Impact of Oil Palm Empty Fruit Bunch Biochar Enriched with Chicken Manure Extract on Phosphorus Retention in Sandy Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar and Manure Source
2.2. Preparation of Chicken Manure Extract
2.3. Preparation of Enriched Biochar
2.4. Soil Sampling and Soil Physico-Chemical Analysis
2.5. Incubation Experiment
2.6. Leaching Experiment
2.7. Statistical Analyses
3. Results and Discussion
3.1. Chemical Characterization of Chicken Manure Solid and Liquid
3.2. Chemical Characterization of Biochar and Enriched Biochar
3.3. Soil Physico-Chemical Characterization
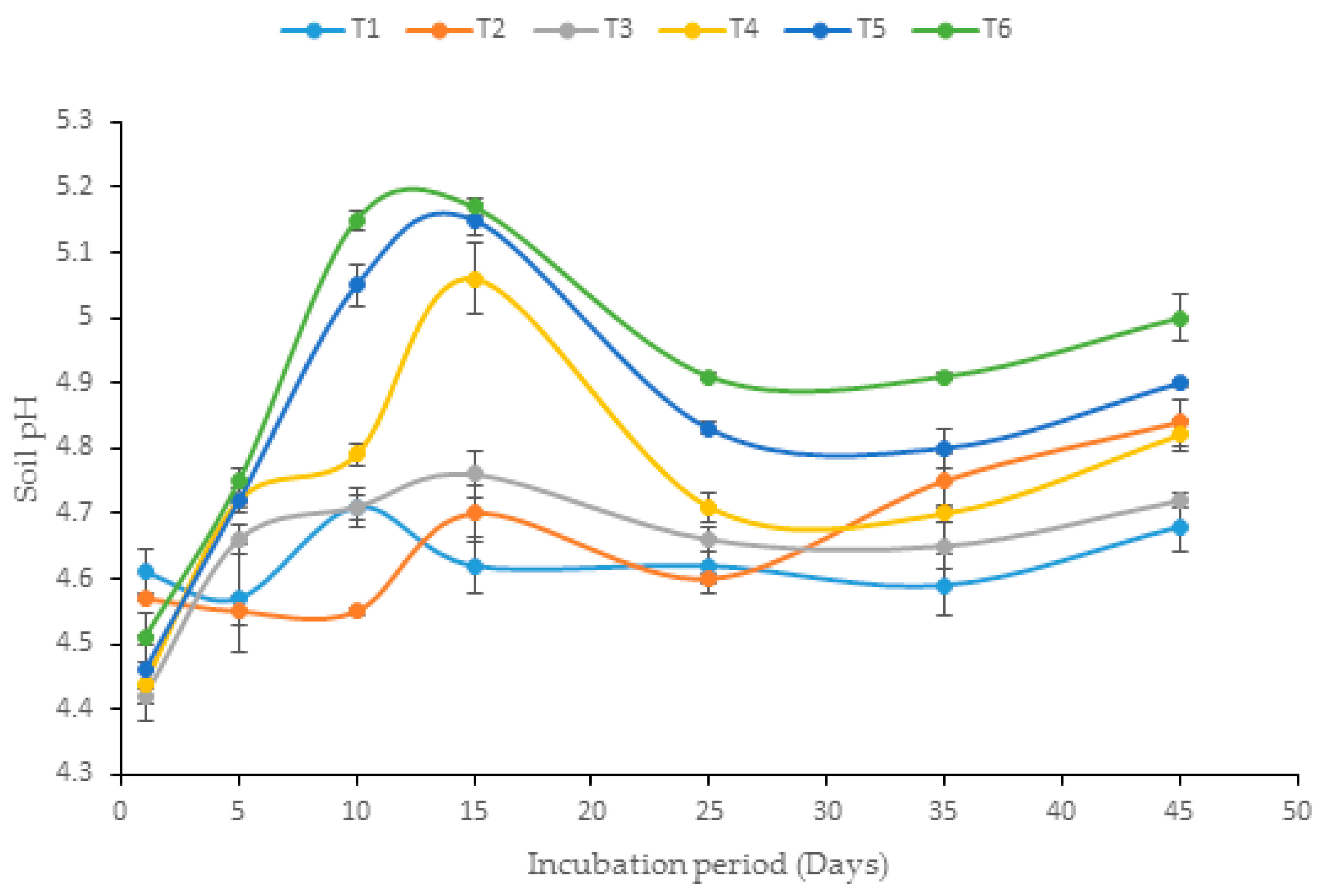
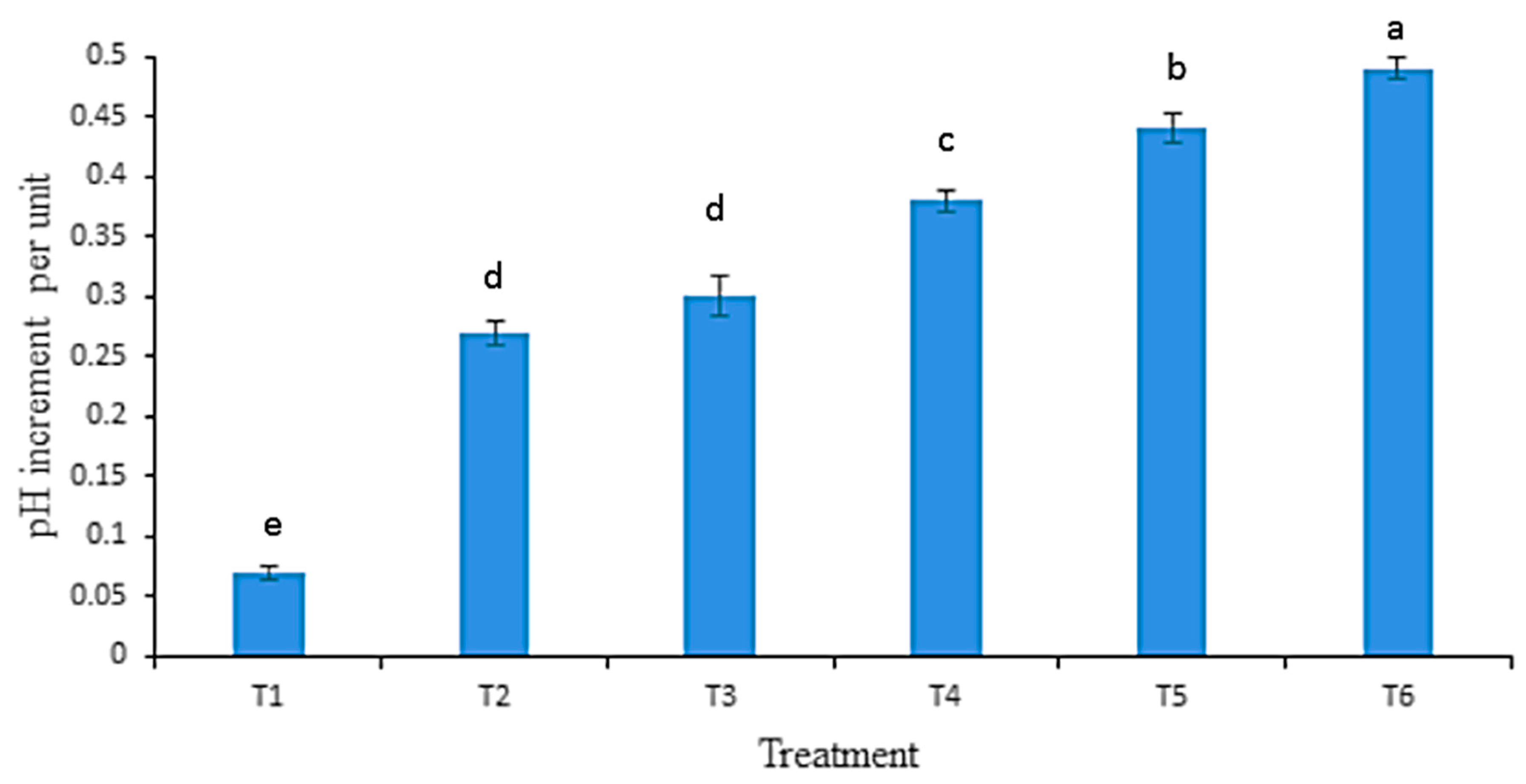
3.4. Influence of Biochar Enrichment on Soil pH
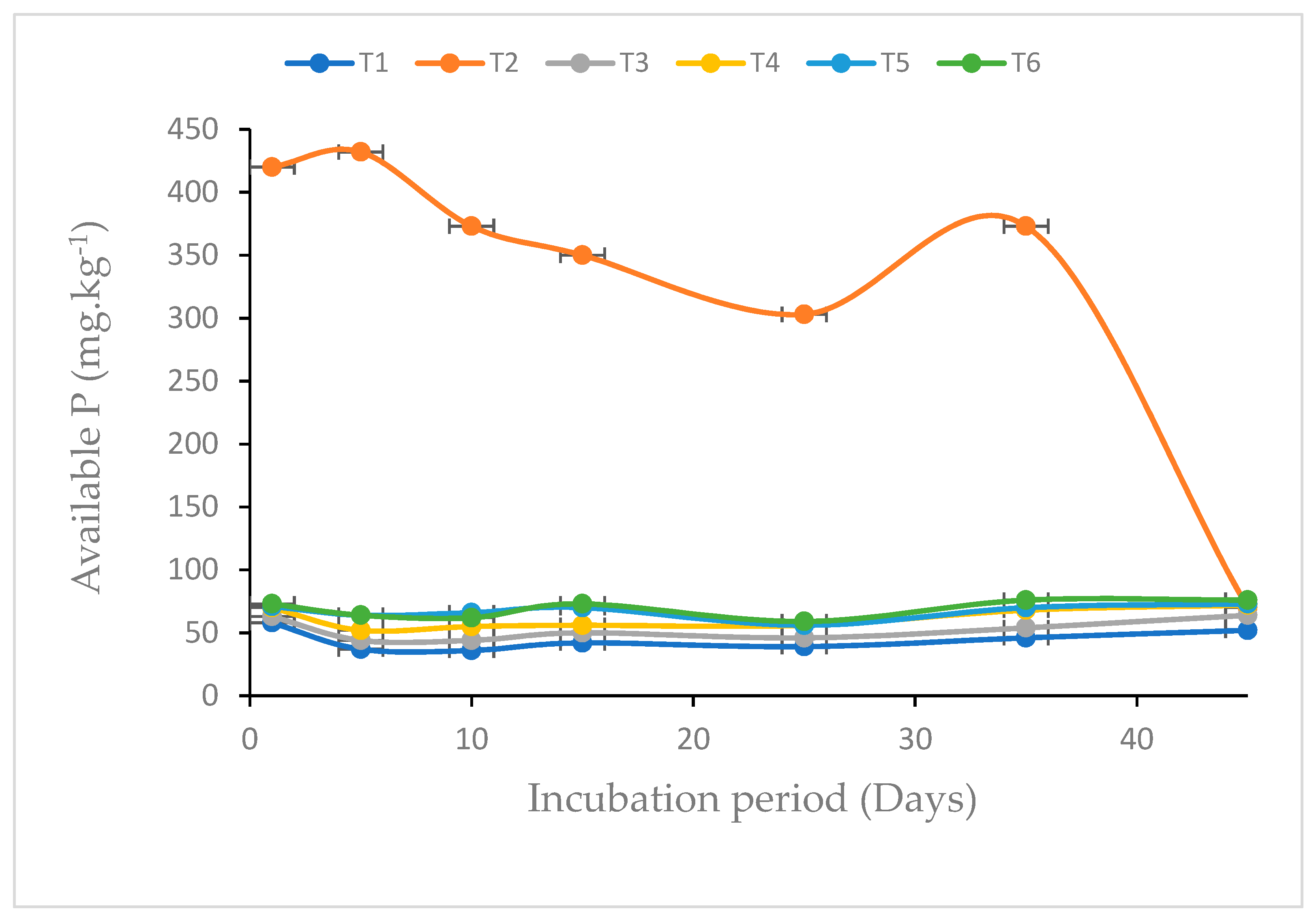
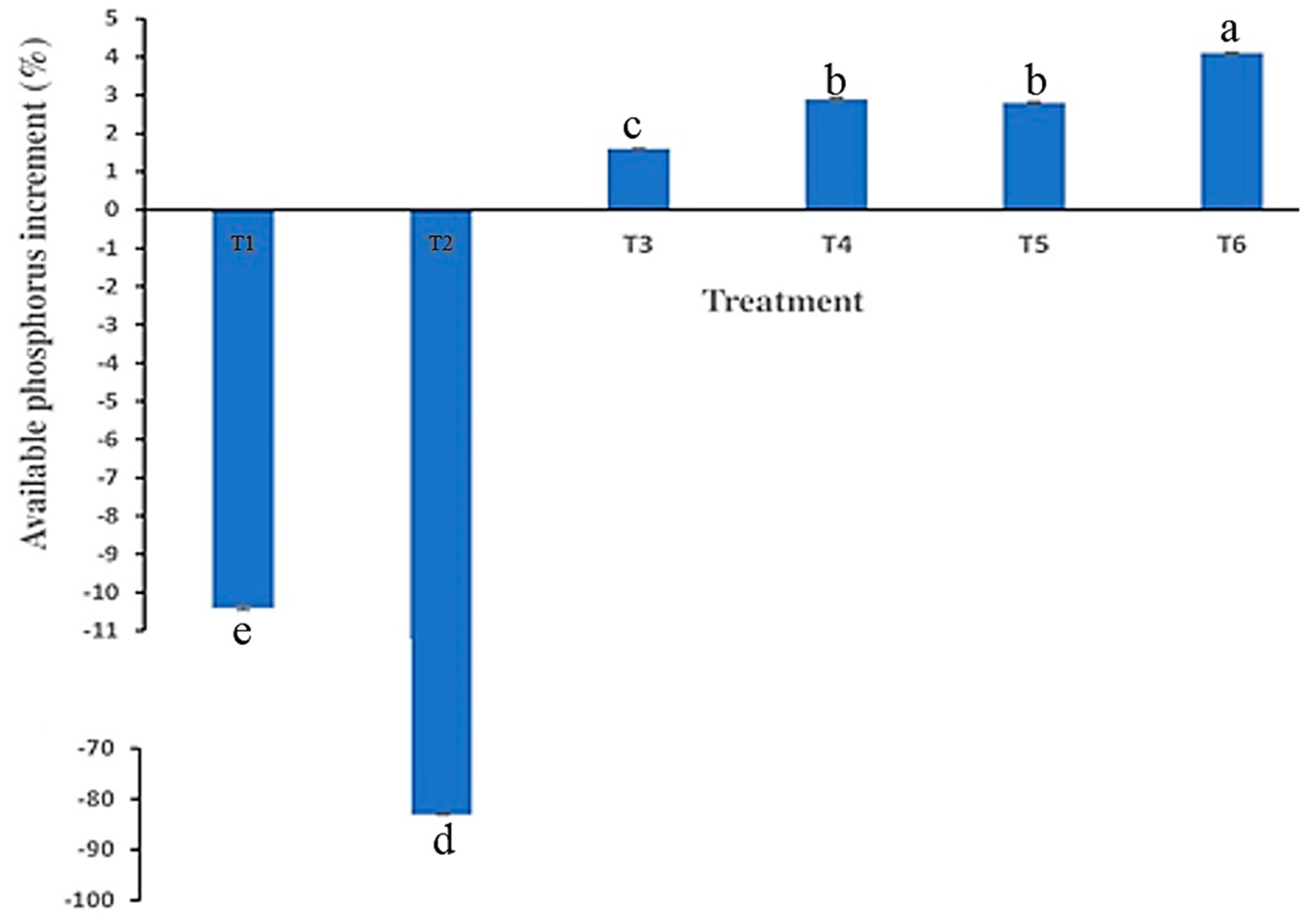
3.5. Influence of Biochar Enrichment on Phosphorus Availability
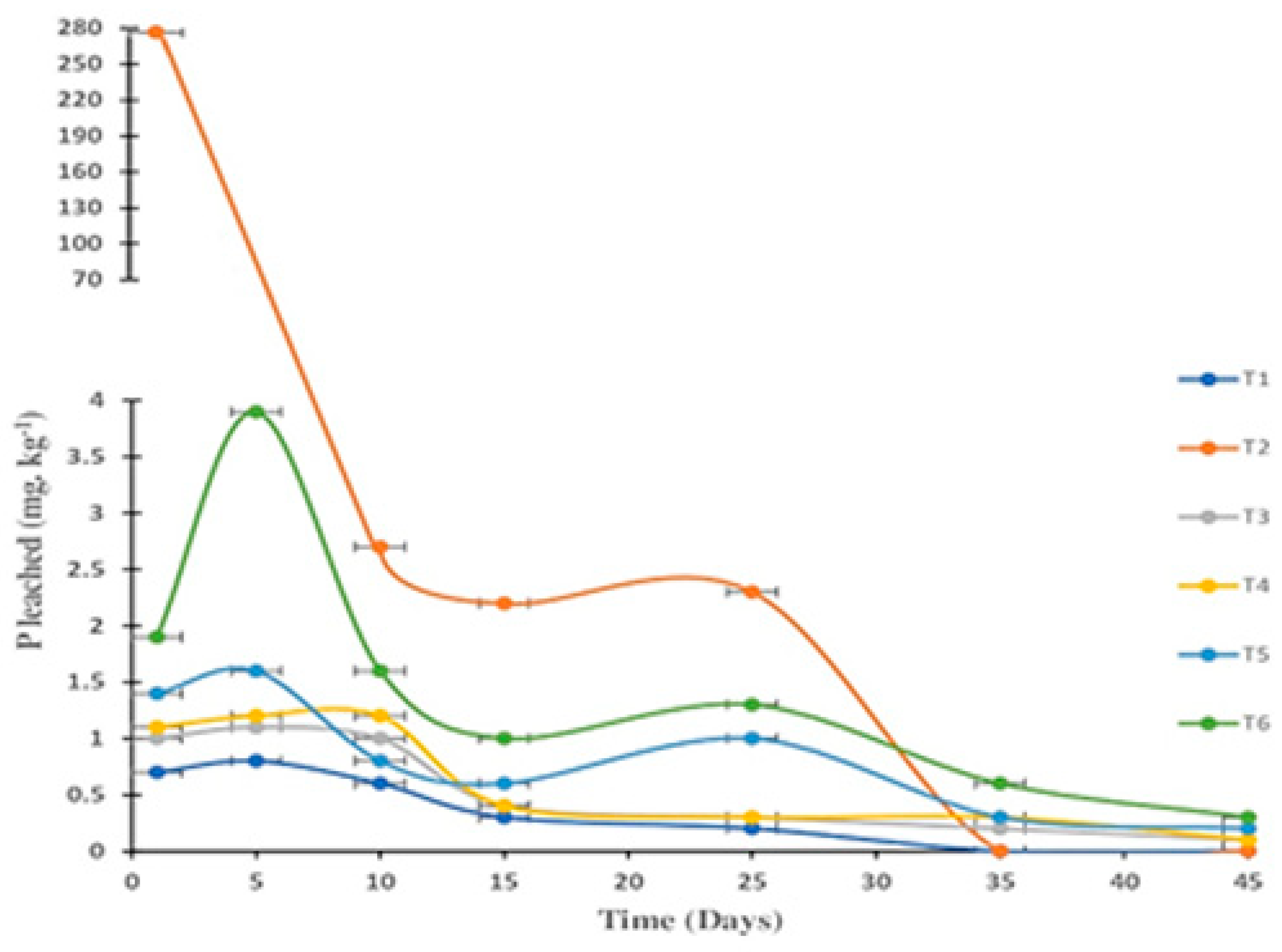
3.6. Influence of Biochar Enrichment on Leaching Losses of Phosphorus
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanafi, M.M. Managing Phosphorus: Under Acid Soils Environment; Universiti Putra Malaysia Press: Serdang, Selangor, Malaysia, 2010. [Google Scholar]
- Asma, I.W.; Rasidah, K.W.; Rosenani, A.B.; Aminuddin, H.; Rozita, A. Effects of mulching and fertilizer on nutrient dynamics of sand tailings grown with Acacia hybrid seedlings. J. Trop. For. Sci. 2011, 1, 440–452. [Google Scholar]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, R.S.; Cruse, R.M.; Ghaffarzadeh, M.; Bakhsh, A.; Karlen, D.L.; Bailey, T.B. Corn-soybean and alternative cropping systems effects on NO3-N leaching losses in subsurface drainage water. Appl. Eng. Agric. 2005, 21, 181–188. [Google Scholar] [CrossRef]
- Stuart, D. Sandy tin Tailings in Malaysia: Characterization and Rehabilitation; University of Plymouth: Plymouth, UK, 2003. [Google Scholar]
- Teh, C.; Sung, B.; Ishak, C.F. Soil Properties (Physical, Chemical, Biological, Mechanical). In Soils of Malaysia, 1st ed.; Ashraf, M.C., Othman, R., Ishak, Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 103–154. [Google Scholar]
- Shamshuddin, J.; Fauziah, C.I. Weathered Tropical Soils. The Ultisols and Oxisols; University Putra Malaysia Press: Serdang, Malaysia, 2010. [Google Scholar]
- Cunha, T.J.; Madari, B.E.; Canellas, L.P.; Ribeiro, L.P.; Benites, V.D.; Santos, G.D. Soil organic matter and fertility of anthropogenic dark earths (Terra Preta de Índio) in the Brazilian Amazon basin. Rev. Bras. Cienc. Solo. 2009, 33, 85–93. [Google Scholar] [CrossRef]
- Lehmann, J.; Da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Topoliantz, S.; Ponge, J.F.; Ballof, S. Manioc peel and charcoal: A potential organic amendment for sustainable soil fertility in the tropics. Biol. Fertil. Soils 2005, 41, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H., Jr. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Jindo, K.; Suto, K.; Matsumoto, K.; García, C.; Sonoki, T.; Sanchez-Monedero, M.A. Chemical and biochemical characterisation of biochar-blended composts prepared from poultry manure. Bioresour. Technol. 2012, 110, 396–404. [Google Scholar] [CrossRef]
- Yoo, G.; Kang, H. Effects of biochar addition on greenhouse gas emissions and microbial responses in a short-term laboratory experiment. J. Environ. Qual. 2012, 41, 1193–1202. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef]
- Lentz, R.D.; Ippolito, J.A. Biochar and manure affect calcareous soil and corn silage nutrient concentrations and uptake. J. Environ. Qual. 2012, 41, 1033–1043. [Google Scholar] [CrossRef]
- Macdonald, L.M.; Farrell, M.; Van Zwieten, L.; Krull, E.S. Plant growth responses to biochar addition: An Australian soils perspective. Biol. Fertil. Soils 2014, 50, 1035–1045. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. Biochar for environmental management. Sci. Technol. 2009, 1, 85–105. [Google Scholar]
- Moore, P.A.; Daniel, T.C.; Sharpley, A.N.; Wood, C.W. Poultry manure management: Environmentally sound options. J. Soil Water Conserv. 1995, 50, 321–327. [Google Scholar]
- Guohua, L.; Li, H.; Leffelaar, P.A.; Shen, J.; Zhang, F. Characterization of phosphorus in animal manures collected from three (dairy, swine, and broiler) farms in China. PLoS ONE 2014, 22, e102698. [Google Scholar]
- Peng, F.; He, P.W.; Luo, Y.; Lu, X.; Liang, Y.; Fu, J. Adsorption of phosphate by biomass char deriving from fast pyrolysis of biomass waste. Clean Soil Air Water 2012, 40, 493–498. [Google Scholar] [CrossRef]
- Streubel, J.D.; Collins, H.P.; Tarara, J.M.; Cochran, R.L. Biochar produced from anaerobically digested fiber reduces phosphorus in dairy lagoons. J. Environ. Qual. 2012, 41, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Chen, J.; Zhang, M.; Inyang, M.; Li, Y.; Alva, A.; Yang, L. Engineered carbon (biochar) prepared by direct pyrolysis of Mg-accumulated tomato tissues: Characterization and phosphate removal potential. Bioresour. Technol. 2013, 138, 8–13. [Google Scholar] [CrossRef]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Biochar pH, electrical conductivity and liming potential. In Biochar: A Guide to Analytical Methods; CRC Press: Boca Raton, FL, USA, 2017; Volume 1, p. 23. [Google Scholar]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- John, P.; Combs, S.; Hoskins, B.; Jarman, J.; Kovar, J.; Watson, M.; Wolf, A.; Wolf, N. Recommended Methods of Manure Analysis; University of Wisconsin Cooperative Extension Publishing: Madison, WI, USA, 2003. [Google Scholar]
- Campbell, C.R.; Plank, C.O. Preparation of plant tissue for laboratory analysis. Methods for Plant Analysis; CRC Press: Boca Raton, FL, USA, 1998; p. 37. [Google Scholar]
- Zarcinas, B.A.; Ishak, C.F.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia. Environ. Geochem. Health 2004, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Landon, J.R. Booker Tropical Soil Manual: A Handbook for Soil Survey and Agricultural Land Evaluation in the Tropics and SUBTROPICS; Routledge: Abingdon, UK, 2014. [Google Scholar]
- Song, C.T.B.; Talib, J. Soil Physics Analyses; Universiti Putra Malaysia Press: Serdang, Selangor, Malaysia, 2006; Volume 1. [Google Scholar]
- Murphy, J.A.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Aini, Z.; Sivapragasam, A.; Vimala, P.; Roff, M.N. Organic Vegetable Cultivation in Malaysia, 1st ed.; Malaysian Agricultural Research and Development Institute (MARDI): Serdang, Malaysia, 2005. [Google Scholar]
- Rodriguez, C.M. Performance of chicken layers as affected by calcium supplement. Int. J. Sci. Res. 2013, 2, 1749–2094. [Google Scholar]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results: What Do all the Numbers Mean? CSIRO Publishing: Collingwood, Australia; Clayton, Australia, 2016. [Google Scholar]
- Shi, R.Y.; Li, J.Y.; Ni, N.I.; Xu, R.K. Understanding the biochar’s role in ameliorating soil acidity. J. Integr. Agric. 2019, 18, 1508–1517. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B.; Hu, D. Effective alleviation of aluminum phytotoxicity by manure-derived biochar. Environ. Sci. Technol. 2013, 47, 2737–2745. [Google Scholar] [CrossRef] [PubMed]
- Berek, A.K.; Hue, N.V. Characterization of biochars and their use as an amendment to acid soils. Soil Sci. 2016, 181, 412–426. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, P.; Quilliam, R.S.; DeLuca, T.H.; Vamerali, T.; Jones, D.L. Does biochar application alter heavy metal dynamics in agricultural soil? Agric. Ecosyst. Environ. 2014, 184, 149–157. [Google Scholar] [CrossRef]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and quantification of biochar alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, C.; Sánchez-Monedero, M.A.; Kammann, C. Biochar as an additive to compost and growing media. In Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015. [Google Scholar]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Boil. Sci. 2009, 1, 48–58. [Google Scholar]
- Ayanda, A.F.; Shamshuddin, J.; Fauziah, C.I.; Radziah, O. Utilization of magnesium-rich synthetic gypsum as magnesium fertilizer for oil palm grown on acidic soil. PLoS ONE 2020, 15, e0234045. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Rate of EEFB t ha−1 | Amount of P (kg. ha−1) | In Experiments g. g Soil−1 |
|---|---|---|---|
| T1(control) | 0 | 0 | 0 |
| T2 | 91 kg ha−1 (TSP) | 18 | 0.0007(TSP) |
| T3 | 1 | 12.87 | 0.0065 |
| T4 | 2 | 25.74 | 0.013 |
| T5 | 3 | 38.61 | 0.020 |
| T6 | 5 | 64.35 | 0.033 |
| Soil Properties | Solid Chicken Manure | Chicken Manure Extract |
|---|---|---|
| pH | 8.20 | 6.25 |
| EC (dS m−1) | 14.7 | 0.0005 |
| C | 21.7 | 3.86 |
| Total N (%) | 2.8 | 0.06 |
| Total P (%) | 2.95 | 1.29 |
| Dissolved P (%) | 2 | |
| K (%) | 3.3 | 0.09 |
| Ca (%) | 14.5 | 1.07 |
| Mg (%) | 3.7 | 0.66 |
| Cu (mg g−1) | 1.06 | 0.18 |
| Zn (mg g−1) | 4.8 | 0.09 |
| Dry matter | 87.2 | 0.69 |
| Moisture content | 12.8 | 7.42 |
| Properties | Before | After |
|---|---|---|
| Ph (1:5) | 8.85 | 8.50 |
| EC (1:5) (dS m−1) | 2.98 | 0.49 |
| Total C (%) | 48.7 | 60.5 |
| Total N (%) | 1.19 | 1.23 |
| Total P (%) | 0.03 | 1.24 |
| K (%) | 1.8 | 0.7 |
| Ca (%) | 0.4 | 1.2 |
| Mg (%) | 0.1 | 0.3 |
| CEC (cmol (+) kg−1) | 73.7 | - |
| AEC (cmol (−) kg−1) | 40.24 | - |
| S (%) | 0.07 | 0.14 |
| Cu (ppm) | 0.1 | 0.2 |
| Zn (ppm) | 0.2 | 0.3 |
| Soil Properties | Values |
|---|---|
| Sand (%) | 88.36 |
| Silt (%) | 7.25 |
| Clay (%) | 4.37 |
| Textural class | Sandy |
| Bulk density (g cm−3) | 1.6 |
| pH (water) | 4.31 |
| EC (dS m−1) | 0.09 |
| Available P (mg kg−1) | 56 |
| Total P (%) | 0.50 |
| Total C (%) | 0.50 |
| Total N (%) | 0.07 |
| C:N | 7:1 |
| K (cmol (+) kg−1) | 0.10 |
| Ca (cmol (+) kg−1) | 0.01 |
| Mg (cmol (+) kg−1) | 0.01 |
| CEC | 0.66 |
| Cu (ppm) | 19.4 |
| Zn (ppm) | 17.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dady, Y.; Ismail, R.; Jol, H.; Arolu, F. Impact of Oil Palm Empty Fruit Bunch Biochar Enriched with Chicken Manure Extract on Phosphorus Retention in Sandy Soil. Sustainability 2021, 13, 10851. https://doi.org/10.3390/su131910851
Dady Y, Ismail R, Jol H, Arolu F. Impact of Oil Palm Empty Fruit Bunch Biochar Enriched with Chicken Manure Extract on Phosphorus Retention in Sandy Soil. Sustainability. 2021; 13(19):10851. https://doi.org/10.3390/su131910851
Chicago/Turabian StyleDady, Yossif, Roslan Ismail, Hamdan Jol, and Fatai Arolu. 2021. "Impact of Oil Palm Empty Fruit Bunch Biochar Enriched with Chicken Manure Extract on Phosphorus Retention in Sandy Soil" Sustainability 13, no. 19: 10851. https://doi.org/10.3390/su131910851





