Factors Affecting Carbonation Depth in Foamed Concrete Bricks for Accelerate CO2 Sequestration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Mix Proportion
2.2. Fresh Stage Tests (Fresh Density Test/Inverted Slump Test)
- d1 = Maximum diameter of slump flow;
- d2 = Perpendicular diameter of d1.
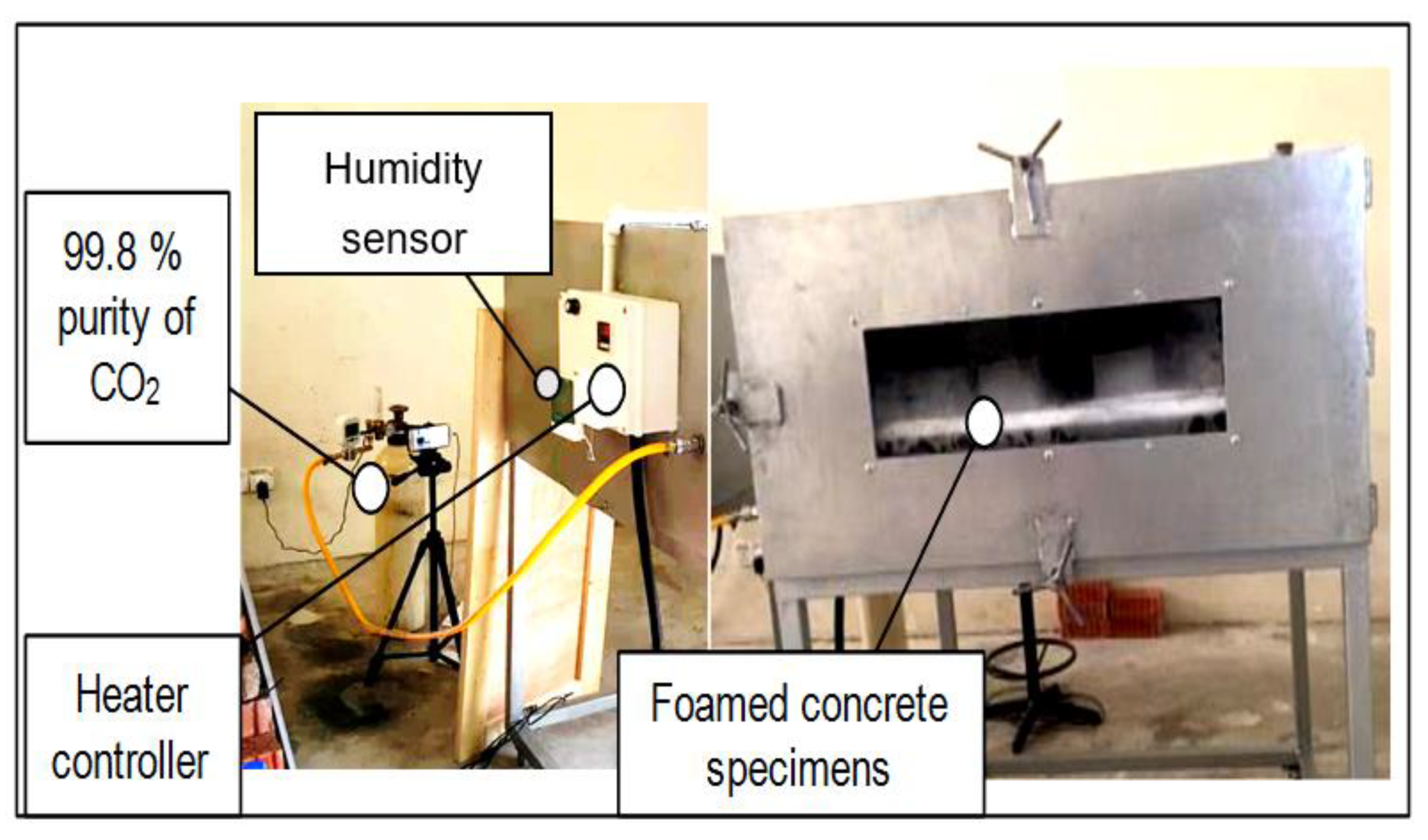
2.3. Sample Preparation and Chamber Curing
2.4. Hardened Stage Test (Carbonation Depth Test)
- d1 = is the carbonation depth from the first side specimens;
- d2 = is the carbonation depth from the second side specimens;
- d3 = is the carbonation depth from the third side specimens.
3. Results
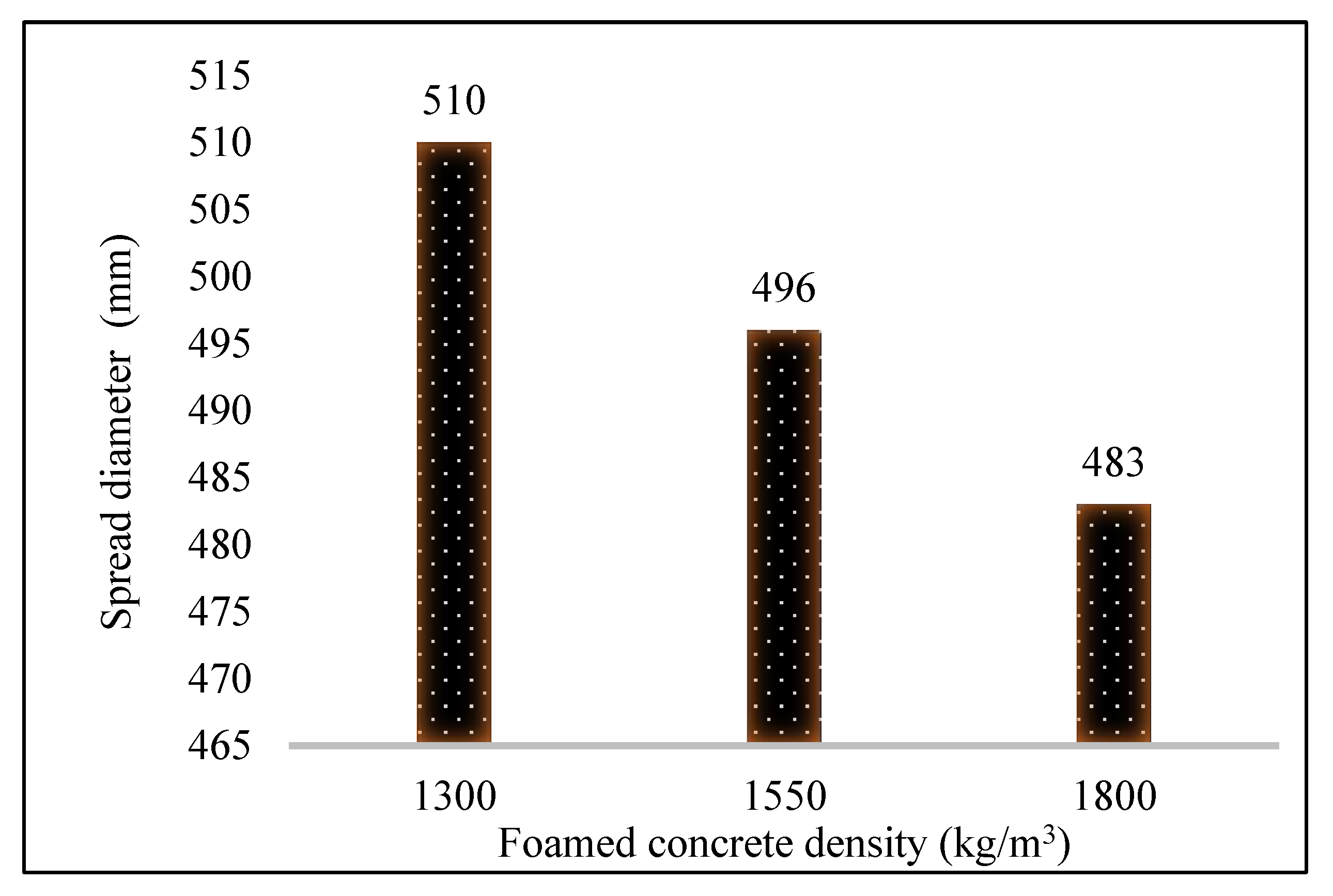
3.1. Fresh and Inverted Slump Tests
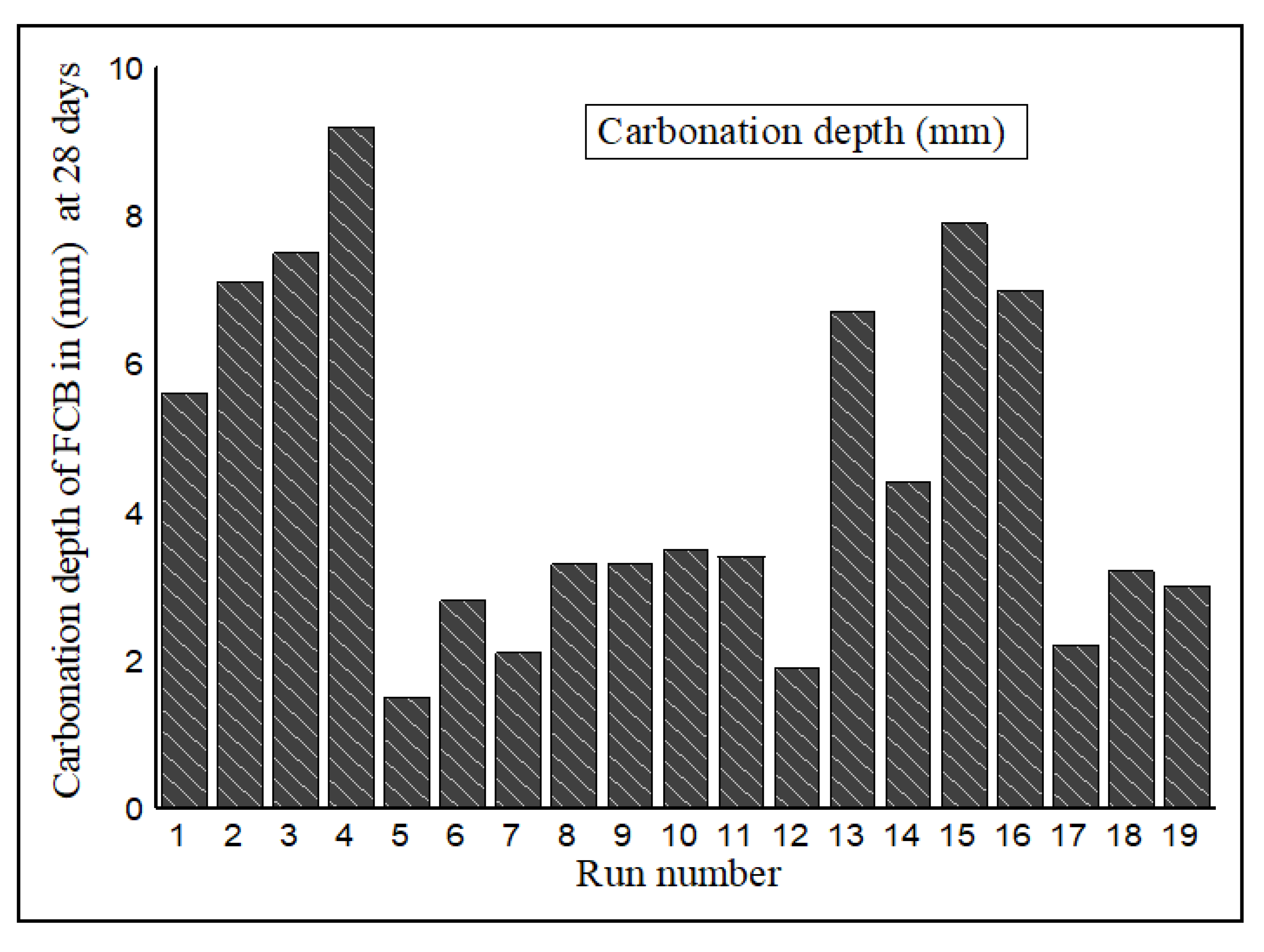
3.2. Carbonation Depth of FCB
3.3. Factorial and RSM Analyses
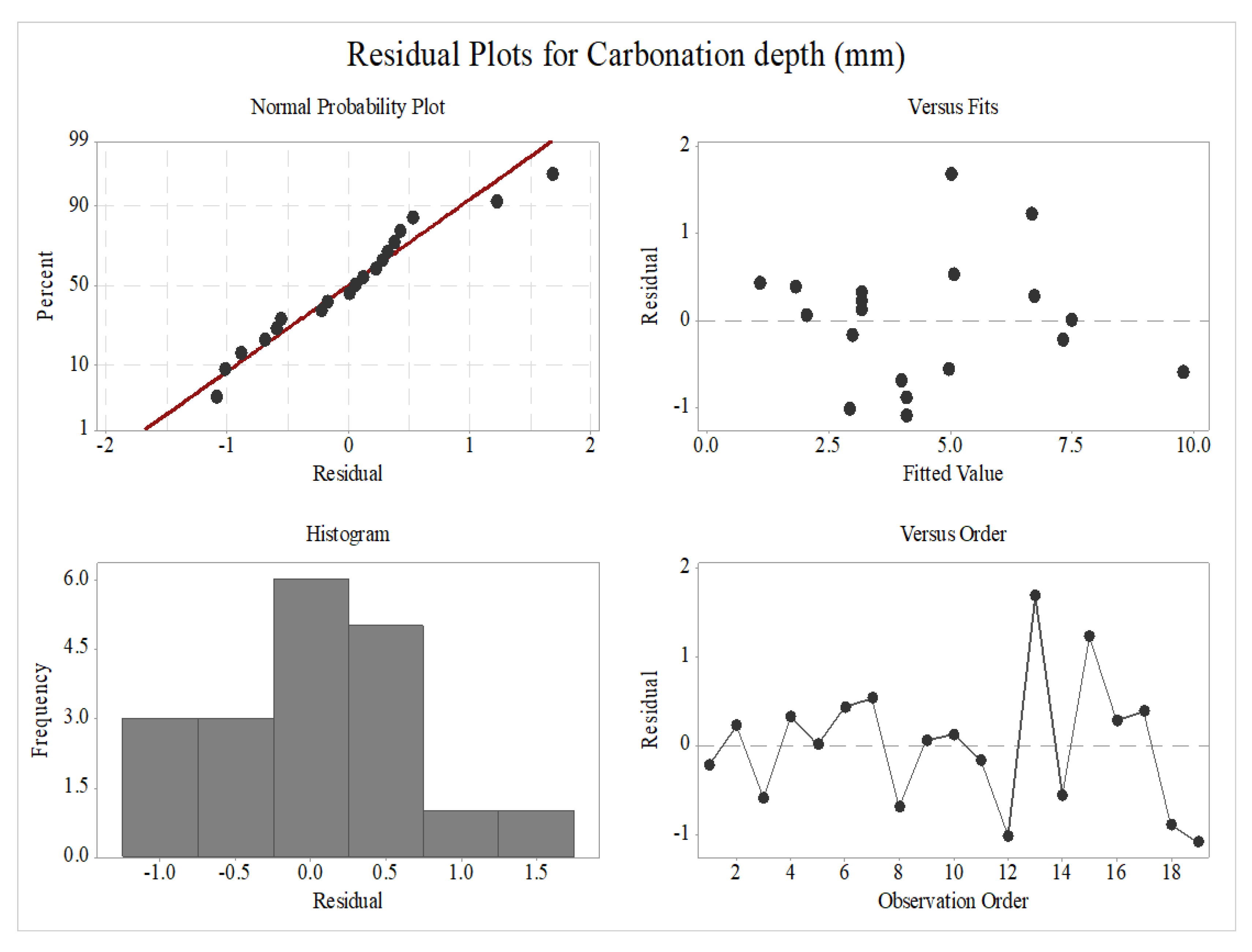
3.3.1. Residual Plots of Carbonation Depth
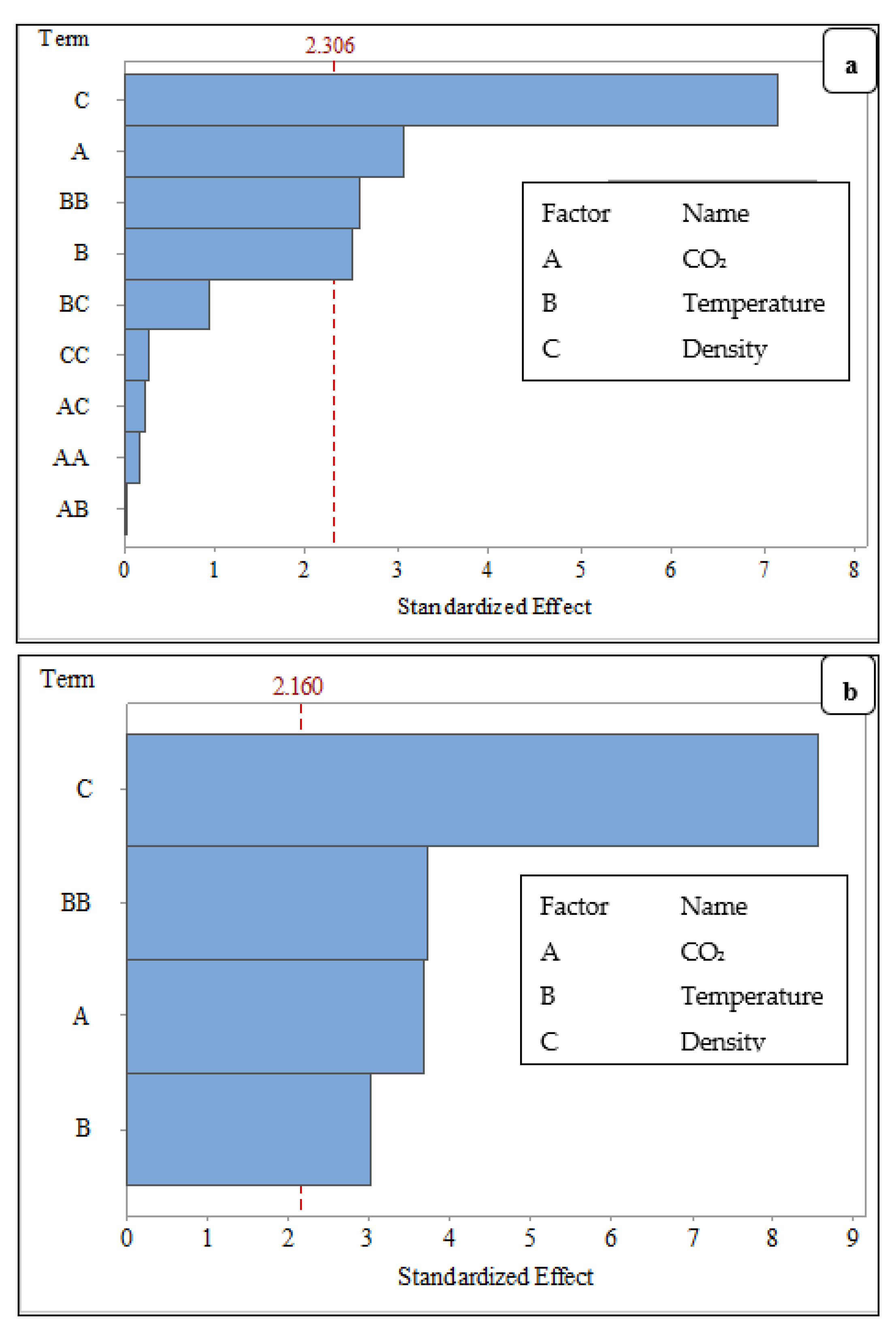
3.3.2. Significance of the Factors to Carbonation Depth of FCB
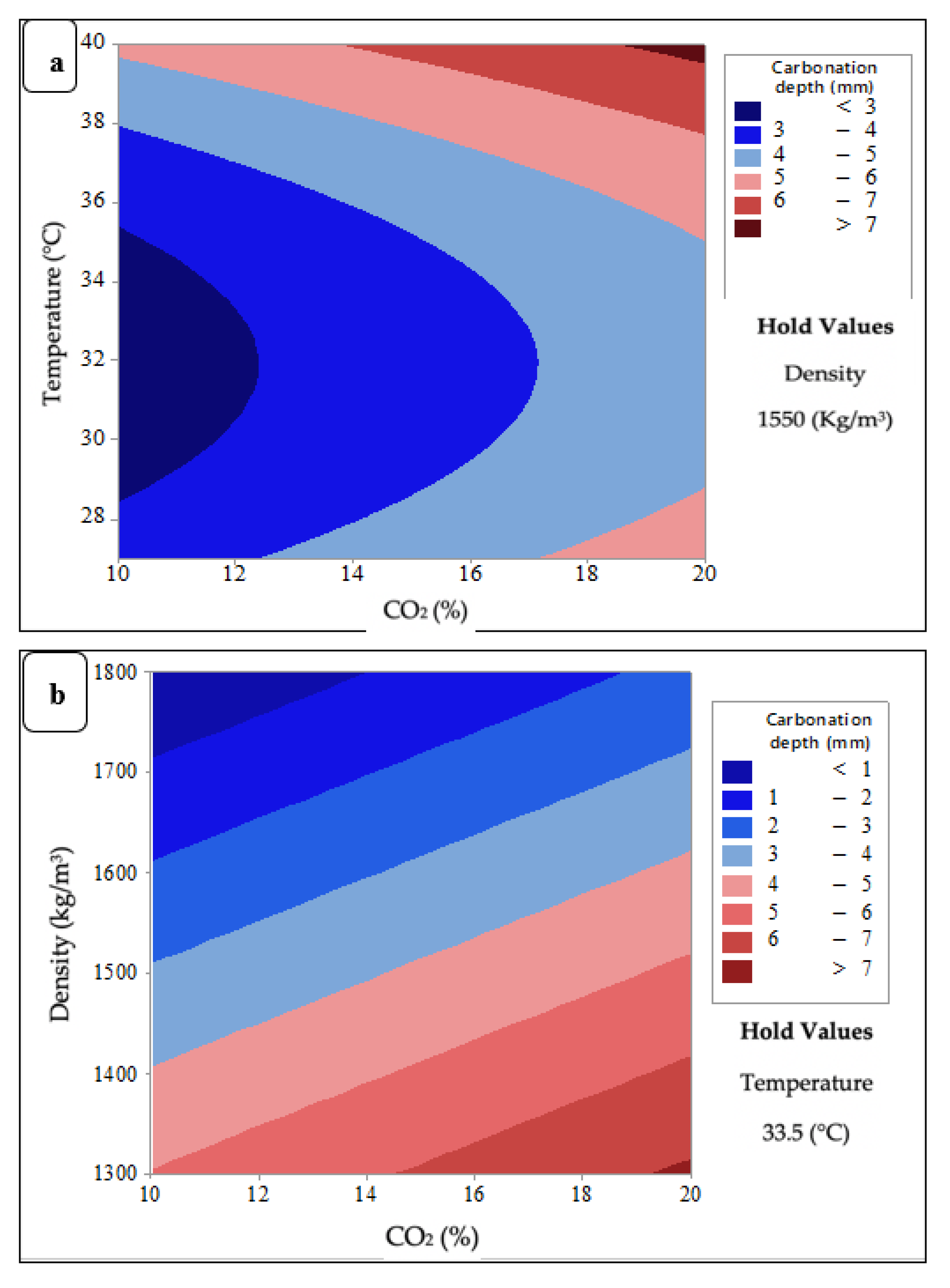
3.3.3. Contour Plots of Carbonation Depth of FCB
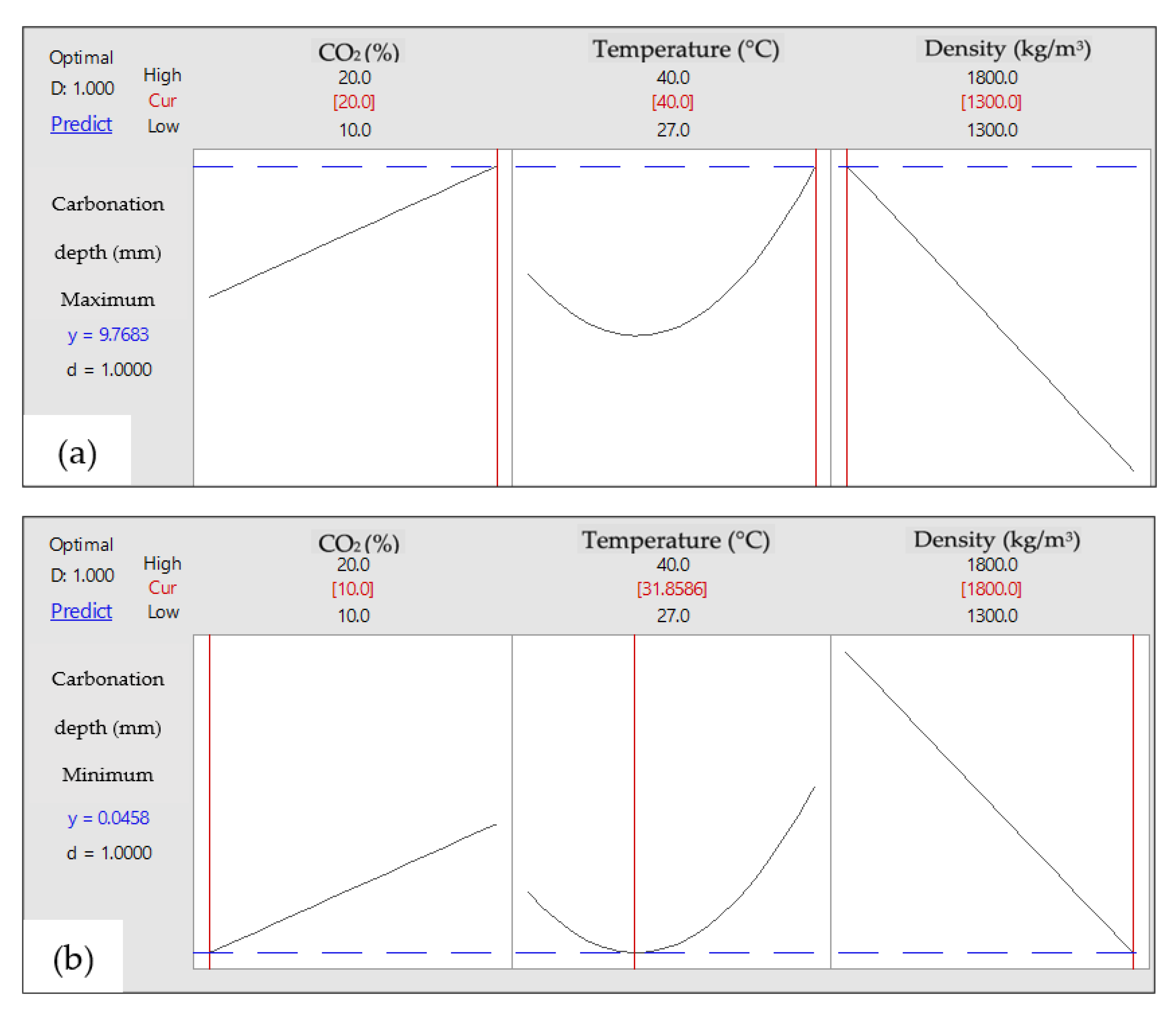
3.3.4. Optimum Conditions of Carbonation Depth of FCB
3.3.5. Development of Initial and Final Regression Equation
* Density − 0.000005 CO2 * Temperature * Density − 1.4875 Ct Pt
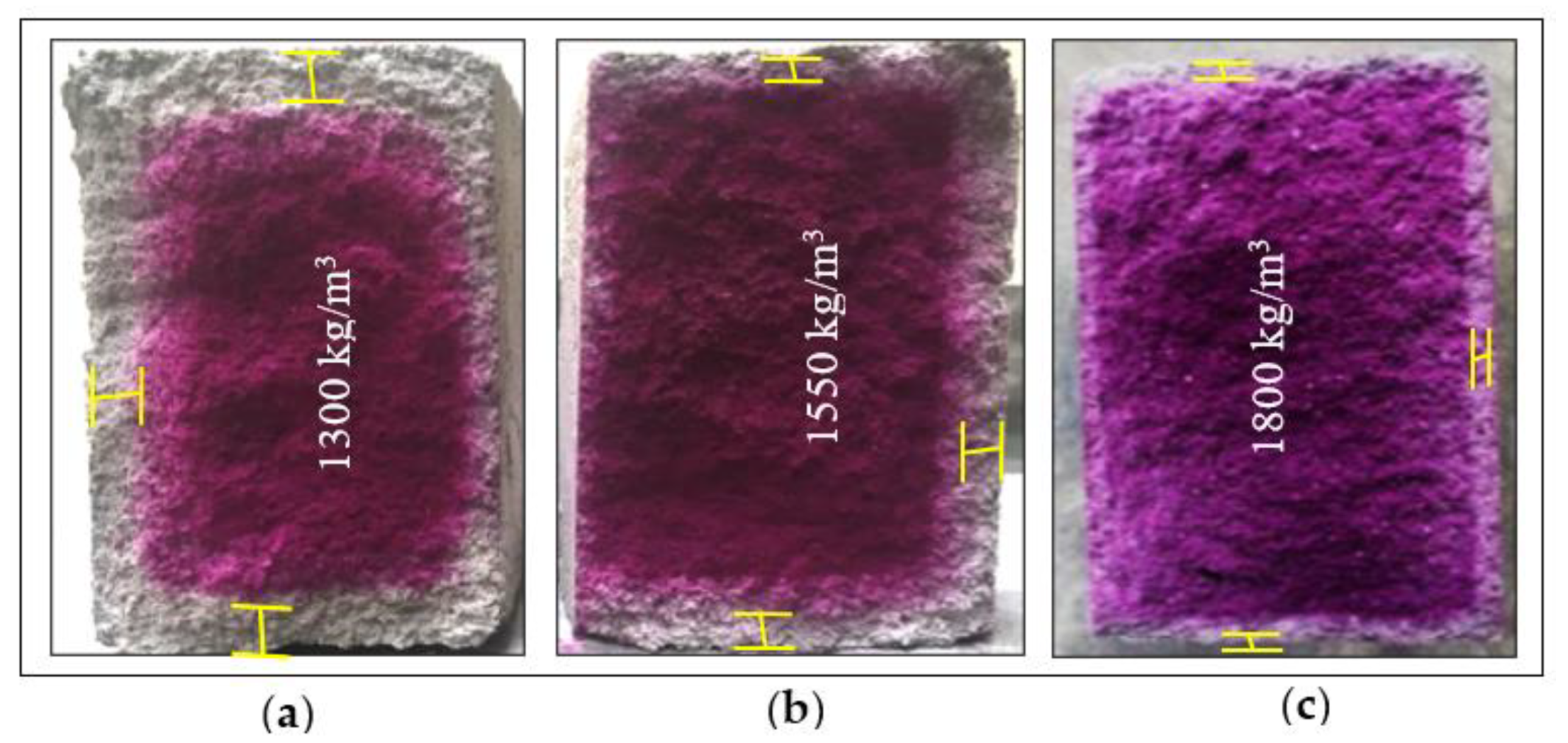
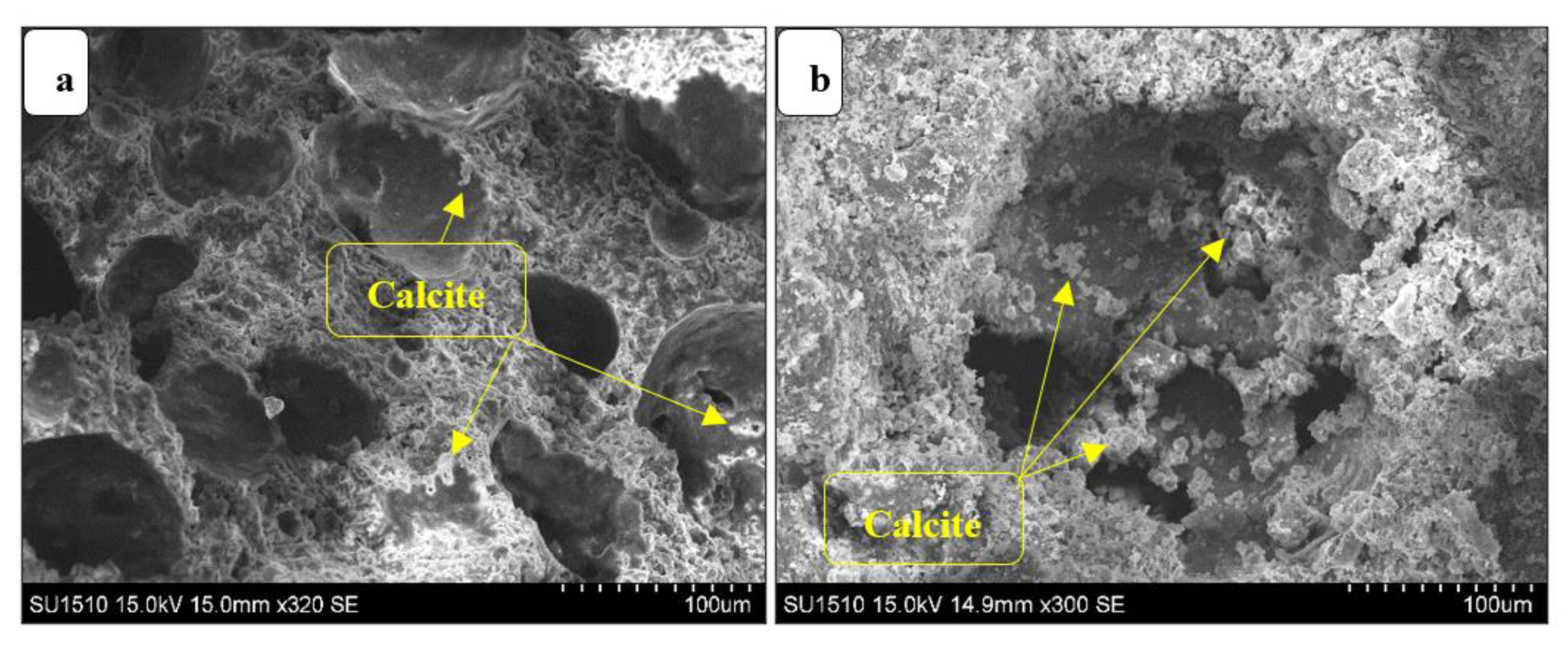
3.3.6. Microstructure Analysis (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narayanan, N.; Ramamurthy, K. Structure and properties of aerated concrete: A review. Cem. Concr. Compos. 2000, 22, 321–329. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Farzadnia, N.; Ali, A.A.A. Properties and applications of foamed concrete; A review. Constr. Build. Mater. 2015, 101, 990–1005. [Google Scholar] [CrossRef]
- Lim, S.K.; Tan, C.S.; Zhao, X.; Ling, T.C. Strength and toughness of lightweight foamed concrete with different sand grading. KSCE J. Civ. Eng. 2015, 19, 2191–2197. [Google Scholar] [CrossRef] [Green Version]
- Mindess, S. Developements in the Formulation and Reinforcement of Concrete; Woodhead Publishing: Sawston, UK, 2019; ISBN 9781845692636. [Google Scholar]
- Song, Y.; Li, B.; Yang, E.; Liu, Y.; Ding, T. Cement & Concrete Composites Feasibility study on utilization of municipal solid waste incineration bottom ash as aerating agent for the production of autoclaved aerated concrete. Cem. Concr. Compos. 2015, 56, 51–58. [Google Scholar]
- Costa, B.L.D.S.; Freitas, J.C.D.O.; Melo, D.M.D.A.; Araujo, R.G.D.S.; de Oliveira, Y.H.; Simão, C.A. Evaluation of density influence on resistance to carbonation process in oil well cement slurries. Constr. Build. Mater. 2019, 197, 331–338. [Google Scholar] [CrossRef]
- Kellouche, Y.; Boukhatem, B.; Ghrici, M.; Tagnit-Hamou, A. Exploring the major factors affecting fly-ash concrete carbonation using artificial neural network. Neural Comput. Appl. 2019, 31, 969–988. [Google Scholar] [CrossRef]
- Namsone, E.; Šahmenko, G.; Korjakins, A. Durability Properties of High Performance Foamed Concrete. Procedia Eng. 2017, 172, 760–767. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Z.; Niu, Y.; Li, J.; Zhang, Y. Study on the preparation and properties of high-porosity foamed concretes based on ordinary Portland cement. Mater. Des. 2016, 92, 949–959. [Google Scholar] [CrossRef]
- Wang, T.; Huang, H.; Hu, X.; Fang, M.; Luo, Z.; Guo, R. Accelerated mineral carbonation curing of cement paste for CO2 sequestration and enhanced properties of blended calcium silicate. Chem. Eng. J. 2017, 323, 320–329. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of early carbonation curing on chloride penetration and weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- Ekolu, S.O. A review on effects of curing, sheltering, and CO2 concentration upon natural carbonation of concrete. Constr. Build. Mater. 2016, 127, 306–320. [Google Scholar] [CrossRef]
- Alshalif, A.F.; Irwan, J.M.; Othman, N.; Al-Gheethi, A.A.; Shamsudin, S. A systematic review on bio-sequestration of carbon dioxide in bio-concrete systems: A future direction. Eur. J. Environ. Civ. Eng. 2020, 1–20. [Google Scholar] [CrossRef]
- Yoon, I.S.; Çopuroǧlu, O.; Park, K.B. Effect of global climatic change on carbonation progress of concrete. Atmos. Environ. 2007, 41, 7274–7285. [Google Scholar] [CrossRef]
- Pacheco Torgal, F.; Miraldo, S.; Labrincha, J.A.; De Brito, J. An overview on concrete carbonation in the context of eco-efficient construction: Evaluation, use of SCMs and/or RAC. Constr. Build. Mater. 2012, 36, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Lovato, P.S.; Possan, E.; Molin, D.C.C.D.; Masuero, Â.B.; Ribeiro, J.L.D. Modeling of mechanical properties and durability of recycled aggregate concretes. Constr. Build. Mater. 2012, 26, 437–447. [Google Scholar] [CrossRef]
- Drouet, E.; Poyet, S.; Le Bescop, P.; Torrenti, J.M.; Bourbon, X. Carbonation of hardened cement pastes: Influence of temperature. Cem. Concr. Res. 2019, 115, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Ha, J.; Hashida, T.; Letters, S.T. Development of a CO2 solidification method for recycling autoclaved lightweight concrete waste. J. Mater. Sci. Lett. 2001, 1791–1794. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Yu, B.; Zhang, R.; French, D.; Grigore, M.; Wang, X.; Chen, Z.; Zhao, S. Insights into Carbonation Kinetics of Fly Ash from Victorian Lignite for CO2 Sequestration. Energy Fuels 2018, 32, 4569–4578. [Google Scholar] [CrossRef]
- Muthu Kumar, E.; Ramamurthy, K. Effect of fineness and dosage of aluminium powder on the properties of moist-cured aerated concrete. Constr. Build. Mater. 2015, 95, 486–496. [Google Scholar] [CrossRef]
- Kumar, R.; Lakhani, R.; Tomar, P. A Simple Novel Mix Design Method and Properties Assessment of Foamed Concretes with Limestone Slurry Waste; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 171, ISBN 9113322834. [Google Scholar] [CrossRef]
- Alshalif, A.F.; Irwan, J.M.; Tajarudin, H.A.; Othman, N.; Al-Gheethi, A.A.; Shamsudin, S.; Altowayti, W.A.H.; Abo Sabah, S. Optimization of Bio-Foamed Concrete Brick Strength via Bacteria Based Self-Healing and Bio-Sequestration of CO2. Materials 2021, 14, 4575. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Z.; Liu, N. Experimental Research on Properties of High-Strength Foamed Concrete. J. Mater. Civ. Eng. 2012, 24, 113–118. [Google Scholar] [CrossRef]
- Zambrano Leal, A. Sociedad de control y profesión docente. Las imposturas de un discurso y la exigencia de una nueva realidad. Antimicrob. Agents Chemother. 2012, 95, 45–52. [Google Scholar] [CrossRef]
- Panesar, D.K. Cellular concrete properties and the effect of synthetic and protein foaming agents. Constr. Build. Mater. 2013, 44, 575–584. [Google Scholar] [CrossRef]
- Possan, E.; Thomaz, W.A.; Aleandri, G.A.; Felix, E.F.; dos Santos, A.C.P. CO2 uptake potential due to concrete carbonation: A case study. Case Stud. Constr. Mater. 2017, 6, 147–161. [Google Scholar] [CrossRef]
- Lo, T.Y.; Nadeem, A.; Tang, W.C.P.; Yu, P.C. The effect of high temperature curing on the strength and carbonation of pozzolanic structural lightweight concretes. Constr. Build. Mater. 2009, 23, 1306–1310. [Google Scholar] [CrossRef]
- Chang, C.F.; Chen, J.W. The experimental investigation of concrete carbonation depth. Cem. Concr. Res. 2006, 36, 1760–1767. [Google Scholar] [CrossRef]
- De Ceukelaire, L.; Van Nieuwenburg, D. Accelerated carbonation of a blast-furnace cement concrete. Cem. Concr. Res. 1993, 23, 442–452. [Google Scholar] [CrossRef]
- Meima, J.A.; Comans, R.N.J. Geochemical modelling of weathering reactions in MSWI bottom ash. Environ. Sci. Technol. 1997, 31, 1269–1276. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Shamsudin, S.; Lajis, M.A.; Zhong, Z.W.; Ahmad, A.; Wagiman, A. Weld strength in solid–state recycling of aluminum chips: Schweißnahtfestigkeit im Festkörper-Recycling von Aluminium-Spänen. Mater. Werkst. 2017, 48, 290–298. [Google Scholar] [CrossRef]
- Biglarijoo, N.; Nili, M.; Hosseinian, S.M.; Razmara, M.; Ahmadi, S.; Razmara, P. Modelling and optimisation of concrete containing recycled concrete aggregate and waste glass. Mag. Concr. Res. 2017, 69, 306–316. [Google Scholar] [CrossRef]
- Yang, K.-H.; Seo, E.-A.; Tae, S.-H. Carbonation and CO2 uptake of concrete. Environ. Impact Assess. Rev. 2014, 46, 43–52. [Google Scholar] [CrossRef]
- Talebi, A.; Razali, Y.S.; Ismail, N.; Rafatullah, M.; Azan Tajarudin, H. Selective adsorption and recovery of volatile fatty acids from fermented landfill leachate by activated carbon process. Sci. Total Environ. 2020, 707, 134533. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X. Recycling of waste autoclaved aerated concrete powder in Portland cement by accelerated carbonation. Waste Manag. 2019, 89, 254–264. [Google Scholar] [CrossRef]
- Sharma, D.; Goyal, S. Accelerated carbonation curing of cement mortars containing cement kiln dust: An effective way of CO2 sequestration and carbon footprint reduction. J. Clean. Prod. 2018, 192, 844–854. [Google Scholar] [CrossRef]
- Branch, J.L.; Kosson, D.S.; Garrabrants, A.C.; He, P.J. The impact of carbonation on the microstructure and solubility of major constituents in microconcrete materials with varying alkalinities due to fly ash replacement of ordinary Portland cement. Cem. Concr. Res. 2016, 89, 297–309. [Google Scholar] [CrossRef] [Green Version]



| Chemical Compound | SiO2 | Al2O3 | Fe2O3 | SO3 | K2O | CaO | MgO |
|---|---|---|---|---|---|---|---|
| Concentration (%) | 20.6 | 5.4 | 4.2 | 2.2 | 0.6 | 64.8 | 2.2 |
| Run No. | Density (kg/m3) | Cement (kg/m3) | Fine Sand (kg/m3) | Water (L/m3) | T (°C) | CO2 (%) |
|---|---|---|---|---|---|---|
| 1 | 1300 | 553.2 | 746.8 | 276.6 | 27.0 | 10 |
| 2 | 1300 | 553.2 | 746.8 | 276.6 | 27.0 | 20 |
| 3 | 1300 | 553.2 | 746.8 | 276.6 | 40.0 | 10 |
| 4 | 1300 | 553.2 | 746.8 | 276.6 | 40.0 | 20 |
| 5 | 1800 | 766 | 1034 | 383 | 27.0 | 10 |
| 6 | 1800 | 766 | 1034 | 383 | 27.0 | 20 |
| 7 | 1800 | 766 | 1034 | 383 | 40.0 | 10 |
| 8 | 1800 | 766 | 1034 | 383 | 40.0 | 20 |
| 9 | 1550 | 659.5 | 890.4 | 329.7 | 33.5 | 15 |
| 10 | 1550 | 659.5 | 890.4 | 329.7 | 33.5 | 15 |
| 11 | 1550 | 659.5 | 890.4 | 329.7 | 33.5 | 15 |
| 12 | 1550 | 659.5 | 890.4 | 329.7 | 33.5 | 10 |
| 13 | 1550 | 659.5 | 890.4 | 329.7 | 33.5 | 20 |
| 14 | 1550 | 659.5 | 890.4 | 329.7 | 27.0 | 15 |
| 15 | 1550 | 659.5 | 890.4 | 329.7 | 40.0 | 15 |
| 16 | 1300 | 553.2 | 746.8 | 276.6 | 33.5 | 15 |
| 17 | 1800 | 766 | 1034 | 383 | 33.5 | 15 |
| 18 | 1550 | 659.5 | 890.4 | 329.7 | 33.5 | 15 |
| 19 | 1550 | 659.5 | 890.4 | 329.7 | 33.5 | 15 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value | Effect |
|---|---|---|---|---|---|---|
| Model | 5 | 89.832 | 17.966 | 22.000 | 0.000 | - |
| Blocks | 1 | 2.880 | 2.880 | 3.530 | 0.083 | - |
| Linear | 3 | 78.446 | 26.148 | 32.020 | 0.000 | - |
| CO2 | 1 | 11.025 | 11.025 | 13.500 | 0.003 | 3.670 |
| Temperature | 1 | 7.396 | 7.396 | 9.060 | 0.010 | 3.010 |
| Density | 1 | 60.025 | 60.025 | 73.490 | 0.000 | −8.570 |
| Square | 1 | 11.371 | 11.371 | 13.920 | 0.003 | 3.730 |
| Temperature * Temperature | 1 | 11.371 | 11.371 | 13.920 | 0.003 | 3.670 |
| Error | 13 | 10.618 | 0.816 | - | - | - |
| Lack-of-Fit | 10 | 10.578 | 1.057 | 79.330 | 0.002 | - |
| Pure Error | 3 | 0.040 | 0.013 | - | - | - |
| Total | 18 | 100.449 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshalif, A.F.; Irwan, J.M.; Tajarudin, H.A.; Othman, N.; Al-Gheethi, A.A.; Shamsudin, S.; Altowayti, W.A.H.; Sabah, S.A. Factors Affecting Carbonation Depth in Foamed Concrete Bricks for Accelerate CO2 Sequestration. Sustainability 2021, 13, 10999. https://doi.org/10.3390/su131910999
Alshalif AF, Irwan JM, Tajarudin HA, Othman N, Al-Gheethi AA, Shamsudin S, Altowayti WAH, Sabah SA. Factors Affecting Carbonation Depth in Foamed Concrete Bricks for Accelerate CO2 Sequestration. Sustainability. 2021; 13(19):10999. https://doi.org/10.3390/su131910999
Chicago/Turabian StyleAlshalif, Abdullah Faisal, J. M. Irwan, Husnul Azan Tajarudin, N. Othman, A. A. Al-Gheethi, S. Shamsudin, Wahid Ali Hamood Altowayti, and Saddam Abo Sabah. 2021. "Factors Affecting Carbonation Depth in Foamed Concrete Bricks for Accelerate CO2 Sequestration" Sustainability 13, no. 19: 10999. https://doi.org/10.3390/su131910999
APA StyleAlshalif, A. F., Irwan, J. M., Tajarudin, H. A., Othman, N., Al-Gheethi, A. A., Shamsudin, S., Altowayti, W. A. H., & Sabah, S. A. (2021). Factors Affecting Carbonation Depth in Foamed Concrete Bricks for Accelerate CO2 Sequestration. Sustainability, 13(19), 10999. https://doi.org/10.3390/su131910999









