Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture
Abstract
:Highlights
- PGPR offer an eco-friendly and green alternative to synthetic agrochemicals and conventional agricultural practices.
- PGPR accomplish sustainable agriculture by boosting growth and stress tolerance in plants.
- PGPR inhabit in the rhizosphere of soil and exhibit positive interaction with plant roots.
- PGPR have the potential to curb the adverse effects of various stresses.
1. Introduction
2. Mechanism of Action
2.1. Direct Plant Growth Promotion
2.1.1. Biological Nitrogen Fixation
2.1.2. Phosphate Solubilization
2.1.3. Siderophore Production
2.1.4. Phytohormone Production
2.2. Indirect Mechanisms
2.2.1. Non-Volatile Biocidals (Antibiotics and Fungicidals)
2.2.2. Volatiles Biocidal
2.2.3. Hydrolytic Enzymes
2.2.4. Induced Systemic Resistance
2.2.5. Stress Tolerance
2.2.6. Osmoprotectants
2.2.7. Ion Homeostasis
2.2.8. Antioxidant Enzymes
3. PGPR as a Sink for ACC Deaminase Enzyme
4. PGPR and Disease Suppression
5. PGPR and Quorum Quenching System
6. PGPR Mitigating Stress in Plants
7. PGPR Impact on Plant Gene Expression
8. Triggers for PGPR Colonization
9. Molecular Mechanisms of PGPR
10. Genetically Engineered PGPR Strains
11. Impact of Environmental Changes on Growth and Development of Microorganism
12. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Carvalho, F.P. Pesticides, environment and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Chávez-Dulanto, P.C.; Thiry, A.A.; Glorio-Paulet, P.; Vögler, O.; Carvalho, F.P. Increasing the impact of science and technology to provide more people with healthier and safer food. Food Energy Secur. 2021, 10, e259. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Meena, M.; Dubey, M.K.; Swapnil, P.; Zehra, A.; Singh, S.; Kumari, P.; Upadhyay, R.S. The rhizosphere microbial community and methods of its analysis. In Advances in PGPR Research; Singh, H.B., Sarma, B.K., Keswani, C., Eds.; CAB International: Wallingford, UK, 2017; pp. 275–295. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Tariq, H.; Latif, S.; Yasmin, H.; Mehmood, A.; Shahid, M.A. Water conservation and plant survival strategies of rhizobacteria under drought stress. Agronomy 2020, 10, 1683. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant growth-promoting rhizobacteria—Alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic insights of the interaction of plant growth-promoting rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Bouremani, N.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Tailoring next generation plant growth promoting microorganisms as versatile tools beyond soil desalinization: A road map towards field application. Sustainability 2021, 13, 4422. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.P.; Sharma, S.; Singh, N.K.; Singh, V.; Tiwari, K.; Singh, S. Nature and role of root exudates: Efficacy in bioremediation. Afr. J. Biotechnol. 2011, 10, 9717–9724. [Google Scholar] [CrossRef] [Green Version]
- Drogue, B.; Combes-Meynet, E.; Moënne-Loccoz, Y.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Control of the cooperation between plant growth-promoting rhizobacteria and crops by rhizosphere signals. In Molecular Microbial Ecology of the Rhizosphere; de Bruijn, F.J., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; Volume 1. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Kumar, A.; Dubey, A. Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J. Adv. Res. 2020, 24, 337–352. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, R.M.; Diaz, P.A.E.; Lobo, L.L.B.; Rigobelo, E.C. Use of plant growth-promoting rhizobacteria in maize and sugarcane: Characteristics and applications. Front. Sustain. Food Syst. 2020, 4, 136. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Meena, M.; Gupta, P.; Dubey, M.K.; Nath, G.; Upadhyay, R.S. Plant growth promoting rhizobacteria and their biopriming for growth promotion in mung bean (Vigna radiata (L.) R. Wilczek). Biocatal. Agric. Biotechnol. 2018, 16, 163–171. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, M.; Upadhyay, R.S. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean). Biocatal. Agric. Biotechnol. 2018, 16, 155–162. [Google Scholar] [CrossRef]
- Martínez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.M.L.M.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, M.V.B.; Seldin, L.; de Araujo, F.F.; Mariano, R.L.R. Plant growth promoting rhizobacteria: Fundamentals and applications. In Plant Growth and Health Promoting Bacteria. Microbiology Monographs; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 18, pp. 21–43. [Google Scholar] [CrossRef]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Lavakush, S.V. Impact of plant growth promoting rhizobacteria on crop production. Int. J. Agric. Res. 2010, 5, 954–983. [Google Scholar] [CrossRef] [Green Version]
- Merzaeva, O.V.; Shirokikh, I.G. Colonization of plant rhizosphere by actinomycetes of different genera. Microbiology 2006, 75, 226–230. [Google Scholar] [CrossRef]
- García-Fraile, P.; Menéndez, E.; Rivas, R. Role of bacterial biofertilizers in agriculture and forestry. AIMS Bioeng. 2015, 2, 183–205. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of tenuazonic acid, alternariol and alternariol monomethyl ether produced by Alternaria species phytopathogenic on plants. Sci. Rep. 2017, 7, 8777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meena, M.; Swapnil, P.; Zehra, A.; Aamir, M.; Dubey, M.K.; Upadhyay, R.S. Beneficial microbes for disease suppression and plant growth promotion. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 395–432. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Sharma, K. Microbial biodiversity and bioremediation assessment through omics approaches. Front. Environ. Chem. 2020, 1, 570326. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, V.K.; Tripathi, V.; Singh, P.P.; Singh, A.K. Plant growth-promoting rhizobacteria (PGPR): Perspective in agriculture under biotic and abiotic Stress. In New and Future Developments in Microbial Biotechnology and Bioengineering: Crop Improvement through Microbial Biotechnology, 1st ed.; Prasad, R., Gill, S.S., Tuteja, N., Eds.; Elsevier: Hoboken, NJ, USA, 2018; pp. 333–342. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, J.S.; Meena, V.S.; Ramteke, P.W. Plant growth-promoting rhizobacteria: Strategies to improve abiotic stresses under sustainable agriculture. J. Plant Nutr. 2019, 42, 1402–1415. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.H.; Huang, E.; Huang, C.C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A Review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [Green Version]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P.; et al. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Saharan, B.S.; Nehra, V. Plant growth promoting rhizobacteria: A critical review. Life Sci. Med. Res. 2011, 21, 1–30. [Google Scholar]
- Tak, H.I.; Ahmad, F.; Babalola, O.O. Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer Science Business Media: NewYork, NY, USA, 2013; pp. 33–52. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microbiol. Biochem. Technol. 2015, 7, 96–102. [Google Scholar]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Zakry, F.A.A.; Shamsuddin, Z.H.; Rahim, K.A.; Zakaria, Z.Z.; Rahim, A.A. Inoculation of Bacillus sphaericus UPMB-10 to young oil palm and measurement of its uptake of fixed nitrogen using the 15N isotope dilution technique. Microb. Environ. 2012, 27, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Zahran, H.H. Rhizobia from wild legumes: Diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J. Biotechnol. 2001, 91, 143–153. [Google Scholar] [CrossRef]
- Govindasamy, V.; Senthilkumar, M.; Magheshwaran, V.; Kumar, U.; Bose, P.; Sharma, V.; Annapurna, K. Bacillus and Paenibacillus spp.: Potential PGPR for sustainable agriculture. In Plant Growth and Health Promoting Bacteria. Microbiology Monographs; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 18, pp. 333–364. [Google Scholar] [CrossRef]
- Podile, A.R.; Kishore, G.K. Plant growth-promoting rhizobacteria. In Plant-Associated Bacteria: Rhizosphere Bacteria; Gnanamanickam, S.S., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 195–230. [Google Scholar] [CrossRef]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, R.A.; Mahmood, I.; Rizvi, R.; Sumbul, A.; Safiuddin. Siderophores: Augmentation of soil health and crop productivity. In Probiotics in Agroecosystem, 1st ed.; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer Nature: Singapore, 2017; pp. 291–312. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Chebotar, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Jayaprakashvel, M.; Mathivanan, N. Management of plant diseases by microbial metabolites. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2011; pp. 237–265. [Google Scholar]
- Zhou, D.; Feng, H.; Schuelke, T.; De Santiago, A.; Zhang, Q.; Zhang, J.; Luo, C.; Wei, L. Rhizosphere microbiomes from root-knot nematode non-infested plants suppress nematode infection. Microb. Ecol. 2019, 78, 470–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, R.; Lee, S.M.; Ryu, C.M. Microbe-induced plant volatiles. New Phytol. 2018, 220, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabood, F.; Zhou, X.; Smith, D.L. Microbial signaling and plant growth promotion. Can. J. Plant Sci. 2014, 94, 1051–1063. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, Y.; Subramanian, R.B. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol. Mol. Biol. Plants 2014, 20, 201–207. [Google Scholar] [CrossRef]
- Saraf, M.; Pandya, U.; Thakkar, A. Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol. Res. 2014, 169, 18–29. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P. Regulation of WRKY genes in plant defense with beneficial fungus Trichoderma: Current perspectives and future prospects. Arch. Phytopathol. Plant Protect. 2019, 52, 1–17. [Google Scholar] [CrossRef]
- Kumar, A. Phosphate solubilizing bacteria in agriculture biotechnology: Diversity, mechanism and their role in plant growth and crop yield. Int. J. Adv. Res. 2016, 4, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Tairo, E.V.; Ndakidemi, P.A. Possible benefits of rhizobial inoculation and phosphorus supplementation on nutrition, growth and economic sustainability in grain legumes. Am. J. Res. Commun. 2013, 1, 532–556. [Google Scholar]
- Smith, B.E.; Richards, R.L.; Newton, W.E. (Eds.) Catalysts for Nitrogen Fixation: Nitrogenases, Relevant Chemical Models and Commercial Processes; Springer Science & Business Media: Berlin, Germany, 2013; Volume 1, p. 340. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Evaluation of plant growth promoting activities of rhizobacterium Pseudomonas putida under herbicidestress. Ann. Microbiol. 2012, 62, 1531–1540. [Google Scholar] [CrossRef]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Swapnil, P.; Harish; Marwal, A.; Yadav, G.; Sonigra, P. Endophytic nanotechnology: An approach to study scope and potential applications. Front. Chem.-Nanosci. 2021, 9, 613343. [Google Scholar] [CrossRef]
- Damam, M.; Kaloori, K.; Gaddam, B.; Kausar, R. Plant growth promoting substances (phytohormones) produced by rhizobacterial strains isolated from the rhizosphere of medicinal plants. Int. J. Pharm. Sci. Rev. 2016, 37, 130–136. [Google Scholar]
- Anand, K.; Kumari, B.; Mallick, M.A. Phosphate solubilizing microbes: An effective and alternative approach as biofertilizers. Int. J. Pharm. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Youssef, M.M.; Eissa, M.F. Biofertilizers and their role in management of plant parasitic nematodes. A review. E3 J. Biotechnol. Pharm. Res. 2014, 5, 1–6. [Google Scholar]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Navathe, S.; Chand, R.; Pandey, S.P. A halotolerant growth promoting rhizobacteria triggers induced systemic resistance in plants and defends against fungal infection. Sci. Rep. 2019, 9, 4054. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sohrabi, Y. Bacterial biofertilizers for sustainable crop production: A review. ARPN J. Agric. Biol. Sci. 2012, 7, 307–316. [Google Scholar]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential ofsiderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Assessment of plant growth promoting activities of rhizobacterium Pseudomonas putida under insecticide stress. J. Microbiol. 2011, 1, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef]
- Thomine, S.; Lanquar, V. Iron transport and signaling in plants. In Transporters and Pumps in Plant Signaling: Signaling and Communication in Plants; Geisler, M., Venema, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 7, pp. 99–131. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Merten, D.; Svatos, A.; Büchel, G.; Kothe, E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J. Appl. Microbiol. 2009, 107, 1687–1696. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Pseudomonas siderophores: A mechanism explaining disease-suppressive soils. Curr. Microbiol. 1980, 4, 317–320. [Google Scholar] [CrossRef]
- Trapet, P.; Avoscan, L.; Klinguer, A.; Pateyron, S.; Citerne, S.; Chervin, C.; Mazurier, S.; Lemanceau, P.; Wendehenne, D.; Besson-Bard, A. The Pseudomonas fluorescens siderophore pyoverdine weakens Arabidopsis thaliana defense in favor of growth in iron-deficient conditions. Plant Physiol. 2016, 171, 675–693. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Ren, H.; Ahmed, T.; Luo, J.; An, Q.; Qi, X.; Li, B. Antifungal effects of rhizospheric Bacillus species against bayberry twig blight pathogen Pestalotiopsis versicolor. Agronomy 2020, 10, 1811. [Google Scholar] [CrossRef]
- Schippers, B.; Bakker, A.W.; Bakker, P.A.H. Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Ann. Rev. Phytopathol. 1987, 25, 339–358. [Google Scholar] [CrossRef]
- Pal, K.K.; Tilak, K.V.; Saxena, A.K.; Dey, R.; Singh, C.S. Suppression of maize root diseases caused by Macrophomina phaseolina, Fusarium moniliforme and Fusarium graminearum by plant growth promoting rhizobacteria. Microbiol. Res. 2001, 156, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud. Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Bano, A.; Ali, S.; Md Babar, A. Cross-talk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Etesami, H.A.; Alikhani, H.A.; Akbari, A.A. Evaluation of plant growth hormones production (IAA) ability by Iranian soils rhizobial strains and effects of superior strains application on wheat growth indexes. World Appl. Sci. J. 2009, 6, 1576–1584. [Google Scholar]
- Kiyohara, S.; Honda, H.; Shimizu, N.; Ejima, C.; Hamasaki, R.; Sawa, S. Tryptophan auxotroph mutants suppress the super-root 2 phenotypes, modulating IAA biosynthesis in Arabidopsis. Plant Signal. Behav. 2011, 6, 1351–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patten, C.L.; Glick, B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Ouzari, H.; Khsairi, A.; Raddadi, N.; Jaoua, L.; Hassen, A.; Zarrouk, M.; Daffonchio, D.; Boudabous, A. Diversity of auxin-producing bacteria associated to Pseudomonas savastanoi- induced olive knots. J. Basic Microbiol. 2008, 48, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plackett, A.R.; Wilson, Z.A. Gibberellins and plant reproduction. In Annual Plant Reviews, Gibberellins; Hedden, P., Thomas, S.G., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; Volume 49, pp. 323–358. [Google Scholar] [CrossRef]
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and seed germination. In Annual Plant Reviews, Gibberellins; Hedden, P., Thomas, S.G., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; Volume 49, pp. 253–284. [Google Scholar] [CrossRef]
- Reid, M.S. The role of ethylene in flower senescene. Acta Hortic. 1981, 261, 157–169. [Google Scholar]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Shelke, G.M.; Kumar, A.; Jha, P.N. Biochemistry and genetics of ACC deaminase: A weapon to “stress ethylene” produced in plants. Front. Microbiol. 2015, 6, 937. [Google Scholar] [CrossRef]
- Galland, M.; Gamet, L.; Varoquaux, F.; Touraine, B.; Desbrosses, G. The ethylene pathway contributes to root hair elongation induced by the beneficial bacteria Phyllobacterium brassicacearum STM196. Plant Sci. 2012, 190, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Poupin, M.J.; Greve, M.; Carmona, V.; Pinedo, I. A complex molecular interplay of auxin and ethylene signaling pathways is involved in Arabidopsis growth promotion by Burkholderia phytofirmans PsJN. Front. Plant Sci. 2016, 7, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fravel, D. Role of antibiosis in the biocontrol of plant diseases. Annu. Rev. Phytopathol. 1988, 26, 75–91. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Aamir, M.; Patel, C.B.; Upadhyay, R.S. Virulence factors and their associated genes in microbes. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Genes Biochemistry and Applications, 1st ed.; Singh, H.B., Gupta, V.K., Jogaiah, S., Eds.; Elsevier: Hoboken, NJ, USA, 2019; pp. 181–208. [Google Scholar] [CrossRef]
- Almoneafy, A.A.; Moustafa-Farag, M.; Mohamed, H.I. The auspicious role of plant growth-promoting rhizobacteria in the sustainable management of plant diseases. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management, 1st ed.; Mohamed, H.I., El-Beltagi, H.E.D.S., Abd-Elsalam, K.A., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 251–283. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Mohd Hata, E.; Zulperi, D.; Ismail, S.I.; Ismail, M.R.; Mohd Zainudin, N.A.I.; Saidi, N.B.; Yusof, M.T. Plant growth-promoting bacteria as an emerging tool to manage bacterial rice pathogens. Microorganisms 2021, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.; Johri, B.N. Antifungals from fluorescent pseudomonads: Biosynthesis and regulation. Curr. Sci. 2003, 85, 1693–1703. [Google Scholar]
- Weller, D.M.; Landa, B.B.; Mavrodi, O.V.; Schroeder, K.L.; De La Fuente, L.; Blouin Bankhead, S.; Allende Molar, R.; Bonsall, R.F.; Mavrodi, D.V.; Thomashow, L.S. Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Boil. 2007, 9, 4–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, J.T.; Arnould, C.; Deulvot, C.; Lemanceau, P.; Gianinazzi-Pearson, V.; Raaijmakers, J.M. Effect of 2,4-diacetylphloroglucinol on Pythium: Cellular responses and variation in sensitivity among propagules and species. Phytopathology 2003, 93, 966–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McSpadden Gardener, B.B. Diversity and ecology of biocontrol Pseudomonas in agricultural systems. Phytopathology 2007, 97, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.; Ruppel, S.; Behrendt, U.; Lentzsch, P.; Müller, M.E.H. Antagonistic potential of fluorescent pseudomonads colonizing wheat heads against mycotoxin producing Alternaria and Fusaria. Front. Microbiol. 2018, 9, 2124. [Google Scholar] [CrossRef]
- Chen, S.; Zou, J.; Hu, Z.; Chen, H.; Lu, Y. Global annual soil respiration in relation to climate, soil properties and vegetation characteristics: Summary of available data. Agric. For. Meteorol. 2014, 198, 335–346. [Google Scholar] [CrossRef]
- Dasgupta, D.; Kumar, A.; Mukhopadhyay, B.; Sengupta, T.K. Isolation of phenazine 1,6-di-carboxylic acid from Pseudomonas aeruginosa strain HRW.1-S3 and its role in biofilm-mediated crude oil degradation and cytotoxicity against bacterial and cancer cells. Appl. Microbiol. Biotechnol. 2015, 99, 8653–8665. [Google Scholar] [CrossRef]
- Jung, B.K.; Hong, S.J.; Park, G.S.; Kim, M.C.; Shin, J.H. Isolation of Burkholderia cepacia JBK9 with plant growth-promoting activity while producing pyrrolnitrin antagonistic to plant fungal diseases. Appl. Biol. Chem. 2018, 61, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Pawar, S.; Chaudhari, A.; Prabha, R.; Shukla, R.; Singh, D.P. Microbial pyrrolnitrin: Natural metabolite with immense practical utility. Biomolecules 2019, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [Green Version]
- Malviya, D.; Sahu, P.K.; Singh, U.B.; Paul, S.; Gupta, A.; Gupta, A.R.; Singh, S.; Kumar, M.; Paul, D.; Rai, J.P.; et al. Lesson from ecotoxicity: Revisiting the microbial lipopeptides for the management of emerging diseases for crop protection. Int. J. Environ. Res. Public Health 2020, 17, 1434. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, T.H.; Sørensen, D.; Tobiasen, C.; Andersen, J.B.; Christophersen, C.; Givskov, M.; Sørensen, J. Antibiotic and biosurfactant properties of cyclic lipopeptides producedby fluorescent Pseudomonas spp. from the sugar beet rhizosphere. Appl. Environ. Microbiol. 2002, 68, 3416–3423. [Google Scholar] [CrossRef] [Green Version]
- Farace, G.; Fernandez, O.; Jacquens, L.; Coutte, F.; Krier, F.; Jacques, P.; Clément, C.; Barka, E.A.; Jacquard, C.; Dorey, S. Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defence responses in grapevine. Mol. Plant Pathol. 2015, 16, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Bakker, P.A.H.M., Raaijmakers, J.M., Bloemberg, G., Höfte, M., Lemanceau, P., Cooke, B.M., Eds.; Springer: Dordrecht, The Newtherland, 2007; Volume 119, pp. 329–339. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Piechulla, B. Plant growth promotion due to rhizobacterial volatiles—An effect of CO2. FEBS Lett. 2009, 583, 3473–3477. [Google Scholar] [CrossRef] [Green Version]
- Piechulla, B.; Lemfack, M.C.; Kai, M. Effects of discrete bioactive microbial volatiles on plants and fungi. Plant Cell Environ. 2017, 40, 2042–2067. [Google Scholar] [CrossRef]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic volatile emissions from the soil. Plant Cell Environ. 2014, 37, 1866–1891. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.M. Revisiting bacterial volatile-mediated plant growth promotion: Lessons from the past and objectives for the future. Ann. Bot. 2018, 122, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Kim, M.S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch. Phytopathol. Plant Protect. 2017, 50, 629–648. [Google Scholar] [CrossRef]
- Ossowicki, A.; Jafra, S.; Garbeva, P. The antimicrobial volatile power of the rhizospheric isolate Pseudomonas donghuensis P482. PLoS ONE 2017, 12, e0174362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castulo-Rubio, D.Y.; Alejandre-Ramírez, N.A.; del Carmen Orozco-Mosqueda, M.; Santoyo, G.; Macías-Rodríguez, L.I.; Valencia-Cantero, E. Volatile organic compounds produced by the rhizobacterium Arthrobacter agilis UMCV2 modulate Sorghum bicolor (strategy II plant) morphogenesis and SbFRO1 transcription in vitro. J. Plant Growth Regul. 2015, 34, 611–623. [Google Scholar] [CrossRef]
- Farag, M.A.; Ryu, C.M.; Sumner, L.W.; Paré, P.W. GC–MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 2006, 67, 2262–2268. [Google Scholar] [CrossRef]
- Tyc, O.; de Jager, V.C.L.; van den Berg, M.; Gerards, S.; Janssens, T.K.S.; Zaagman, N.; Kai, M.; Svatos, A.; Zweers, H.; Hordijk, C.; et al. Exploring bacterial interspecific interactions for discovery of novel antimicrobial compounds. Microb. Biotechnol. 2017, 10, 910–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, C.; Li, Z.; Yu, D. Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran. J. Microbiol. 2010, 48, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Dutta, S.; Ann, M.; Raaijmakers, J.M.; Park, K. Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem. Biophys. Res. Commun. 2015, 461, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.; Holsters, M.; Vereecke, D. The gram-positive side of plant-microbe interactions. Environ. Microbiol. 2010, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Budi, S.W.; van Tuinen, D.; Arnould, C.; Dumas-Gaudot, E.; Gianinazzi-Pearson, V.; Gianinazzi, S. Hydrolytic enzyme activity of Paenibacillus sp. strain B2 and effects of the antagonistic bacterium on cell integrity of two soil borne pathogenic bacteria. Appl. Soil Ecol. 2000, 15, 191–199. [Google Scholar] [CrossRef]
- Chet, I.; Inbar, J. Biological control of fungal pathogens. Appl. Biochem. Biotechnol. 1994, 48, 37–43. [Google Scholar] [CrossRef]
- Frankowski, J.; Lorito, M.; Scala, F.; Schmid, R.; Berg, G.; Bahl, H. Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch. Microbiol. 2001, 176, 421–426. [Google Scholar] [CrossRef]
- Someya, N.; Tsuchiya, K.; Yoshida, T.; Noguchi, M.T.; Akutsu, K.; Sawada, H. Co-inoculation of an antibiotic-producing bacterium and a lytic enzyme-producing bacterium for the biocontrol of tomato wilt caused by Fusarium oxysporum f. sp. lycopersici. Biocontrol. Sci. 2007, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Hameed, S.; Shahid, M.; Iqbal, M.; Lazarovits, G.; Imran, A. Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol. Res. 2020, 232, 126389. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U.; Mitra, A.; Mallick, I.; Haldar, A.; Haldar, S.; Ghosh, A.; Ghosh, A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.V.N.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B.; et al. The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 2021, 10, 101. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Gen. Mol. Biol. 2012, 35 (Suppl. 1), 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- Yadav, G.; Meena, M. Bioprospecting of endophytes in medicinal plants of Thar Desert: An attractive resource for biopharmaceuticals. Biotechnol. Rep. 2021, 30, e00629. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Noorieh, B.; Arzanesh, M.H.; Mahlegha, G.; Maryam, S. The effect of plant growth promoting rhizobacteria on growth parameters, antioxidant enzymes and microelements of canola under salt stress. J. Appl. Environ. Biol. Sci. 2013, 3, 17–27. [Google Scholar]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M. Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom. Biotech. Lett. 2013, 18, 8132–8143. [Google Scholar]
- Azarmi, F.; Mozafari, V.; Dahaji, P.A.; Hamidpour, M. Biochemical, physiological and antioxidant enzymatic activity responses of pistachio seedlings treated with plant growth promoting rhizobacteria and Zn to salinity stress. Acta Physiol. Plant 2016, 38, 21. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Paul, D.; Nair, S. Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J. Basic Microbiol. 2008, 48, 378–384. [Google Scholar] [CrossRef]
- Street, T.O.; Bolen, D.W.; Rose, G.D. A molecular mechanism for osmolyte-induced protein stability. Proc. Natl. Acad. Sci. USA 2006, 103, 13997–14002. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef] [Green Version]
- Czarnes, S.; Hallett, P.D.; Bengough, A.G.; Young, I.M. Root- and microbial-derived mucilages affect soil structure and water transport. Eur. J. Soil Sci. 2000, 51, 435–443. [Google Scholar] [CrossRef]
- Khan, F.; Ahmed, K.B.M.; Shariq, M.; Siddiqui, M.A. Potentiality of plant growth-promoting rhizobacteria in easing of soil salinity and environmental sustainability. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Akhtar, M., Ed.; Springer: Singapore, 2019; pp. 21–58. [Google Scholar] [CrossRef]
- Schmidt, R.; Köberl, M.; Mostafa, A.; Ramadan, E.M.; Monschein, M.; Jensen, K.B.; Bauer, R.; Berg, G. Effects of bacterial inoculants on the indigenous microbiome and secondary metabolites of chamomile plants. Front. Microbiol. 2014, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Atouei, M.T.; Pourbabaee, A.A.; Shorafa, M. Alleviation of salinity stress on some growth parameters of wheat by exopolysaccharide-producing bacteria. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 2725–2733. [Google Scholar] [CrossRef]
- Jouve, L.; Hoffmann, L.; Hausman, J.F. Polyamine, carbohydrate, and proline content changes during salt stress exposure of aspen (Populus tremula L.): Involvement of oxidation and osmoregulation metabolism. Plant Biol. 2004, 6, 74–80. [Google Scholar] [CrossRef]
- Sandhya, V.S.K.Z.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Redillas, M.C.; Park, S.H.; Lee, J.W.; Kim, Y.S.; Jeong, J.S.; Jung, H.; Bang, S.W.; Hahn, T.R.; Kim, J.K. Accumulation of trehalose increases soluble sugar contents in rice plants conferring tolerance to drought and salt stress. Plant Biotechnol. Rep. 2012, 6, 89–96. [Google Scholar] [CrossRef]
- López, M.; Tejera, N.A.; Iribarne, C.; Lluch, C.; Herrera-Cervera, J.A. Trehalose and trehalase in root nodules of Medicago truncatula and Phaseolus vulgaris in response to salt stress. Physiol. Plant 2008, 134, 575–582. [Google Scholar] [CrossRef]
- Rodriguez, S.J.; Suarez, R.; Caballero, M.J.; Itturiaga, G. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. FEMS Microbiol. Lett. 2009, 296, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio, A.; Miyazaki, S.; Veronese, P.; Fujita, T.; Ibeas, J.I.; Damsz, B.; Narasimhan, M.L.; Hasegawa, P.M.; Joly, R.J.; Bressan, R.A. Does proline accumulation play an active role in stress-induced growth reduction? Plant J. 2002, 31, 699–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Khan, A.; Zhao, X.Q.; Javed, M.T.; Khan, K.S.; Bano, A.; Shen, R.F.; Masood, S. Bacillus pumilus enhances tolerance in rice (Oryza sativa L.) to combined stresses of NaCl and high boron due to limited uptake of Na+. Environ. Exp. Bot. 2016, 124, 120–129. [Google Scholar] [CrossRef]
- Shintu, P.V.; Jayaram, K.M. Phosphate solubilising bacteria (Bacillus polymyxa)—An effective approach to mitigate drought in tomato (Lycopersicon esculentum Mill). Trop. Plant Res. 2015, 2, 17–22. [Google Scholar]
- Munns, R.; and Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; James, R.A.; Gilliham, M.; Flowers, T.J.; Colmer, T.D. Tissue tolerance: An essential but elusive trait for salt-tolerant crops. Funct. Plant Biol. 2016, 43, 1103–1113. [Google Scholar] [CrossRef] [Green Version]
- Serrano, R.; Mulet, J.M.; Rios, G.; Marquez, J.A.; de Larrinoa, I.F.; Leube, M.P.; Mendizabal, I.; Pascual-Ahuir, A.; Proft, M.; Ros, R.; et al. A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot. 1999, 50, 1023–1036. [Google Scholar] [CrossRef]
- Neel, J.P.S.; Alloush, G.A.; Belesky, D.P.; Clapham, W.M. Influence of rhizosphere ionic strength on mineral composition, dry matter yield and nutritive value of forage chicory. J. Agron. Crop. Sci. 2002, 188, 398–407. [Google Scholar] [CrossRef]
- Hameed, M.; Ashraf, M.; Ahmad, M.S.A.; Naz, N. Structural and functional adaptations in plants for salinity tolerance. In Plant Adaptation and Phytoremediation; Ashraf, M., Ozturk, M., Ahmad, M.S.A., Eds.; Springer Science Business Media: New York, NY, USA, 2010; pp. 151–170. [Google Scholar] [CrossRef]
- Sivritepe, N.; Sivritepe, H.O.; Eris, A. The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci. Hortic. 2003, 97, 229–237. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plantarum 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, A.; Munns, R.; Poustini, K.; Watt, M. A screening method to identify genetic variation in root growth response to a salinity gradient. J. Exp. Bot. 2011, 62, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, K.J.; Tavakoli, N.; Kluge, C.; Mimura, T.; Sharma, S.; Harris, G.; Chardonnens, A.; Golldack, D. Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level. J. Exp. Bot. 2001, 52, 1969–1980. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.H.; Lee, S.Y.; Bressan, R.A.; Yun, D.J.; Bohnert, H.J. Intracellular consequences of SOS1 deficiency during salt stress. J. Exp. Bot. 2010, 61, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Chen, J.G.; Gao, Y.Q.; Li, X.; Chao, Z.F.; Chen, Z.R.; Li, Q.Q.; Han, M.L.; Wang, Y.L.; Wang, Y.F.; et al. AtHKT1 drives adaptation of Arabidopsis thaliana to salinity by reducing floral sodium content. PLoS Genet. 2017, 13, e1007086. [Google Scholar] [CrossRef] [Green Version]
- Ali, Z.; Park, H.C.; Ali, A.; Oh, D.H.; Aman, R.; Kropornicka, A.; Hong, H.; Choi, W.; Chung, W.S.; Kim, W.Y.; et al. TsHKT1; 2, a HKT1 homolog from the extremophile Arabidopsis relative Thellungiella salsuginea, shows K+ specificity in the presence of NaCl. Plant Physiol. 2012, 158, 1463–1474. [Google Scholar] [CrossRef] [Green Version]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Ahmed, A.H. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174, 104023. [Google Scholar] [CrossRef]
- Hammer, E.C.; Nasr, H.; Pallon, J.; Olsson, P.A.; Wallander, H. Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 2011, 21, 117–129. [Google Scholar] [CrossRef]
- Niu, S.Q.; Li, H.R.; Paré, P.W.; Aziz, M.; Wang, S.M.; Shi, H.; Li, J.; Han, Q.Q.; Guo, S.Q.; Li, J.; et al. Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil 2016, 407, 217–230. [Google Scholar] [CrossRef]
- Rafiq, K.; Akram, M.S.; Shahid, M.; Qaisar, U.; Rashid, N. Enhancement of salt tolerance in maize (Zea mays L.) using locally isolated Bacillus sp. SR-2-1/1. Biologia 2020, 75, 1425–1436. [Google Scholar] [CrossRef]
- Vaishnav, A.; Singh, J.; Singh, P.; Rajput, R.S.; Singh, H.B.; Sarma, B.K. Sphingobacterium sp. BHU-AV3 induces salt tolerance in tomato by enhancing antioxidant activities and energy metabolism. Front. Microbiol. 2020, 11, 443. [Google Scholar] [CrossRef]
- Zushi, K.; Matsuzoe, N. Seasonal and cultivar differences in salt-induced changes in antioxidant system in tomato. Sci. Hortic. 2009, 120, 181–187. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Khan, M.R.; Siddiqui, Z.A. Potential of Pseudomonas putida, Bacillus subtilis, and their mixture on the management of Meloidogyne incognita, Pectobacterium betavasculorum, and Rhizoctonia solani disease complex of beetroot (Beta vulgaris L.). Egypt J. Biol. Pest Control 2019, 29, 73. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Susuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.A. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 2016, 124, 39–63. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, U.; Chakraborty, B.N.; Chakraborty, A.P.; Dey, P.L. Water stress amelioration and plant growth promotion in wheat plants by osmotic stress tolerant bacteria. World J. Microbiol. Biotechnol. 2013, 29, 789–803. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Balusamy, S.R.; Kim, Y.J.; Lee, C.H.; Kim, Y.J.; Koh, S.C.; Yang, D.C. A growth-promoting bacteria, Paenibacillus yonginensis DCY 84T enhanced salt stress tolerance by activating defense-related systems in Panax ginseng. Front. Plant Sci. 2018, 9, 813. [Google Scholar] [CrossRef] [Green Version]
- Zahir, Z.A.; Nadeem, S.M.; Khan, M.Y.; Binyamin, R.; Waqas, M.R. Role of halotolerant microbes in plant growth promotion under salt stress conditions. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Kumar, M., Ed.; Springer: Singapore, 2019; pp. 209–253. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.D.R.; Sosa Alderete, L.G.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Amby, D.B.; Hegelund, J.N.; Fimognari, L.; Großkinsky, D.K.; Westergaard, J.C.; Müller, R.; Moelbak, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Abiri, R.; Shaharuddin, N.A.; Maziah, M.; Yusof, Z.N.B.; Atabaki, N.; Sahebi, M.; Valdiani, A.; Kalhori, N.; Azizi, P.; Hanafi, M.M. Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environ. Exp. Bot. 2017, 134, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Pandey, S. ACC Deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Raghuwanshi, R.; Prasad, J.K. Perspectives of rhizobacteria with ACC deaminase activity in plant growth under abiotic stress. In Root Biology. Soil Biology; Giri, B., Prasad, R., Varma, A., Eds.; Springer Nature: Cham, Switzerland, 2018; Volume 52, pp. 303–321. [Google Scholar] [CrossRef]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Glick, B.R.; Pasternak, J.J. Molecular Biotechnology: Principles and Applications of Recombinant DNA, 3rd ed.; ASM Press: Washington, DC, USA, 2003; pp. 163–189. [Google Scholar]
- Yu, Y.B.; Adams, D.O.; Yang, S.F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch. Biochem. Biophys. 1979, 198, 280–286. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Gupta, V.V.S.R.; Franco, C.M.M.; Sharma, A.K. Evaluation of ACC-deaminase-producing rhizobacteria to alleviate water-stress impacts in wheat (Triticum aestivum L.) plants. Can. J. Microbiol. 2019, 65, 387–403. [Google Scholar] [CrossRef]
- Maxton, A.; Singh, P.; Masih, S.A. ACC-Deaminase-producing bacteria mediated drought and salt tolerance in Capsicum annuum. J. Plant Nutr. 2018, 41, 574–583. [Google Scholar] [CrossRef]
- Mhatre, P.H.; Karthik, C.; Kadirvelu, K.; Divya, K.L.; Venkatasalam, E.P.; Srinivasan, S.; Ramkumar, G.; Saranya, C.; Shanmuganathan, R. Plant growth promoting rhizobacteria (PGPR): A potential alternative tool for nematodes bio-control. Biocatal. Agric. Biotechnol. 2018, 17, 119–128. [Google Scholar] [CrossRef]
- Santoyo, G.; Orozco-Mosqueda, M.D.C.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Alizadeh, H.; Behboudi, K.; Ahmadzadeh, M.; Javan-Nikkhah, M.; Zamioudis, C.; Pieterse, C.M.; Bakker, P.A. Induced systemic resistance in cucumber and Arabidopsis thaliana by the combination of Trichoderma harzianum Tr6 and Pseudomonas sp. Ps14. Biol. Control 2013, 65, 14–23. [Google Scholar] [CrossRef]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-Dependent signaling pathway and activates PAMP-triggered immunity in Arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Patel, C.B.; Singh, V.K.; Singh, A.P.; Meena, M.; Upadhyay, R.S. Microbial genes involved in interaction with plants. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Genes Biochemistry and Applications; Singh, H.B., Gupta, V.K., Jogaiah, S., Eds.; Elsevier: Hoboken, NJ, USA, 2019; pp. 171–180. [Google Scholar] [CrossRef]
- Rizvi, A.; Zaidi, A.; Khan, M.S.; Saif, S.; Ahmed, B.; Shahid, M. Growth improvement and management of vegetable diseases by plant growth-promoting rhizobacteria. In Microbial Strategies for Vegetable Production; Springer Nature: Cham, Switzerland, 2017; pp. 99–123. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Crop protection against Botrytis cinerea by rhizhosphere biological control agent Bacillus velezensis XT1. Microorganisms 2020, 8, 992. [Google Scholar] [CrossRef] [PubMed]
- Redouan, Q.; Rachid, B.; Abderahim, A.; Hind, L.; Abdelhadi, A.; Naima, A.A.; Abdelghani, T.; El Hassan, M.; Bouchra, C. Effect of Pseudomonas as a preventive and curative control of tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae). J. Appl. Sci. 2019, 19, 473–479. [Google Scholar] [CrossRef]
- Kashyap, B.K.; Solanki, M.K.; Pandey, A.K.; Prabha, S.; Kumar, P.; Kumari, B. Bacillus as plant growth promoting rhizobacteria (PGPR): A promising green agriculture technology. In Plant Health under Biotic Stress, 1st ed.; Ansari, R., Mahmood, I., Eds.; Springer: Singapore, 2019; pp. 219–236. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, B.; Dulla, G.; Lindow, S.E. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol. Plant Microbe Interact. 2005, 18, 682–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Castro, R.; López-Bucio, J. Review: Phytostimulation and root architectural responses to quorum-sensing signals and related molecules from rhizobacteria. Plant Sci. 2019, 284, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Tannieres, M.; Morera, S.; Dessaux, Y.; Faure, D. Quorum quenching: Role in nature and applied developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef]
- Uroz, S.; Dessaux, Y.; Oger, P. Quorum sensing and quorum quenching: The yin and yang of bacterial communication. Chembiochem 2009, 10, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gao, Y.; Chen, X.; Yu, Z.; Li, X. Quorum quenching enzymes and their application in degrading signal molecules to block quorum sensing-dependent infection. Int. J. Mol. Sci. 2013, 14, 17477–17500. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, L. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 2005, 43, 101–109. [Google Scholar]
- Pan, J.; Huang, T.; Yao, F.; Huang, Z.; Powell, C.A.; Qiu, S.; Guan, X. Expression and characterization of aiiA gene from Bacillus subtilis BS-1. Microbiol. Res. 2008, 163, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; D’Angelo-Picard, C.; Carlier, A.; Elasri, M.; Sicot, C.; Petit, A.; Oger, P.; Faure, D.; Dessaux, Y. Novel bacteria degrading N-acylhomoserine lactones and their use as quenchers of quorum-sensing-regulated functions of plant-pathogenic bacteria. Microbiology 2003, 149, 1981–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Y.; Liu, X.; Ma, Y.; Chernin, L.; Berg, G.; Gao, K. Induction of systemic resistance, root colonization and control activities of the rhizospheric strain of Serratia plymuthica are dependent on N-acyl homoserine lactones. Eur. J. Plant Pathol. 2009, 124, 261–268. [Google Scholar] [CrossRef]
- GAP Report. Global Agricultural Productivity Report® (GAP Report®) Global Harvest Initiative, Washington. 2018. Available online: https://globalagriculturalproductivity.org/wp-content/uploads/2019/01/GHI_2018-GAP-Report_FINAL-10.03.pdf (accessed on 25 September 2021).
- Nemecek, T.; Gaillard, G. Challenges in assessing the environmental impacts of crop production and horticulture. In Environmental Assessment and Management in the Food Industry; Sonesson, U., Berlin, J., Ziegler, F., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 98–116. [Google Scholar] [CrossRef]
- FAO; ITPS. Status of the World’s Soil Resources (SWSR)–Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015; p. 650. [Google Scholar]
- Shilev, S. Plant-growth-promoting bacteria mitigating soil salinity stress in plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Rütting, T.; Aronsson, H.; Delin, S. Efficient use of nitrogen in agriculture. Nutr. Cycl. Agroecosyst. 2018, 110, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Schirawski, J.; Perlin, M.H. Plant microbe interaction 2017–The good, the bad and the diverse. Int. J. Mol. Sci. 2018, 19, 1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Herger, G.; Nielsen, R.; Margheim, J. Fertilizer History P3: In WWII Nitrogen Production Issues in Age of Modern Fertilizers. 2015. Available online: http://cropwatch.unl.edu/fertilizer-history-p3 (accessed on 25 September 2021).
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, M.; Mishra, J.; Mishra, V. Environmental sustainability: Challenges and viable solutions. Environ. Sustain. 2018, 1, 309–340. [Google Scholar] [CrossRef]
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef] [Green Version]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum. 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Kong, X.; Ge, R.; Liu, T.; Xu, S.; Hao, P.; Zhao, X.; Li, Z.; Lei, X.; Duan, H. Super-stable mineralization of cadmium by calcium-aluminum layered double hydroxide and its large-scale application in agriculture soil remediation. Chem. Eng. J. 2021, 407, 127178. [Google Scholar] [CrossRef]
- Ayyam, V.; Palanivel, S.; Chandrakasan, S. Approaches in land degradation management for productivity enhancement. In Coastal Ecosystems of the Tropics–Adaptive Management; Ayyam, V., Palanivel, S., Chandrakasan, S., Eds.; Springer Nature: Singapore, 2019; pp. 463–491. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth andantioxidants status of wheat plant under salinecondition. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L.) following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef] [PubMed]
- Rajput, L.U.B.N.A.; Imran, A.; Mubeen, F.; Hafeez, F.Y. Salt-tolerant PGPR strain Planococcus rifietoensis promotes the growth and yield of wheat (Triticum aestivum L.) cultivated in saline soil. Pak. J. Bot. 2013, 45, 1955–1962. [Google Scholar]
- Zhou, C.; Zhu, L.; Ma, Z.; Wang, J. Bacillus amyloliquefaciens SAY09 increases cadmium resistance in plants by activation of auxin-mediated signaling pathways. Genes 2017, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Kothari, V.V.; Kothari, R.K.; Kothari, C.R.; Bhatt, V.D.; Nathani, N.M.; Koringa, P.G.; Joshi, C.G.; Vyas, B.R. Genome sequence of salt-tolerant Bacillus safensis strain VK, isolated from Saline Desert Area of Gujarat, India. Genome Announc. 2013, 1, e00671-13. [Google Scholar] [CrossRef] [Green Version]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Hou, J.; Tu, C.; Luo, Y.; Christie, P. Whole genome analysis of halotolerant and alkalotolerant plant growth-promoting rhizobacterium Klebsiella sp. D5A. Sci. Rep. 2016, 6, 26710. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gomez-Cadenas, A.; Perez-Clemente, R.M. Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Habib, S.H.; Kausar, H.; Halimi, M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed. Res. Int. 2016, 2016, 6284547. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current progress in nitrogen fixing plants and microbiome research. Plants 2020, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, K.; Shinagawa, E.; Ameyama, M. D-Gluconate dehydrogenase from bacteria, 2-keto-d-gluconate-yielding, membrane-bound. Methods Enzymol. 1982, 89, 187–193. [Google Scholar] [CrossRef]
- Alaylar, B.; Egamberdieva, D.; Gulluce, M.; Karadayi, M.; Arora, N.K. Integration of molecular tools in microbial phosphate solubilization research in agriculture perspective. World J. Microbiol. Biotechnol. 2020, 36(7), 93. [Google Scholar] [CrossRef]
- Ovaa, W.; Bitter, W.; Weisbeek, P.; Koster, M. Multiple outer membrane receptors for uptake of ferric pseudobactins in Pseudomonas putida WCS358. Mol. Gen. Genet. 1995, 248, 735–743. [Google Scholar] [CrossRef]
- Calvo, P.; Zebelo, S.; McNear, D.; Kloepper, J.; Fadamiro, F. Plant growth-promoting rhizobacteria induce changes in Arabidopsis thaliana gene expression of nitrate and ammonium uptake genes. J. Plant Interact. 2019, 14, 224–231. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.S.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional alterations reveal Bacillus amyloliquefaciens-rice cooperation under salt stress. Sci. Rep. 2019, 9, 11912. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.; Seo, S.G.; Lee, C.; Woo, S.Y.; Kim, S.H. Gene expression profile affected by volatiles of new plant growth promoting rhizobacteria, Bacillus subtilis strain JS, in tobacco. Genes Genom. 2015, 37, 387–397. [Google Scholar] [CrossRef]
- Kerff, F.; Amoroso, A.; Herman, R.; Sauvage, E.; Petrella, S.; Filée, P.; Charlier, P.; Joris, B.; Tabuchi, A.; Nikolaidis, N.; et al. Crystal structure and activity of Bacillus subtilis YoaJ (EXLX1), a bacterial expansin that promotes root colonization. Proc. Natl. Acad. Sci. USA 2008, 105, 16876–16881. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanan, V.; Bais, H.P. Factors other than root secreted malic acid that contributes toward Bacillus subtilis FB17 colonization on Arabidopsis roots. Plant Signal. Behav. 2013, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Blake, C.; Christensen, M.N.; Kovács, A.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Rekha, K.; Kumar, R.M.; Ilango, K.; Rex, A.; Usha, B. Transcriptome profiling of rice roots in early response to Bacillus subtilis (RR4) colonization. Botany 2018, 96, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Wang, J.; Zhu, L.; Liao, D.; Gu, M.; Ren, L.; Kapulnik, Y.; Xu, G. An active factor from tomato root exudates plays an important role in efficient establishment of mycorrhizal symbiosis. PLoS ONE 2012, 7, e43385. [Google Scholar] [CrossRef] [Green Version]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, M.M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agriculture: A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.M.; Michalet, S.; Fossou, R.; Putnik-Delić, M.; Crèvecoeur, M.; Meyer, J.; de Malézieux, C.; Hopfgartner, G.; Maksimović, I.; Perret, X. Loss of NifQ leads to accumulation of porphyrins and altered metal-homeostasis in nitrogen-fixing symbioses. Mol. Plant Microbe Interact. 2018, 32, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, A.C.; Jiang, T.; Liu, Y.X.; Bai, Y.C.; Reed, J.; Qu, B.; Goossens, A.; Nützmann, H.W.; Bai, Y.; Osbourn, A. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 2019, 364, eaau6389. [Google Scholar] [CrossRef]
- Cotton, T.E.A.; Petriacq, P.; Cameron, D.D.; Meselmani, M.A.; Schwarzenbacher, R.; Rolfe, S.A.; Ton, J. Metabolic regulation of the maize rhizobiome by benzoxazinoids. ISME J. 2019, 13, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Voges, M.; Bai, Y.; Schulze-Lefert, P.; Sattely, E.S. Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 12558–12565. [Google Scholar] [CrossRef] [Green Version]
- Marschner, P.; Crowley, D.; Yang, C.H. Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 2004, 261, 199–208. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Vandana, U.K.; Singha, B.; Gulzar, A.B.M.; Mazumder, P.B. Molecular mechanisms in plant growth promoting bacteria (PGPR) to resist environmental stress in plants. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Sharma, V., Salwan, R., Tawfeeq, L., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Hoboken, NJ, USA, 2020; pp. 221–233. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent advances in pgpr and molecular mechanisms involved in drought stress tolerance. Preprints 2021, 2021, 2021050331. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, R.; Shimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, L.; Xie, Y.; Li, F.; Xiao, X.; Ma, Z.; Wang, J. Bacillus licheniformis SA03 confers increased saline-alkaline tolerance in Chrysanthemum plants by induction of abscisic acid accumulation. Front. Plant Sci. 2017, 8, 1143. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Luo, S.; Wu, Y.; Ye, J.; Wang, Q.; Xu, X.; Pan, F.; Khan, K.Y.; Feng, Y.; Yang, X. The effects of the endophytic bacterium Pseudomonas fluorescens sasm05 and IAA on the plant growth and cadmium uptake of Sedum alfredii Hance. Front. Microbiol. 2017, 8, 2538. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Luo, S.; Shen, J.; Wang, Q.; Ye, J.; Meng, Q.; Wu, Y.; Chen, B.; Cao, X.; Yang, X.; et al. The effects of endophytic bacterium SaMR12 on Sedum alfredii Hance metal ion uptake and the expression of three transporter family genes after cadmium exposure. Environ. Sci. Pollut. Res. 2017, 24, 9350–9360. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, H.R.; Mostajeran, A. TASOS1 and TATM20 genes expression and nutrient uptake in wheat seedlings may be altered via excess cadmium exposure and inoculation with Azospirillum brasilense sp7 under saline condition. Appl. Ecol. Environ. Res. 2018, 16, 1797–1817. [Google Scholar] [CrossRef]
- Jebara, S.H.; Chiboub, M.; Jebara, M. Antioxidant responses and gene level expressions of Sulla coronaria inoculated by heavy metals resistant plant growth promoting bacteria under cadmium stress. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions. EMCEI 2017. Advances in Science, Technology & Innovation (IEREK Interdisciplinary Series for Sustainable Development); Kallel, A., Ksibi, M., Ben Dhia, H., Khélifi, N., Eds.; Springer Nature: Chem, Switerland, 2018; pp. 335–337. [Google Scholar] [CrossRef]
- Gururani, M.A.; Upadhyaya, C.P.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 2012, 32, 245–258. [Google Scholar] [CrossRef]
- Ambreetha, S.; Chinnadurai, C.; Marimuthu, P.; Balachandar, D. Plant-associated Bacillus modulates the expression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere 2018, 5, 57–66. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Kohli, S.K.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Abd Allah, E.F.; Hashem, A.; Ahmad, P. Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 2019, 217, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Singh, J.S.; Abhilash, P.C.; Singh, H.B.; Singh, R.P.; Singh, D.P. Genetically engineered bacteria: An emerging tool for environmental remediation and future research perspectives. Gene 2011, 480, 1–9. [Google Scholar] [CrossRef]
- Joutey, N.T.; Bahafid, W.; Sayel, H.; El Ghachtouli, N. Biodegradation: Involved microorganisms and genetically engineered microorganisms. In Biodegradation-Life of Science; Intech Publishers: London, UK, 2013; Volume 14, pp. 289–320. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.C.; Cheung, K.C.; Luo, Y.M.; Wong, M.H. Effects of inoculation of plant growth-promoting rhizobacteria on metal uptake by Brassica juncea. Environ. Pollut. 2006, 140, 124–135. [Google Scholar] [CrossRef]
- Qiu, Z.; Tan, H.; Zhou, S.; Cao, L. Enhanced phytoremediation of toxic metals by inoculating endophytic Enterobacter sp. CBSB1 expressing bifunctional glutathione synthase. J. Hazard. Mater. 2014, 267, 17–20. [Google Scholar] [CrossRef]
- Xu, D.; Pei, J. Construction and characterization of a photosynthetic bacterium genetically engineered for Hg2+ uptake. Bioresour. Technol. 2011, 102, 3083–3088. [Google Scholar] [CrossRef]
- Yong, X.; Chen, Y.; Liu, W.; Xu, L.; Zhou, J.; Wang, S.; Chen, P.; Ouyang, P.; Zheng, T. Enhanced cadmium resistance and accumulation in Pseudomonas putida KT2440 expressing the phytochelatin synthase gene of Schizosaccharomyces pombe. Lett. Appl. Microbiol. 2014, 58, 255–261. [Google Scholar] [CrossRef]
- Whitaker, J.; Ostle, N.; Nottingham, A.T.; Ccahuana, A.; Salinas, N.; Bardgett, R.D.; Meir, P.; McNamara, N.P. Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes-to-Amazon elevation gradient. J. Ecol. 2014, 102, 1058–1071. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Cao, H.; Peng, H.; Hu, H.; Wang, W.; Zhang, X. Reaction kinetics for the biocatalytic conversion of phenazine-1-carboxylic acid to 2-hydroxyphenazine. PLoS ONE 2014, 9, e98537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeAngelis, K.M.; Pold, G.; Topçuoğlu, B.D.; van Diepen, L.T.; Varney, R.M.; Blanchard, J.L.; Melillo, J.; Frey, S.D. Long-term forest soil warming alters microbial communities in temperate forest soils. Front. Microbiol. 2015, 6, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcão Salles, J.; Le Roux, X.; Poly, F. Relating phylogenetic and functional diversity among denitrifiers and quantifying their capacity to predict community functioning. Front. Microbiol. 2012, 3, 209. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.U.; Chapin Iii, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Hagerty, S.B.; Van Groenigen, K.J.; Allison, S.D.; Hungate, B.A.; Schwartz, E.; Koch, G.W.; Kolka, R.K.; Dijkstra, P. Accelerated microbial turnover but constant growth efficiency with warming in soil. Nat. Clim. Chang. 2014, 4, 903–906. [Google Scholar] [CrossRef]
- Briones, M.J.I.; McNamara, N.P.; Poskitt, J.; Crow, S.E.; Ostle, N.J. Interactive biotic and abiotic regulators of soil carbon cycling: Evidence from controlled climate experiments on peatland and boreal soils. Glob. Chang. Biol. 2014, 20, 2971–2982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccio, E.E.; Hodge, A.; Pett-Ridge, J.; Herman, D.J.; Weber, P.K.; Firestone, M.K. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ. Microbiol. 2013, 15, 1870–1881. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Moore, J.A.M.; Jiang, J.; Post, W.M.; Classen, A.T. Decomposition by ectomycorrhizal fungi alters soil carbon storage in a simulation model. Ecosphere 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Nazir, R.; Warmink, J.A.; Boersma, H.; Van Elsas, J.D. Mechanisms that promote bacterial fitness in fungal-affected soil microhabitats. FEMS Microbiol. Ecol. 2009, 71, 169–185. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, C.V.; Hartley, I.P.; Ineson, P.; Fitter, A.H. Soil temperature affects carbon allocation within arbuscular mycorrhizal networks and carbon transport from plant to fungus. Glob. Chang. Biol. 2008, 14, 1181–1190. [Google Scholar] [CrossRef]
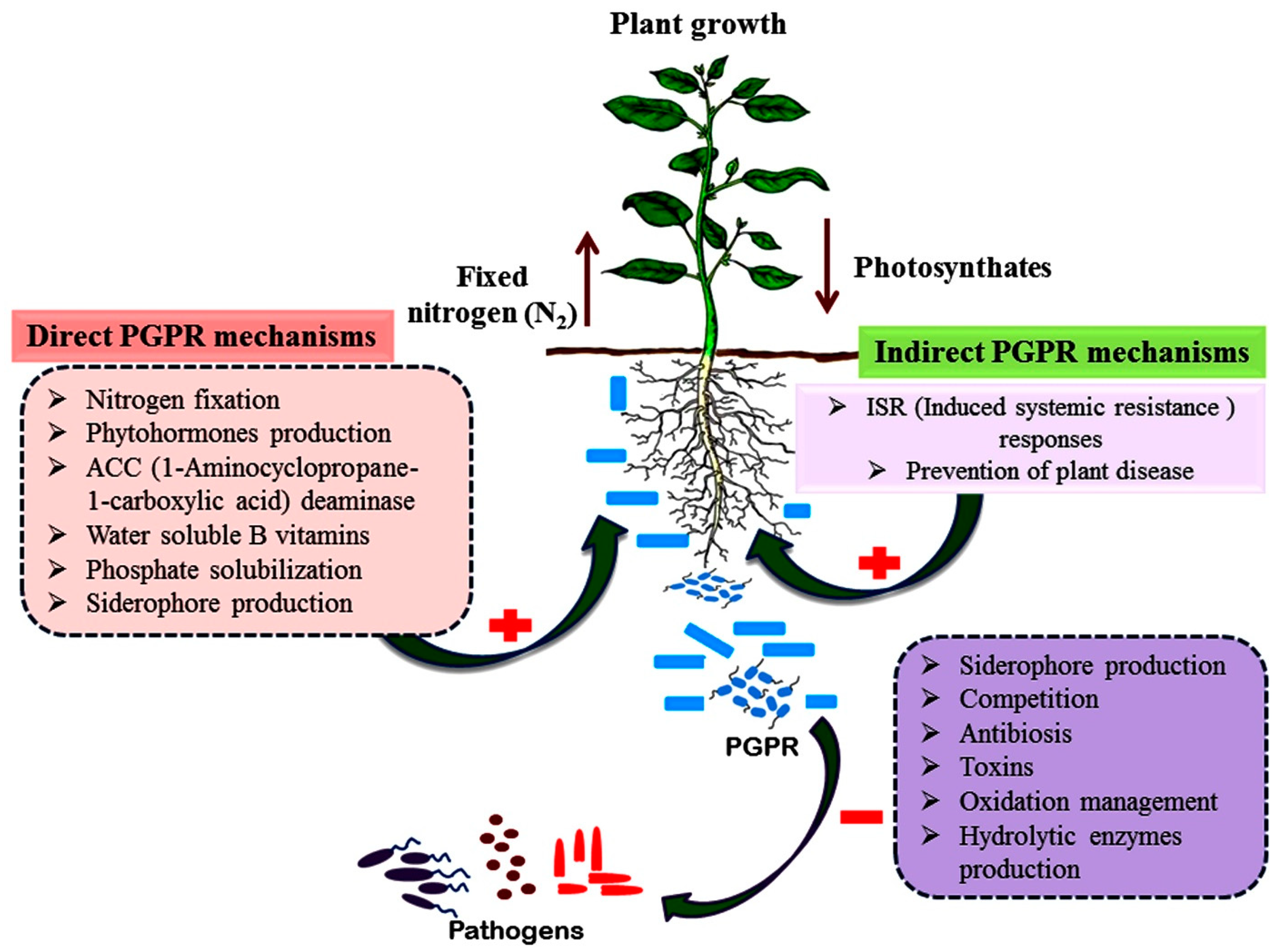


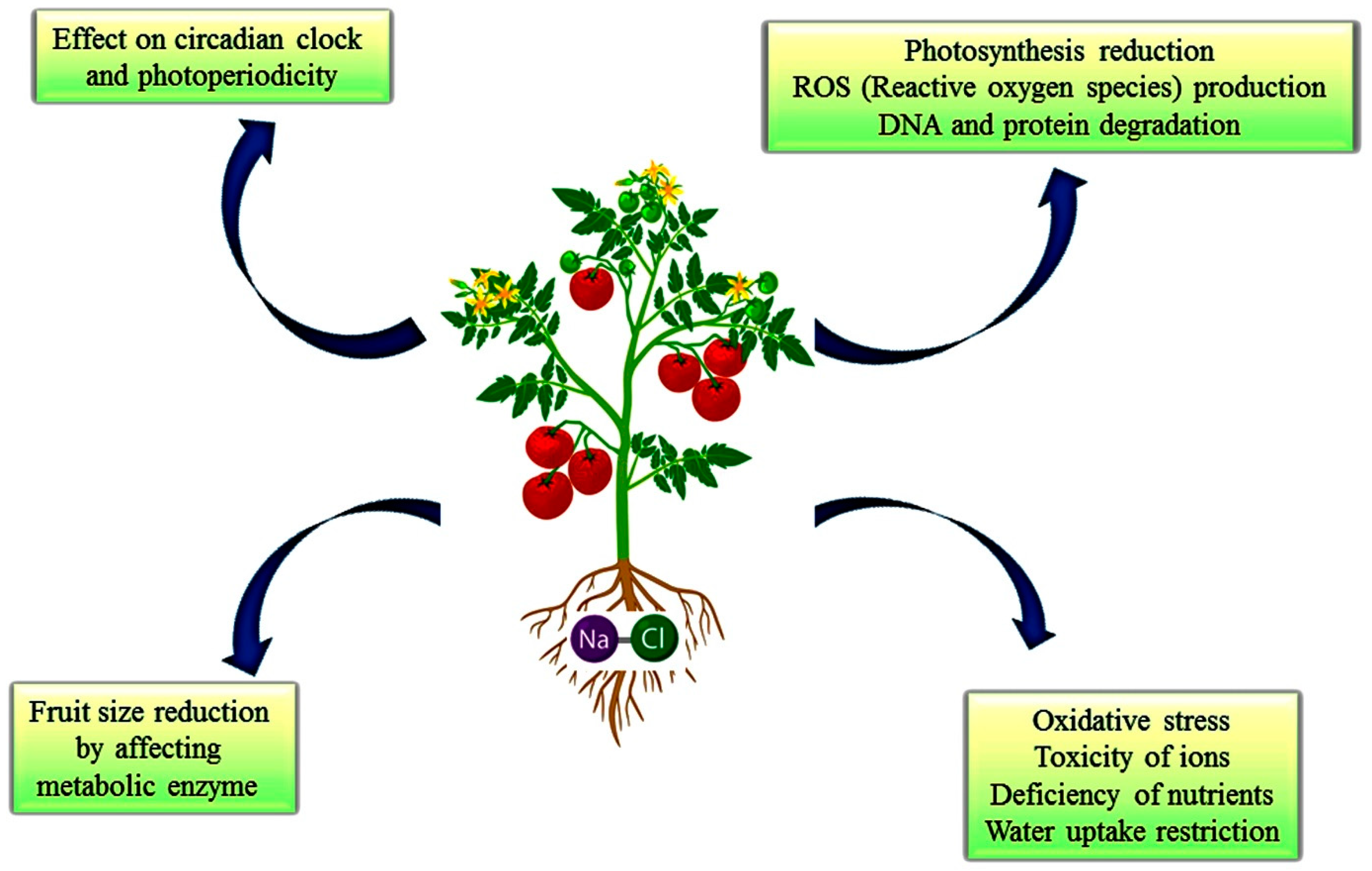
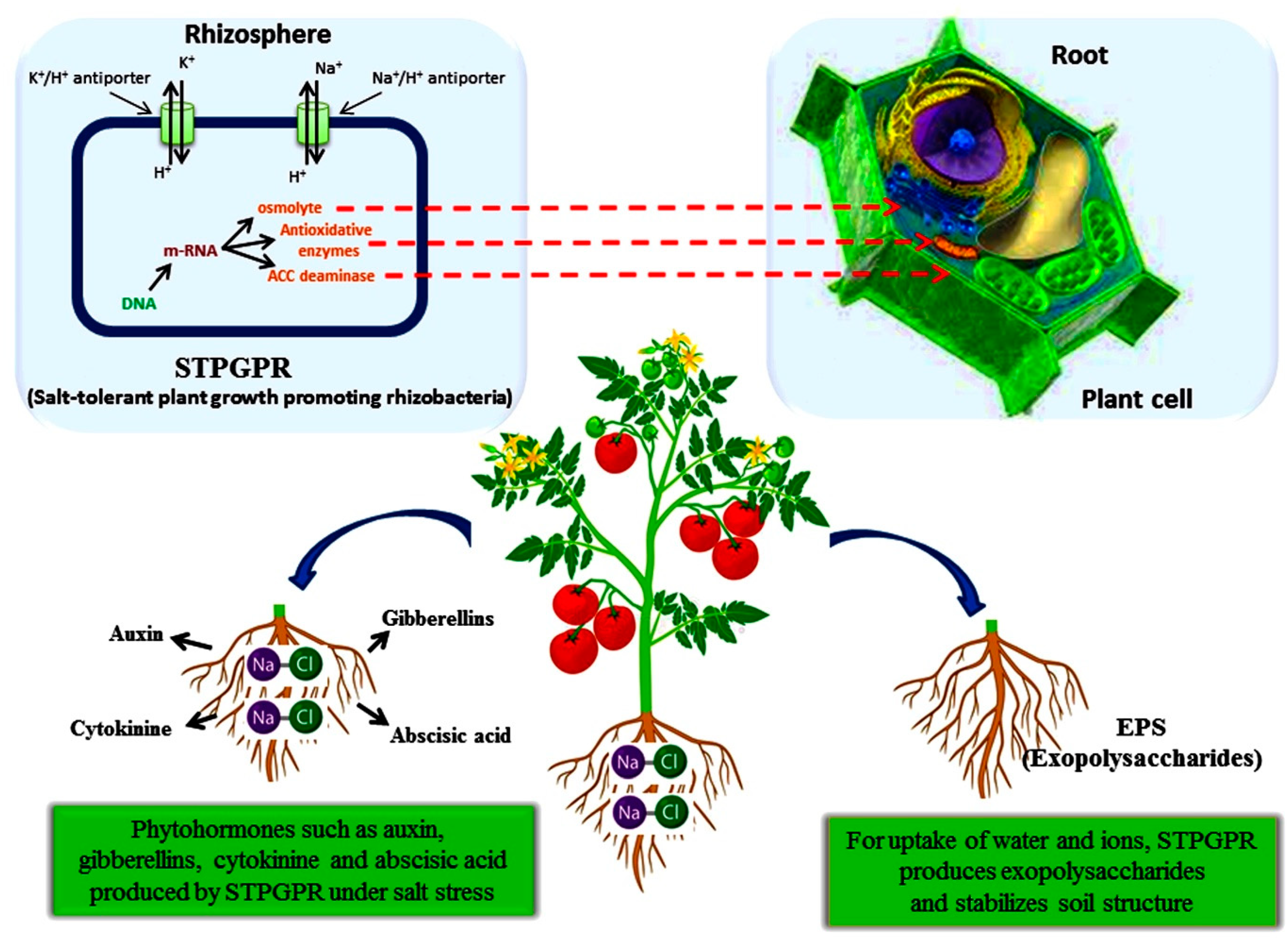
| PGPR Mechanism | Microorganism | References |
|---|---|---|
| Nitrogen fixation | Bacillus, Rhizobium, Azotobacter, Azospirillum, Frankia, Gluconacetobacter, Burkholderia, Azorhizobium, Beijerinckia, Cyanobacteria | [21,38,44,45] |
| Phosphate solubilzation | Arthrobacter, Burkholderia, Enterobacter, Microbacterium Pseudomonas, Bacillus, Erwinia, Rhizobium, Mesorhizobium, Flavobacterium, Rhodococcus, Serratia | [46,47] |
| Siderophore production | Pseudomonas, Bacillus, Rhizobium, Azotobactor, Enterobacter, Serratia | [48] |
| Phytohormone production | Rhizobium, Bradyrhizobium, Mesorhizobium, Bacillus, Pantoea, Arthrobacter Pseudomonas, Enterobacter, Burkholderia, Agrobacterium, Xanthomonas, Azospirillum, | [49,50] |
| Antibiotic production | Bacillus species, Pseudomonas species, Burkholderia, Brevibacterium, Streptomyces | [51,52] |
| Volatile metabolite production | Pseudomonas, Bacillus, Burkholderia, Agrobacterium, Paenibacillus polymyxa, Xanthomonas | [53] |
| Lytic enzyme production | Bacillus, Pseudomonas species | [54] |
| Induced systemic resistance | Pseudomonas, Bacillus, Serratia, Azospirillum, Trichoderma | [55] |
| Stress tolerance | Pseudomonas, Bacillus, Pantoea, Burkholderia, Rhizobium | [36,56] |
| Biocontrol agents | Pseudomonas, Bacillus, Trichoderma | [57,58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandran, H.; Meena, M.; Swapnil, P. Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture. Sustainability 2021, 13, 10986. https://doi.org/10.3390/su131910986
Chandran H, Meena M, Swapnil P. Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture. Sustainability. 2021; 13(19):10986. https://doi.org/10.3390/su131910986
Chicago/Turabian StyleChandran, Hema, Mukesh Meena, and Prashant Swapnil. 2021. "Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture" Sustainability 13, no. 19: 10986. https://doi.org/10.3390/su131910986
APA StyleChandran, H., Meena, M., & Swapnil, P. (2021). Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture. Sustainability, 13(19), 10986. https://doi.org/10.3390/su131910986








