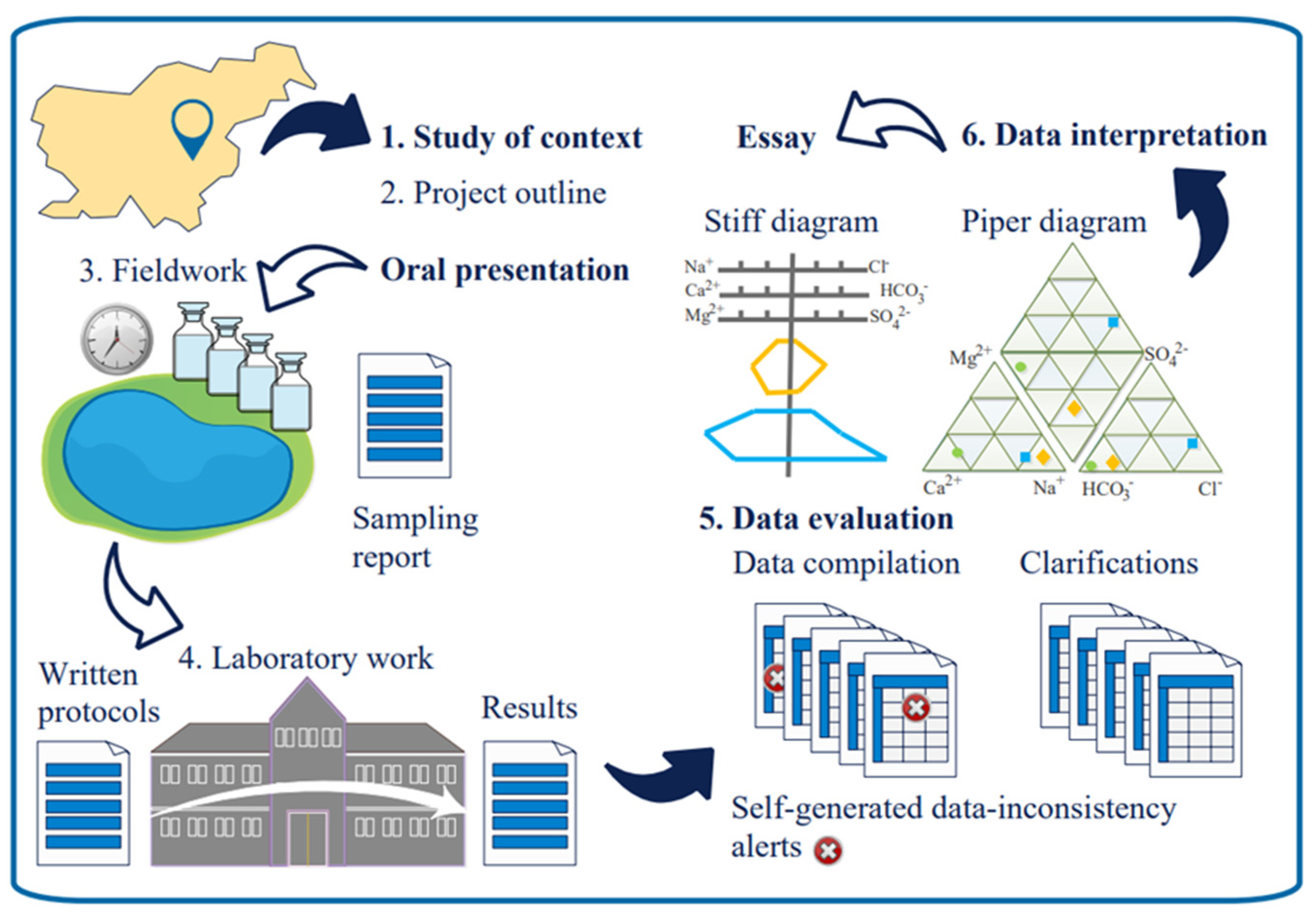
1. Introduction
Water research as a multidisciplinary area can turn professionals from different disciplines into better scientists [
1]. Given that for the closely related branches already, there are distinctions in terminology and methodology [
2], and some countries are organising interdisciplinary networks for graduate students [
3] or encouraging early interaction and integration to improve graduate skills in an increasingly diverse research landscape requiring a science-to-solutions approach [
4,
5]. However, students do not have equal opportunities everywhere.
Nevertheless, chemists and chemical engineers often become involved in water research and management, even though they might not have been extensively trained in these subjects during their studies. Elective subjects can ease into the transition. We describe a collaborative project-centred elective subject “Water as a Hydrogeological, Ecological, and Analytical System” that provides knowledge and skills in water research and helps students perceive their profession and one of its core disciplines, analytical chemistry, differently. We ensure efficient collaboration among students by incorporating the elements of agile management into the organisational scheme of the subject.
Several disciplines need chemical analyses to test their hypotheses. Within a professional context, results are not only numbers that might be lower or higher, if we compare them among themselves or with some reference, but they also bear meaning and have a mission. One achieves a deeper understanding of a process, event, or phenomenon by relating, combining, and confronting them. Consequently, the quality of the results and their fitness for purpose become more than just assumed accuracy, precision, and uncertainty of individual numeric values. Students of chemistry and chemical engineering usually lack such insight. For many of them, analytical chemistry remains mostly about analytical-methods choice, sampling, validation, laboratory work, and statistical evaluation of results, which they might find exhausting and not very appealing. The exploitation of results might be very poor or even remain out of the scope.
EuroCurriculum II for analytical chemistry recommends a problem-solving approach covering a total analytical path of a sample from sampling to data analysis, interpretation, and communication of results. Data analysis based on the fundamentals of statistics, including chemometrics and graphical data-interpretation techniques, and promoting the concepts of quality assurance and optimization are expected [
6].
A sample can follow a total analytical path but might still be very weakly associated with a meaningful professional context, and not all contexts are equally powerful when it comes to data exploitation and interpretation. Waterbodies as a topic can make a difference. They are of different kinds; water composition reflects natural and artificial influences and varies spatially and temporarily. Students feel connected to them. To be able to define research questions, state hypotheses, detail a sampling plan, and fully exploit the potential of the results of chemical analyses, it requires of students to profoundly study the environment and all possible impacts.
By a considerate choice of parameters, we enable not only rich data interpretations that go beyond judgments on compliance with legislation or confirmation of water pollution or suitability for a particular use, but also make specialised analytical data reliability checks possible. By relating parameters with water-research-specific data-evaluation and data-interpretation techniques including graphical approaches such as the Piper and Stiff diagram [
7,
8,
9], students can explain water characteristics, water genesis, processes in water, and external influences. Waterbody evaluations are broader than water-samples evaluations that focus on a specific aspect of water quality and are prevalent in project-based learning, and this is where we tend to contribute.
Several project-based laboratory classes introduce water samples to provide a research-like experience and raise motivation in students while they are becoming acquainted with the analytical process, analytical methods, and instrumentation [
10,
11,
12,
13]. In addition to already stated objectives, diverse authors tend to develop collaborative skills [
14], to outreach to private well-owners and provide community service [
15], to give students experience regarding how professional environmental chemists work and contribute to community health [
16], to familiarise them with Environmental Protection Agency procedures and trans-program cooperation with state agencies [
17], to help them understand performance-based analytical chemistry and the differences between analytical variability and scientific and legal uncertainty, and to build skills to communicate scientific data in a policy and legal context [
18].
Project-centred curricula ensure that content and process become integral parts of the learning experience through which students gain higher employability skills [
19], enhance their motivation, and achieve more in-depth learning [
20,
21]. Project-based learning becomes doing with understanding if students comprehend the goals correctly, self-assess, improve through repeated attempts, and have a social environment that supports their sustained engagement [
22]. High-quality group processes are profitable for all the participants [
23], and task-related conflicts contribute to the value of the final product [
24].
Project-based collaborative learning, if complex enough, requires organisational scheme and strategy of management, but this point of view is usually not so clearly exposed. The awareness is different with the degrees that require the project management knowledge and skills as their learning outcomes, they recognise project-based learning as an opportunity for students to obtain experience in project management too.
Agile management expressed as 12 principles was initiated in computer engineering through the Manifesto for Agile Software Development in 2001 [
25] to make programming more efficient and adaptive to stakeholders’ needs. The justification was that all details of every project cannot be clearly foreseen, and some of the requirements might change or become more profoundly expressed only during the course of the project. Consequently, the effort to precisely plan everything from the early beginning and to write very detailed documentation cannot make the project any more successful. Agile management, in contrast to traditional management, accepts uncertainties and transitions from negotiating with the stakeholders to collaborating with them tightly. It tends to release as early as possible the working versions of the software created during the short periods of intensive developments, later called “sprints”. The software is further developed and perfected through a sequence of iterations involving the stakeholders. The process is believed to lead to a better product in a shorter time with less effort and requires motivated and competent team members with complementary abilities and knowledge. The agile process promotes sustainable development.
Even though agile management is still the most prevalent in computer engineering, other professions and companies are starting to recognise its potential. It is adopted either in its original form or tailored to the needs of the project or organisation and sometimes combined with the more traditional forms [
26,
27]. The companies that have agile management implemented into their organisational scheme can use it for the recruitment of the employees that value the personal empowerment it provides [
28]. It is suitable for small teams and academia where the nature of work also involves elements of uncertainty and unpredictability [
29].
The Agile Manifesto in Education was declared a response to the deficiencies in university education and the demands of the employment market in 2012. It requested: “Teachers and Students over Administration and Infrastructure; Competence and Collaboration over Compliance and Competition; Employability and Marketability over Syllabus and Marks; Attitude and Learning skills over Aptitude and Degree” [
30]. There are appeals for agile management to be taught to engineering students more widely, not only through the information technology projects [
29], and there is evidence that it can improve online project management and collaboration among students [
31]. We tend to demonstrate its value for water research and analytical chemistry educational areas.
The aim of this paper is to present an elective master-level subject that merges water research with analytical chemistry and focuses on waterbodies rather than water samples (i) to broaden students’ perception of a total analytical process and (ii) to introduce water-research-specific data-evaluation and data-interpretation techniques. Further, the goal is (iii) to suggest the organisational scheme with the elements of agile management and control mechanisms that help students collaborate efficiently with success and satisfaction and facilitates the guidance and supervision for the staff. The final objectives are (iv) to evaluate students’ responses and (v) to indicate the implications, limitations, and transferability of the approach.
Innovative contributions and insights:
We provide full support for the implementation of the approach that merges water research, analytical chemistry, and agile management into teaching practice. The students’ responses reflect the instrument that we used to judge their perception of the project-centred collaborative approach and self-efficacy beliefs.
We present the projects’ recruitment mechanism and organisational scheme with the elements of agile management, which helped students collaborate and master their projects efficiently and enabled us to omit the classical exam with no adverse effect. We wish to inspire other educators to test the Principles of the Manifesto for Agile Software Development and the Agile Manifesto in Higher Education and translate then to their environment.
2. Materials and Methods
2.1. Elective Subject
“Water as a Hydrogeological, Ecological and Analytical System” is an elective subject of the Chemistry Master Study Programme at the University of Ljubljana. It is awarded 5 European Credit Transfer System (ECTS) credits and comprises 30 h of lectures, 15 h of seminar, and 30 h of practical.
2.2. Participants and Procedures
Our faculty decided to reduce costs by offering every elective subject only every second year. By suddenly having to deal with 40 students in 2015/2016 instead of 15, it became evident that single-student projects are not going to be feasible anymore. To keep a project-based approach, we introduced a collaborative-learning component with the elements of agile management. Students’ responses were encouraging, and so we upgraded and finalised the scheme in 2017/2018 when the enrolment rate was 19. The subject’s popularity rose, 60 students registered in 2019/2020, but the Coronavirus crisis hindered the realisation to some extent. Consequently, we present the responses of the generation 2017/2018 that we assessed by developing a five-section paper-form questionnaire. Students provided their feedback individually and anonymously by filling it in immediately after they accomplished the subject. Statistical evaluation was performed with MS Excel.
The first two sections gathered responses to the project-centred and cooperative learning experience. The third section tested students’ opinions on how close to a real-profession situation and how profitable particular aspects of their project work were in their opinion. For all three, the Likert scale was used, with one meaning the lowest, and five the highest level of agreement. The fourth and fifth parts were open-ended questions. The former collected opinions on how the fact that there was no classical exam affected students’ performance; the final part was open for expressions of any opinions or suggestions.
2.3. Choice of Parameters, Analytical Methods, and Reporting of Results
We made a compromise between a reasonable workload and the interpretative potential of the selected parameters. Students performed a potentiometric neutralisation titration with an automatic titrator to determine hydrogen carbonate. They used a suppressed ion chromatography for the determination of chloride, nitrate, and sulphate, quantified potassium and calcium by flame photometry, and calcium and magnesium by atomic absorption spectrometry. Lanthanum nitrate and caesium chloride were applied as releasing and ionisation-suppressing agents, and lithium-ion was applied as an internal standard. A molecular absorption spectrometry was employed for silicic acid, hydrogen phosphate, and nitrite ions determinations. The first two procedures are ammonium molybdate based; the latter involves the Griess reaction. For the determination of the dissolved oxygen concentration, the Winkler titrimetric method was applied. Chemical oxygen demand (COD) was determined titrimetrically to estimate the content of organic pollutants that can be readily oxidised. We are always open to students’ suggestions to determine some additional parameters. The generation concerned had no such requests.
All the students were already skilled in performing analyses with different methods. Each subgroup specialised in one or a few analytes, which they determined in all the samples. They prepared everything they needed on their own and agreed with the technicians to obtain access to laboratories and equipment. The deadlines by which analytical results were due had been agreed on in advance. Students reported results through online MS Excel spreadsheet tables.
2.4. The Subject Dynamics
The subject started with an explanation of the objectives, content, procedures, and expected outcomes.
2.4.1. Groups Formation and Choices of Waterbodies
Students were asked to think individually for a few minutes of waterbodies they would like to examine and define what makes their propositions good choices. Afterward, they were invited into subgroups to gather at different areas of the lecturing hall regarding geographical regions of their origin and to discuss their ideas with their peers. Every student could freely choose to make several relocations to recognise inclinations or the best synergies between his own proposition and propositions of the others. The process resulted in the groups’ formation with no intervention from the staff. The students within a group singled out the waterbody for the unified group project and announced the projects’ title and the members of the groups.
The students who felt they did not fit into any of the groups were not obliged to give up on their propositions. They were stimulated to discuss with others in the same position what potentially relates the waterbodies of their personal choices, e.g., making them similar or complementary in terms of a type, some particular feature, or a common problem, and to define combined projects.
2.4.2. Subject Outline
After all the projects were announced, the date for oral presentations was set and expectations explained. Students started working on their projects alongside the lectures that provided background knowledge on the types and characteristics of waterbodies, designs of water research studies, selection and evaluation of water quality variables, field sampling strategies, on-the-spot determination of parameters, and data-evaluation techniques [
32]. During seminars, students were challenged to reflect on how they were going to master particular tasks and situations and supported to develop the necessary data-evaluation and data-interpretation skills.
At the oral presentation, they described waterbody characteristics, explained expected influences on water composition, declared research hypotheses, and suggested sampling sites. A list of samples was discussed and approved, sample codes allocated, and samples that could present extreme cases for particular analytical methods recognized.
Students accomplished water sampling for a preliminary study that was intended to give an initial insight into water-sample composition and to enable testing of analytical methodology. They studied the method protocol, self-organised, and planned how to share laboratory work to accomplish analyses on time. Strategies, organizational schemes, and timelines were discussed, iterative exchanges of observations enabled during seminars, and problems addressed when recognised. Students worked autonomously and independently to the highest possible extent, coordinated their work, and primarily communicated among themselves. During the seminar, any observed inconsistencies in analytical results were challenged and possible causes identified. Students repeated analyses if necessary or used some additional method where possible to test the reliability of their results. Data re-evaluation followed. The core and post-study obeyed the same pattern. The purpose of the post-study is to obtain some insight into temporary variations in water composition.
Students used the results from all three phases and inspect titration curves and their first derivatives to examine their waterbodies and test hypotheses. They used different numerical and graphical evaluation methods that were specific to water research. They submit a written essay for evaluation and optionally also an amended description of analytical methodology if they recognised this as necessary. They passed the module with an oral defence of their essay.
2.5. Water Samples
Students sampled waters during the weekends on the pre-agreed dates. There were 35 sampling sites altogether. The first three projects included five sites; the remaining two consisted of ten sampling sites, respectively. Sampling was performed three times, comprising preliminary, core, and post-study phases. Only during the core study phase were samples collected from all the sampling sites. Samples were taken into 1.5 L polymeric bottles of commercial spring waters of low mineralisation and filtered in a laboratory when required. There were 60 in total. Samples for the determination of dissolved oxygen were collected only from selected sampling sites. Winkler bottles were used, and sample preservation was accomplished at the site. The temperature of air and water was measured. A pH was determined in a laboratory.
Students delivered samples to the laboratory on Monday at a pre-agreed hour. Samples were allocated to a special area, and the workspace was organised for taking analytical portions of different sizes. Students filled in a registration form stating the sample code, waterbody, sampling location and time, and observations or presumptions important for analytical work, e.g., sample turbidity, colour, odour, high pollution, or concentration of particular analytes.
2.6. Hypotheses and Research Questions
We are going to test the hypotheses:
Hypotheses 1 (H1). By merging water research with analytical chemistry and transferring the focus of the project-centred approach from water samples to waterbodies, we strengthen the contextual framework of a total analytical process and give students an opportunity to more extensively examine and discuss something real they feel connected to.
Hypotheses 2 (H2). By a considerate choice of chemical parameters, we enable the introduction of water-research-specific data-evaluation and data-interpretation techniques and maximise the interpretative potential of the results of chemical analyses for an explanation of waterbody characteristics.
Hypotheses 3 (H3). The suggested organisational scheme of the project-centred approach with the elements of agile management helps students collaborate efficiently with success and satisfaction, facilitates the guidance and supervision for the staff, and enables the omission of the final classical exam with no adverse effects.
We tend to answer the research questions:
Q1: How did students perceive the project-centred approach?
Q2: What were their self-efficacy beliefs in relation to the omission of a classical exam?
Q3: What were the students’ opinions on the profitability of the elective subject for their possible prospective career?
4. Discussion
As Helle et al. realised, the purposes of project-based learning can be of three different kinds. Firstly, very concrete experiences regarding a certain process, secondly, opportunities for integration of subject matter, and finally, guided discovery learning, promoting more in-depth learning [
38]. Even though the authors warn not to target within the same project all the three objectives, students’ answers and their essays demonstrate that by the waterbodies-centred approach, they were not only more fruitfully exposed to the total analytical process (H1,
Figure 1) and collaboration (H3,
Figure 3 and
Figure 6), but also integrated water-research-related subject matter (H2,
Figure 1,
Section 4.1), and learned more in-depth (
Table 2,
Section 3.2.4). Some authors raise concerns that in self-driven project-based learning, not all students learn the same and the whole content as they would have in classical learning environments, since their projects usually require specific background and skills. With waterbodies and the project-centred approach, this is not the case. Every group needs to master the same general background and methodology to accomplish the total analytical process and interpret data. Share of work might cause some within-group differentiation, but not in a way that some content would have been entirely missed by some students, and they would have been unaware of it. Some members might go deeper in some areas and others in others, depending on their agreement. Nevertheless, during the preparation of a joint essay, they need to collaborate and exchange knowledge to write a coherent and concise contribution with justifiable conclusions.
For the readers not particularly interested in water research and analytical chemistry,
Section 4.2 is more relevant than
Section 4.1.
4.1. Water-Research-Specific Data-Evaluation and Data-Interpretation Techniques
The main challenge for students was to relate and compare results to explain waterbody characteristics at the professionally sound level. In relation to the H2 hypothesis, we discuss in this section how students are expected to exploit the results of chemical analyses. It is a higher level of complexity examining water genesis or processes in water on the basis of water composition, than judging contamination or compliance with the norms for a particular use. As in every real-sample analysis, especially with a high number of samples, unexpected problems always arise, e.g., unidentifiable peaks in ion chromatograms, irregularities in titration curves that are difficult to explain, or calibration ranges that are not universally applicable and need to be tailored to specific characteristics of particular samples. However, with the insight into disagreements in cation–anion balance (
Section 4.1.1) and unusual ion proportions (
Section 4.1.2), the quality of the results receives an additional dimension. After students resolve inconsistencies, they can extract meaning from the results, as we indicate in
Section 4.1.2 and
Section 4.1.3.
4.1.1. Cation–Anion Balance
The complete analysis of a water sample, as understood in water research, is very extensive. Nevertheless, with the parameters that we selected (
Figure 2), the core composition comprising cations and anions is covered reasonably well and enables the test of the consistency of an analysis as a whole. The cation–anion balance is calculated by Equation (1).
Symbol
ce stands for an equivalent concentration of a cation or anion, distinguished by a plus or minus sign in a subscript. Students might not be familiar with an equivalent (eq) as a non-SI unit (International System of Units) and might need an explanation that it represents the amount of charge directly. One further calculates the equivalent concentration by multiplying the amount concentration of an ion,
c, with its charge,
zA (
Figure 2). They deduce that if an analysis is complete with no grave errors, and the results are high quality, the total equivalent concentrations of cations and anions are supposed to be equal. Of course, a perfect match is only an exception, since the determination of every concentration is associated with some measurement uncertainty.
The expectations derived from experiences are that disagreements up to five percent confirm that analytical results are of a high enough quality [
7]. Otherwise, an explanation should be found. A possible cause might be that an analysis is not complete, so some ions were not considered. Water contamination with organic anions might be the reason. Special cases are waters with a low pH, e.g., volcanic waters or those in which pyrite watering is taking place, and carbonates that can consume the released H
+ ions are not present. The concentration of H
+ ions is normally not included in a cationic section of an analysis. Their contribution is expected to be negligible. The cation–anion balance should be reconsidered. If no explanation can be found, a transcription or calculation error or an analytical error might be the reason.
4.1.2. Watering and Proportions of Ions in Water
Students become acquainted with the processes of watering that affect the natural composition of water, which consequently reflects the geology of the region. Dolomite watering reaction, for example, leaves no solid residual and results in a relative calcium on magnesium on hydrogen carbonate ratio 1:1:4:
In contrast to this, the watering of albite leaves some solid residue. If the residue, for instance, is kaolinite, the reaction goes as follows:
It results in a 1:1:2 relative proportion of sodium to hydrogen carbonate to silicic acid in water. By including SiO2 into water analyses, in addition to cations and anions, students had the opportunity to make such judgements.
Figure 7 gives some further insight into some naturally expected relative amount proportions of ions and silicic acid in water resulting from the watering of some common minerals. A rectangle in a grid represents a unit.
Relative proportions of ions discussed previously regarding water’s hydrogeological origin are also an indicator of the coherency of analytical results. A higher amount concentration of calcium than magnesium, more calcium than sulphate, more sodium than chloride, and more sodium than potassium can be expected for the majority of cases as far as the natural sources of ions are concerned. If one observes a different pattern, an explanation is necessary.
Chloride, for example, can be of a hydrogeological origin coming from halite (NaCl) that is at the same time and to the same extent the source of sodium. Furthermore, sodium has other sources, as can be seen from
Figure 7. Hence, its amount concentration in water is either equal to or higher than chloride concentration. If the contrary is true, additional artificial sources of chloride should be recognized, e.g., sewage, road de-icing, or some industrial source. If no explanation can be found, an analytical or transcription error might be a cause.
Other parameters such as pH, oxygen concentration, nitrite, nitrate, hydrogen phosphate, and COD support discussion on water contamination and water quality.
4.1.3. Graphical Data-Evaluation and Data-Interpretation Techniques
Graphical evaluation techniques such as the Stiff and Piper diagrams can tell us even more [
7]. They were both schematically presented in
Figure 1. From a Stiff diagram, a rock deduction can be made. Usually, it has three horizontal axes dedicated to the pairs of ions. The scales are in meq/L; they might well be logarithmic. The two shapes presented in the Stiff diagram in
Figure 2 (top/bottom) suggest predominantly limestone or gypsum origin, respectively.
A Piper diagram has a different role. A water type can be deduced from it. It has two triangular parts: one dedicated to cations and another to anions. The scales are in meq%, obtained by dividing the equivalent concentration of a particular anion by the sum of the equivalent concentrations of all the anions, for instance. The same is true for cations. The scale runs from 0 to 100%, reaching the latter where the ion mark is. In
Figure 1, each scale of the Piper diagram is divided into only three sections, to ensure that the graph is not too crowded. A dot close to the calcium corner, for instance, represents water with a composition of 65 meq% of calcium, 18 meq% of magnesium, and 17 meq% of sodium. The corresponding dot in the anionic part shows composition 92 meq% of hydrogen carbonate, 5 meq% of chloride, and 3 meq% of sulphate. Accordingly, water is of the calcium-hydrogen carbonate type, common for drinking waters. Every ion reaching at least 20 meq% contribution to cationic or anionic composition is named in the balneological type, cations before anions, and within each part, the sequence is from the highest to the lowest contributor. This gives the first insight into potential use, e.g., for drinking or bathing or industrial use.
The points pertaining to a single waterbody can form specific graphical patterns in each of the triangular parts. By judging them comparatively, one can recognise processes in a waterbody, e.g., dissolution or precipitation of minerals, mixing of waters of different origins, or ion exchange.
In a diamond section of a Piper diagram, cationic and anionic composition come together. Waters group concerning their overall character. Waters characterised by permanent hardness, alkaline carbonates, temporary hardness, or saline, respectively, group separately in the upper, lower, right, or left section.
In relation to the H1 and H2 hypotheses, we demonstrated that waterbodies as a topic in a combination with the choice of chemical parameters that enable water-research-specific data-evaluation and data-interpretation techniques give students the opportunity to extensively examine and discuss their subject. By being much more intensively involved in the initial and the final step of the total analytical process, as is usually possible with other topics, their perception of analytical chemistry changes and extends beyond the results as numbers and towards their meaning and significance.
4.2. Project-Centred Collaborative Approach with the Elements of Agile Management
In the H3 hypothesis, we claimed that the suggested organisational scheme of the project-centred approach with the elements of agile management helps students collaborate efficiently with success and satisfaction, facilitates the guidance and supervision for the staff, and enables the omission of the final classical exam with no adverse effects.
We first analyse the elements of agile management and their significance; in the continuation, we discuss the overall quality of collaboration.
4.2.1. Elements of Agile Project Management and Their Impact
We analyse three main aspects of agile management [
25]: (a) necessity to have a team of motivated and competent individuals with complementary abilities, (b) iterative approach with an early release of working versions and sustainable development, and (c) collaboration between providers and consumers of the results of chemical analyses. We justify how they contributed to confirmation of the hypothesis H3.
(a) Team of competent individuals and recruitment mechanism
According to other authors [
31], by incorporating a preparatory phase into collaborative agile learning, students obtain an insight into what they can expect from their peers; furthermore, relations are not hierarchical, distribution of work is more pragmatic, responsibilities are rotational, and therefore, better perceived by students. As
Figure 3 demonstrates, the project proposition and team formation process as a preparatory phase starts with students’ personal ideas and continues in discussions with their peers, associating first with those who come from the same geographic region. Geographical proximity may help them position as well-informed connoisseurs of the region; otherwise, they needed to define themselves as potentially valuable contributors by some other aspect. Students can shape their proposition through several iterative discussions or even several relocations. This brings them closer to a real-profession situation where project participants are selected by their expertise, and employees are expected to be able to collaborate with different co-workers. The approach follows the fifth principle of the Agile Manifesto [
25], which suggests that we build projects around motivated individuals, who receive proper environment and support, and are trusted to carry the job out.
(b) Iterative approach with an early release of working versions and sustainable development
Apart from the recruitment mechanism, the iterative approach is involved in points 3, 4, 5, and 6 of the total analytical process (
Figure 3). Field and laboratory work are accomplished in three cycles, preliminary, core, and post-study. The preliminary study gives students an initial insight into concentration ranges they can expect for particular chemical parameters. They test and apply analytical methods to fever samples than in the following cycle to recognise potential difficulties and find solutions. During the core study, they have the opportunity to perfect their skills, enhance their analytical efficiency, and obtain an overall insight into the composition of water on all sampling sites. The post-study has a confirmatory nature or indicates how water composition changed over time. Students similarly apply data-evaluation and data-interpretation techniques first to some training sets during the seminars; later, they use them in all three cycles with real data.
The organisational scheme (
Table 1) that associates each step of the total analytical process with the outcomes and control mechanisms dissociates the whole process into periods of intensified activities “sprints” that end in some initial working versions that lead towards the essay accomplishment. This helped students to apply simplified agile management articulated by Nicolls et al. in three points: (i) identification of hard and soft deadlines, (ii) scoping, prioritising and selecting projects, and (iii) managing the tasks to be done now [
29]. Students were capable of handling the situation by not running into a shortage of time that could have compromised the quality of the final result, the problem that some authors observed in students with agile project collaboration [
31].
The third principle of the Agile Manifesto [
25] requires frequent delivery of working software in short intervals. In our case, it reflects in the gradual release of working versions as partial inputs to the final essay. The initial input is an oral presentation comprising the study of the background, project outline, statement of hypotheses, and sampling sites. Documentation of the process of field sampling that they accomplish autonomously follows. They evaluate and judge the results of chemical analyses promptly, since partially self-filling self-notifying tables require an immediate response for the analyses to be repeated. To accomplish an essay, students need to perfect the already existent materials, make a synthesis, and write a discussion of results and conclusions.
Writing the essay for combined waterbodies projects, if compared to the unified waterbody projects, was more demanding. The content was extensive, space very limited, every sentence had to be considered, students acted as experts for individual waterbodies, and had to collaborate tightly and make compromises to prepare a coherent piece of work that did not exceed the allowed number of pages. The combined waterbody projects enhance the overall quality of the essay and collaboration.
(c) Collaboration between providers and consumers
Frequent communication, which is critical also for the simplified agile management [
29], was the essence and driving force of within-group collaboration in our case. Between-group collaboration was necessary to resolve inconsistencies in the results of chemical analyses. Students followed the sixth principle of the Agile Manifesto that recognises face-to-face conversation as the most efficient and effective method of conveying information to and within a development team [
25].
Students found themselves in a double role, being consumers and providers all in one and switching the role depending on the issue that they were resolving. By this, their experience with the provider–customer cooperation as one of the essential agile management characteristics [
25] was even more intensive. In the continuation, we rationalise the benefits.
4.2.2. Collaboration: Commitment, Responsibility, and Satisfaction
As
Figure 6 confirms, students recognised several advantages of collaborative learning and strongly disagreed that they would have preferred working on their own. No-one complained about the fairness of the work share as can be seen from the final open-ended part of the questionnaire (
Table 3). The staff were not made aware of any conflicts, and students were not made to sign any formal code of conduct. Students created a bond and developed a devotion to the team and the project. Group formation was neither staff regulated nor driven by students’ wishes to stay with people they already knew well and were friendly with; it was topic centred. Students qualified as members of a group by being actively involved in discussions with their peers while shaping the project outline. Someone’s dedication is different if participation and a topic are one’s own choices than if one considers being forced into a project against one’s own wishes. Other authors realised that social loafing is minimised when group processes include positive interdependence, individual accountability, equal participation, and good social skills [
23].
The eleventh principle of the Agile Manifesto recognises that the best architectures, requirements, and designs emerge from self-organising teams [
25]. Exchange of arguments among the peers and autonomous decisions were characteristic for all the steps of the project: from the project topic definition, through self-organised field sampling and laboratory work, between-group collaboration in resolving inconsistencies in analytical results, to the essay accomplishment. Students appreciated that they were trusted, allowed to work on their own, self-organise, and address problems (
Table 2 and
Table 3). Other researchers confirmed that project- and problem-based environments that allow students to actively participate in making decisions about what, how, at which level, where, and when are they going to do something, termed as self-regulation, can contribute to deeper-level learning and motivation [
39].
No persuasion skills and effort were required of the staff to promote quality; students were well prepared to seek potential causes of inconsistencies and check or repeat the determinations. They were aware that high-quality results are inevitable for each group to test their hypothesis, and since every group determined only a few parameters, they were interdependent and shared the responsibility not to compromise their own or other people’s projects. It is likely that responsibility towards their peers was supported by the awareness that if one part of the results was missing, nothing would have been accomplished yet. Consistency of analytical results could not have been confirmed and hypotheses not tested. Other authors observed similar behaviour when the outcome was meaningful to students [
22,
35].
Cognitive and social mechanisms contribute to the success or failure of collaborative learning, e.g., if collaboration requires more energy than the task itself, it ends in exhaustion and dissatisfaction [
40]. In our case, on the contrary, students were satisfied with the collaboration, since it was not an artificial task but a necessity. The workload was simply too heavy for an individual student to sustain; consequently, students appreciated the combined effort and support of their peers, and the organisational scheme helped them progress.
Trying to navigate students through the whole process would have been an impossible task for the staff to realise, and the outcomes would certainly not have been equally good. This is in line with the agile management manifesto [
25]: in complex situations with several sources of uncertainty, enhanced regulation does not contribute to better results. Students needed to work independently. Iterative approach and control points were safeguards for students that directed them towards the successful accomplishment of the project.
The ninth principle of the Agile Manifesto recognises that the continuous attention to technical excellence and good design enhances agility [
25]. Partially self-filling online Excel spreadsheet tables with self-generated inconsistency alerts were very important for students, their attitude, and success. These water-research-specific data reliability checks were made possible by a considerate choice of chemical parameters and by putting waterbodies instead of a particular aspect of water at the centre of their projects.
We confirmed the H3 hypothesis; the suggested organisational scheme of the project-centred approach with the elements of agile management helps students collaborate efficiently with success and satisfaction, facilitates the guidance and supervision for the staff, and enables the omission of the final classical exam with no adverse effects.
4.3. Transferability of the Approach, Implications, and Limitations
Even though the analyses were not complete, since we included only the main ions, we confirmed that the choice of parameters was good enough for the great majority of samples to enable the application of water-research-specific data-evaluation and data-interpretation techniques. However, some inconsistencies in results remained difficult or even impossible to resolve. The particulate matter in water was a possible cause. With some investment into additional equipment, filtering of the samples at the site could have been made possible for all the groups. Some additional organic or inorganic contaminants or field tests can be included, either generally or specifically, for some projects or even some sampling sites, as required.
The approach is transferable in its original or reduced form. Waterbodies must remain the central point of the projects, since they allow the recruitment mechanism, which contributes to successful cooperation, students’ interdependence, rich essay content, and discussions. The number of parameters can be reduced, while keeping some that can be associated with hydrogeological origin and enabling at least some judgement on the overall consistency of the analytical results would be advisable. Between-group interdependence that enables students to experience the consumer–provider collaboration from both sides is beneficial for the quality of collaboration and dedication.