmir, Pakistan. J. Wildl. Ecol. 2018, 2, 1–10. [Google Scholar]
- Rehman, E.; Khattak, R.H. Trophy Hunting Impacts on Kashmir Markhor and Changing the Negative Perception of Local Communities about Wildlife in Chitral District, Pakistan. Mammal Tales #20, Zoo’s Print 2020, 35, 12–14. [Google Scholar]
- Roberts, T.J. Mammals of Pakistan; Oxford University Press: New York, NY, USA, 1977. [Google Scholar]
- Adams, C.E. Urban Wildlife Management; CRC Press: Boca Raton, FL, USA, 2009; ISBN 0429111533. [Google Scholar]
- Bíl, M.; Andrášik, R.; Janoška, Z. Identification of Hazardous Road Locations of Traffic Accidents by Means of Kernel Density Estimation and Cluster Significance Evaluation. Accid. Anal. Prev. 2013, 55, 265–273. [Google Scholar] [CrossRef]
- Bíl, M.; Andrášik, R.; Dul’a, M.; Sedoník, J. On Reliable Identification of Factors Influencing Wildlife-Vehicle Collisions along Roads. J. Environ. Manag. 2019, 237, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Khattak, R.H.; Liu, Z.; Teng, L.; ur Rehman, E. The Wild Mammalian Fauna of Nowshera District Khyber Pakhtunkhwa, Pakistan. Pak. J. Zool. 2021. [Google Scholar] [CrossRef]
- Khattak, R.H.; Xin, Z.; Ahmad, S.; Roberts, N.J. An Avi-Faunal Inventory of Miangan Tarakai Game Reserve: A Future Destination for Eco-Tourists. Pak. J. Life Soc. Sci. 2019, 17, 39–45. [Google Scholar]
- White, P.C.L.; Jennings, N.V.; Renwick, A.R.; Barker, N.H.L. Questionnaires in Ecology: A Review of Past Use and Recommendations for Best Practice. J. Appl. Ecol. 2005, 42, 421–430. [Google Scholar] [CrossRef]
- Lunney, D.; Matthews, A. The Contribution of the Community to Defining the Distribution of a Vulnerable Species, the Spotted-Tailed Quoll, Dasyurus Maculatus. Wildl. Res. 2001, 28, 537–545. [Google Scholar] [CrossRef]
- Shima, A.L.; Berger, L.; Skerratt, L.F. Conservation and Health of Lumholtz’s Tree-Kangaroo (Dendrolagus lumholtzi). Aust. Mammal. 2019, 41, 57–64. [Google Scholar] [CrossRef]
- Behdarvand, N.; Kaboli, M.; Ahmadi, M.; Nourani, E.; Mahini, A.S.; Aghbolaghi, M.A. Spatial Risk Model and Mitigation Implications for Wolf–Human Conflict in a Highly Modified Agroecosystem in Western Iran. Biol. Conserv. 2014, 177, 156–164. [Google Scholar] [CrossRef]
- Szabó, L.; Heltai, M.; Lanszki, J.; Szűcs, E. An Indigenous Predator, the Golden Jackal (Canis aureus L., 1758) Spreading like an Invasive Species in Hungary. Bull USAMV-CN 2007, 63, 1–6. [Google Scholar]
- Lanszki, J.; Heltai, M. Feeding Habits of Golden Jackal and Red Fox in South-Western Hungary during Winter and Spring. Mamm. Biol. 2002, 67, 129–136. [Google Scholar] [CrossRef]
- Katuwal, H.B.; Dahal, S. Golden Jackals in Human Dominated Landscapes of the Manaslu Conservation Area, Nepal. Vertebr. Zool. 2013, 63, 329–334. [Google Scholar]
- Feit, B.; Feit, A.; Letnic, M. Apex Predators Decouple Population Dynamics between Mesopredators and Their Prey. Ecosystems 2019, 22, 1606–1617. [Google Scholar] [CrossRef]
- Alexander, J.; Chen, P.; Damerell, P.; Youkui, W.; Hughes, J.; Shi, K.; Riordan, P. Human Wildlife Conflict Involving Large Carnivores in Qilianshan, China and the Minimal Paw-Print of Snow Leopards. Biol. Conserv. 2015, 187, 1–9. [Google Scholar] [CrossRef]
- Din, J.U.; Nawaz, M.A.; Mehmood, T.; Ali, H.; Ali, A.; Adli, D.S.H.; Norma-Rashid, Y. A Transboundary Study of Spatiotemporal Patterns of Livestock Predation and Prey Preferences by Snow Leopard and Wolf in the Pamir. Glob. Ecol. Conserv. 2019, 20, e00719. [Google Scholar] [CrossRef]
- Mishra, C. Livestock Depredation by Large Carnivores in the Indian Trans-Himalaya: Conflict Perceptions and Conservation Prospects. Environ. Conserv. 1997, 24, 338–343. [Google Scholar] [CrossRef]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P. Status and Ecological Effects of the World’s Largest Carnivores. Science 2014, 343. [Google Scholar] [CrossRef]
- Sillero-Zubiri, C.; Laurenson, M.K. Interactions between Carnivores and Local Communities: Conflict or Co-Existence? Conserv. Biol. Ser. 2001, 282–312. Available online: https://ora.ox.ac.uk/objects/uuid:80adf08f-f601-4a74-b62a-7a38772f4942 (accessed on 29 July 2021).
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of Wild Boar (Sus scrofa) in Its Introduced and Native Range: A Review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Thapa, S. Effectiveness of Crop Protection Methods against Wildlife Damage: A Case Study of Two Villages at Bardia National Park, Nepal. Crop Prot. 2010, 29, 1297–1304. [Google Scholar] [CrossRef]
- Sapkota, S.; Aryal, A.; Baral, S.R.; Hayward, M.W.; Raubenheimer, D. Economic Analysis of Electric Fencing for Mitigating Human-Wildlife Conflict in Nepal. J. Resour. Ecol. 2014, 5, 237–243. [Google Scholar] [CrossRef]
- Schley, L.; Dufrêne, M.; Krier, A.; Frantz, A.C. Patterns of Crop Damage by Wild Boar Sus scrofa) in Luxembourg over a 10-Year Period. Eur. J. Wildl. Res. 2008, 54, 589–599. [Google Scholar] [CrossRef]
- Pandey, L.; Arunachalam, A.; Joshi, N. Challenges of Hill Farming Due to Crop-Raiding by Wild Pigs in the Indian Himalayan Region. Curr. Sci. 2019, 116. [Google Scholar] [CrossRef]
- Amici, A.; Serrani, F.; Rossi, C.M.; Primi, R. Increase in Crop Damage Caused by Wild Boar Sus scrofa L.): The “Refuge Effect”. Agron. Sustain. Dev. 2012, 32, 683–692. [Google Scholar]
- Lombardini, M.; Meriggi, A.; Fozzi, A. Factors Influencing Wild Boar Damage to Agricultural Crops in Sardinia (Italy). Curr. Zool. 2017, 63, 507–514. [Google Scholar] [CrossRef]
- Herrero, J.; García-Serrano, A.; Couto, S.; Ortuño, V.M.; García-González, R. Diet of Wild Boar Sus srofa L. and Crop Damage in an Intensive Agroecosystem. Eur. J. Wildl. Res. 2006, 52, 245–250. [Google Scholar] [CrossRef]
- Piekarczyk, P.; Tajchman, K.; Belova, O.; Wójcik, M. Crop Damage by Wild Boar Sus scrofa L.) Depending on the Crop Composition in Central-Eastern Poland. Balt. For. 2021, 27. [Google Scholar] [CrossRef]
- Rutten, A.; Casaer, J.; Strubbe, D.; Leirs, H. Agricultural and Landscape Factors Related to Increasing Wild Boar Agricultural Damage in a Highly Anthropogenic Landscape. Wildl. Biol. 2019, 2020. [Google Scholar] [CrossRef]
- Khan, A.A. Bio-Economic Impacts of Vertebrate Pests on Crops with Special Reference to Rodent Pests in Pakistan and Other Countries. In Proceedings of the Pakistan Congress of Zoology; Zoological Society of Pakistan: Lahore, Pakistan, 2013; Volume 33, pp. 75–108. [Google Scholar]
- Hafeez, S.; Khan, G.S.; Ashfaq, M.; Khan, Z.H. Food Habits of the Indian Crested Porcupine (Hystrix indica) in Faisalabad, Pakistan. Pak. J. Agric. Sci 2011, 48, 205–210. [Google Scholar]
- Khan, A.A.; Ahmad, S.; Hussain, I.; Munir, S. Deterioration Impact of Indian Crested Porcupine, Hystrix indica, on Forestry and Agricultural Systems in Pakistan. Int. Biodeterior. Biodegrad. 2000, 45, 143–149. [Google Scholar] [CrossRef]
- Niu, K.; Liu, W.; Xiao, Z.; Wu, A.; Yang, T.; Riondato, I.; Ellwanger, A.L.; Ang, A.; Gamba, M.; Yang, Y.; et al. Exploring Local Perceptions of and Attitudes toward Endangered François’ Langurs (Trachypithecus francoisi) in a Human-Modified Habitat. Int. J. Primatol. 2019, 40, 331–355. [Google Scholar] [CrossRef]
- Holmern, T.; Nyahongo, J.; Røskaft, E. Livestock Loss Caused by Predators Outside the Serengeti National Park, Tanzania. Biol. Conserv. 2007, 135, 518–526. [Google Scholar] [CrossRef]
- Al-Shawaf, L.; Conroy-Beam, D.; Asao, K.; Buss, D.M. Human Emotions: An Evolutionary Psychological Perspective. Emot. Rev. 2016, 8, 173–186. [Google Scholar] [CrossRef]
- Yasuda, A. Is Sport Hunting a Breakthrough Wildlife Conservation Strategy for Africa? A Case Study of Northern Cameroon. Field Actions Sci. Rep. J. Field Actions 2012, 6, 1–9. [Google Scholar]
- Watanabe, T.; Izumiyama, S.; Gaunavinaka, L.; Anarbaev, M. Wolf Depredation on Livestock in the Pamir. Geogr. Stud. 2010, 85, 26–36. [Google Scholar] [CrossRef][Green Version]
- Nowell, K. An Ounce of Prevention: Snow Leopard Crime Revisited; Traffic: Cambridge, UK, 2016; ISBN 1858504090. [Google Scholar]
- Theile, S. Fading Footprints: The Killing and Trade of Snow Leopards; Traffic International: Cambridge, UK, 2003; ISBN 1858502012. [Google Scholar]
- Hötte, M.; Bereznuk, S. Compensation for Livestock Kills by Tigers and Leopards in Russia. Carniv. Damage Prev. News 2001, 3, 6–7. [Google Scholar]
- Mishra, C.; Allen, P.; McCarthy, T.O.M.; Madhusudan, M.D.; Bayarjargal, A.; Prins, H.H.T. The Role of Incentive Programs in Conserving the Snow Leopard. Conserv. Biol. 2003, 17, 1512–1520. [Google Scholar] [CrossRef]
- Helldin, J.O.; Liberg, O.; Glöersen, G. Lynx (Lynx lynx) Killing Red Foxes (Vulpes vulpes) in Boreal Sweden–Frequency and Population Effects. J. Zool. 2006, 270, 657–663. [Google Scholar] [CrossRef]
- Merkle, J.A.; Stahler, D.R.; Smith, D.W. Interference Competition between Gray Wolves and Coyotes in Yellowstone National Park. Can. J. Zool. 2009, 87, 56–63. [Google Scholar] [CrossRef]
- Leo, V.; Reading, R.P.; Letnic, M. Interference Competition: Odours of an Apex Predator and Conspecifics Influence Resource Acquisition by Red Foxes. Oecologia 2015, 179, 1033–1040. [Google Scholar] [CrossRef] [PubMed]


| Response Variable | Explanatory Variables | Variable Type | Levels |
|---|---|---|---|
| Attitude | Education | Nominal | Illiterate Primary Middle Graduate |
| Occupation | Nominal | Farmer Employee Labour | |
| Attacks on humans | Continuous | n/a | |
| Crop damage | Continuous | n/a | |
| Sighted wildlife species | Continuous | n/a | |
| Age | Continuous | n/a | |
| Earning members | Continuous | n/a | |
| Livestock owned | Nominal | Cattle Goat Sheep Others | |
| Livestock predation | Season | Nominal | Winter Spring Summer Autumn |
| Location | Nominal | Forest Non-forest | |
| Prey type | Nominal | Cattle Goat Sheep Other | |
| Prey sex | Nominal | Male Female | |
| Prey age | Nominal | Young Adult | |
| Management practices | Nominal | Guarded Un-guarded | |
| Circumstances | Nominal | Grazing Non-grazing | |
| Crop damage | Season | Nominal | Winter Spring Summer Autumn |
| Crop type | Nominal | Cereals Fruits Vegetables |
| Model | AIC Full Model | AIC Selected Model |
|---|---|---|
| Attitude toward common leopard | 4256.3 | 2217.2 |
| Attitude towards grey wolf | 3807.8 | 1715.3 |
| Attitude toward golden jackal | 1925.7 | 1073.5 |
| Attitude toward red fox | 2863.1 | 1625.4 |
| Attitude toward Indian porcupine | 3824.4 | 1007.9 |
| Attitude toward wild boar | 1634.7 | 873.1 |
| Livestock predation | 3125.2 | 1047.24 |
| Crop damage | 942.7 | 324.1 |
| Livestock | Price | Common Leopard | Grey Wolf | Golden Jackal | Red Fox | Total |
|---|---|---|---|---|---|---|
| Cow | 442 | 7072 (16) | 19,006 (43) | - | - | 26,078 (59) |
| Donkey | 252 | 504 (2) | 7308 (29) | - | - | 7812 (31) |
| Sheep | 158 | - | 474 (3) | 7426 (47) | - | 7900 (50) |
| Goat | 126 | 1386 (11) | 4032 (32) | 49,392 (392) | - | 54,810 (435) |
| Hen | 4 | - | - | 20 (5) | 360 (90) | 380 (95) |
| Total loss | 8962 (31) | 30,820 (107) | 56,838 (444) | 360 (90) | 96,980 (670) | |
| Annual loss | 4481 (15.5) | 15,410 (53.5) | 28,419 (222) | 180 (45) | 48,490 (335) | |
| Per hh/year loss | 9.0 | 30.9 | 57.1 | 0.4 | 97.4 |
| Factors | Common Leopard | Grey Wolf | Golden Jackal | |||||||||
| Odds Ratio | Coefficient | SD | p Value | Odds Ratio | Coefficient | SD | p Value | Odds Ratio | Coefficient | SD | p Value | |
| ED: graduate | 2.549 | 0.936 | 0.49 | 0.055 | 2.420 | 0.884 | 0.49 | 0.069 | 3.105 | 1.133 | 0.52 | 0.03 |
| ED: middle | 2.266 | 0.818 | 0.4 | 0.039 | 2.183 | 0.781 | 0.39 | 0.048 | 2.286 | 0.827 | 0.42 | 0.05 |
| ED: primary | 2.263 | 0.817 | 0.38 | 0.033 | 2.038 | 0.712 | 0.38 | 0.063 | - | - | - | - |
| Sighted grey wolf | 0.571 | −0.559 | 0.3 | 0.066 | 0.581 | −0.543 | 0.3 | 0.074 | - | - | - | - |
| Sighted golden jackal | 0.924 | −0.079 | 0.02 | <0.001 | 0.927 | −0.075 | 0.02 | 0.001 | 0.922 | −0.081 | 0.03 | 0.001 |
| Sighted red fox | - | - | - | - | - | - | - | - | - | - | - | - |
| Sighted porcupine | 0.938 | −0.064 | 0.04 | 0.09 | 0.939 | −0.062 | 0.04 | 0.1 | - | - | - | - |
| Goat | 0.939 | −0.062 | 0.02 | 0.005 | 0.939 | −0.062 | 0.02 | 0.006 | 0.933 | −0.069 | 0.02 | 0.004 |
| Factors | Red Fox | Indian Porcupine | Wild Boar | |||||||||
| Odds Ratio | Coefficient | SD | p Value | Odds Ratio | Coefficient | SD | p Value | Odds Ratio | Coefficient | SD | p Value | |
| ED: graduate | 3.792 | 1.333 | 0.58 | 0.022 | 3.031 | 1.109 | 0.53 | 0.035 | - | - | - | - |
| ED: middle | - | - | - | - | 2.415 | 0.882 | 0.43 | 0.038 | - | - | - | - |
| ED: primary | - | - | - | - | - | - | - | - | - | - | - | - |
| Sighted grey wolf | - | - | - | - | - | - | - | - | - | - | - | - |
| Sighted golden jackal | - | - | - | - | - | - | - | - | 0.878 | −0.13 | 0.08 | 0.093 |
| Sighted red fox | 0.677 | −0.39 | 0.07 | 0 | - | - | - | - | 1.212 | 0.193 | 0.1 | 0.045 |
| Sighted porcupine | - | - | - | - | 0.843 | −0.17 | 0.06 | 0.005 | - | - | - | - |
| Goat | - | - | - | - | 0.954 | −0.047 | 0.02 | 0.043 | - | - | - | - |
| Crop damaged | - | - | - | - | - | - | - | - | 3.218 | 1.169 | 0.69 | 0.089 |
| Others | - | - | - | - | - | - | - | - | 1.072 | 0.07 | 0.03 | 0.028 |
| Factors | Levels | Odds Ratio | Coefficient | SD | p Value |
|---|---|---|---|---|---|
| Location | Non Forest | 2.373 | 0.864 | 0.377 | 0.022 |
| Prey type | Goat | 8.23 | 3.037 | 0.347 | 0.000 |
| Prey age | Young | 2.623 | 0.964 | 0.368 | 0.009 |
| Guarded | Yes | 12.395 | 2.517 | 0.608 | 0.000 |
| Factors | Levels | Odds Ratio | Coefficient | SD | p Value |
|---|---|---|---|---|---|
| Crop type | Cereal | 0.031 | −3.468 | 1.012 | 0.001 |
| Vegetable | 0.100 | −2.306 | 1.135 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattak, R.H.; Teng, L.; Mehmood, T.; Ahmad, S.; Bari, F.; Rehman, E.U.; Liu, Z. Understanding the Dynamics of Human–Wildlife Conflicts in North-Western Pakistan: Implications for Sustainable Conservation. Sustainability 2021, 13, 10793. https://doi.org/10.3390/su131910793
Khattak RH, Teng L, Mehmood T, Ahmad S, Bari F, Rehman EU, Liu Z. Understanding the Dynamics of Human–Wildlife Conflicts in North-Western Pakistan: Implications for Sustainable Conservation. Sustainability. 2021; 13(19):10793. https://doi.org/10.3390/su131910793
Chicago/Turabian StyleKhattak, Romaan Hayat, Liwei Teng, Tahir Mehmood, Shakeel Ahmad, Fathul Bari, Ejaz Ur Rehman, and Zhensheng Liu. 2021. "Understanding the Dynamics of Human–Wildlife Conflicts in North-Western Pakistan: Implications for Sustainable Conservation" Sustainability 13, no. 19: 10793. https://doi.org/10.3390/su131910793
APA StyleKhattak, R. H., Teng, L., Mehmood, T., Ahmad, S., Bari, F., Rehman, E. U., & Liu, Z. (2021). Understanding the Dynamics of Human–Wildlife Conflicts in North-Western Pakistan: Implications for Sustainable Conservation. Sustainability, 13(19), 10793. https://doi.org/10.3390/su131910793






