Significance of Urban Vegetation on Lawns Regarding the Risk of Fire
Abstract
:1. Introduction
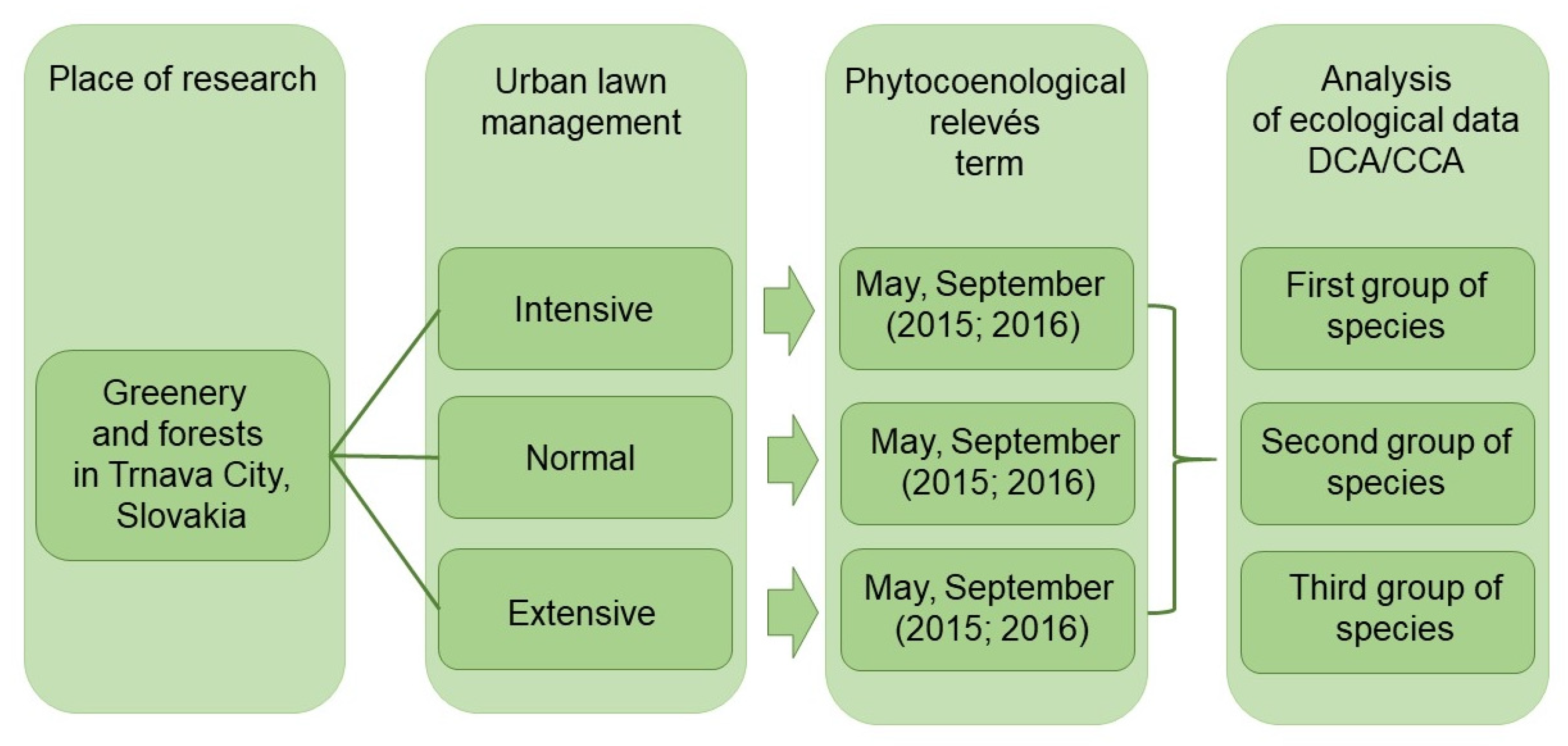
2. Materials and Methods
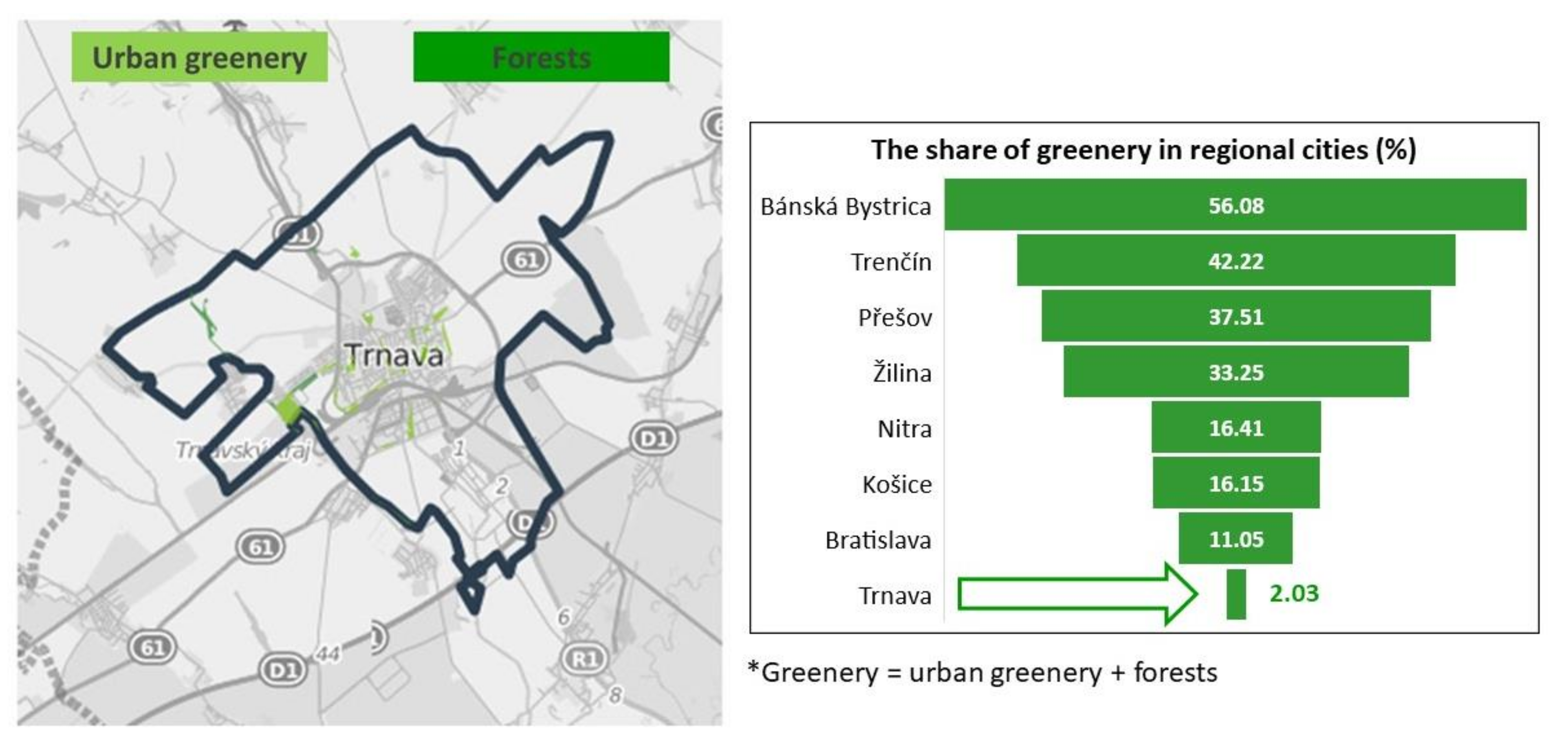
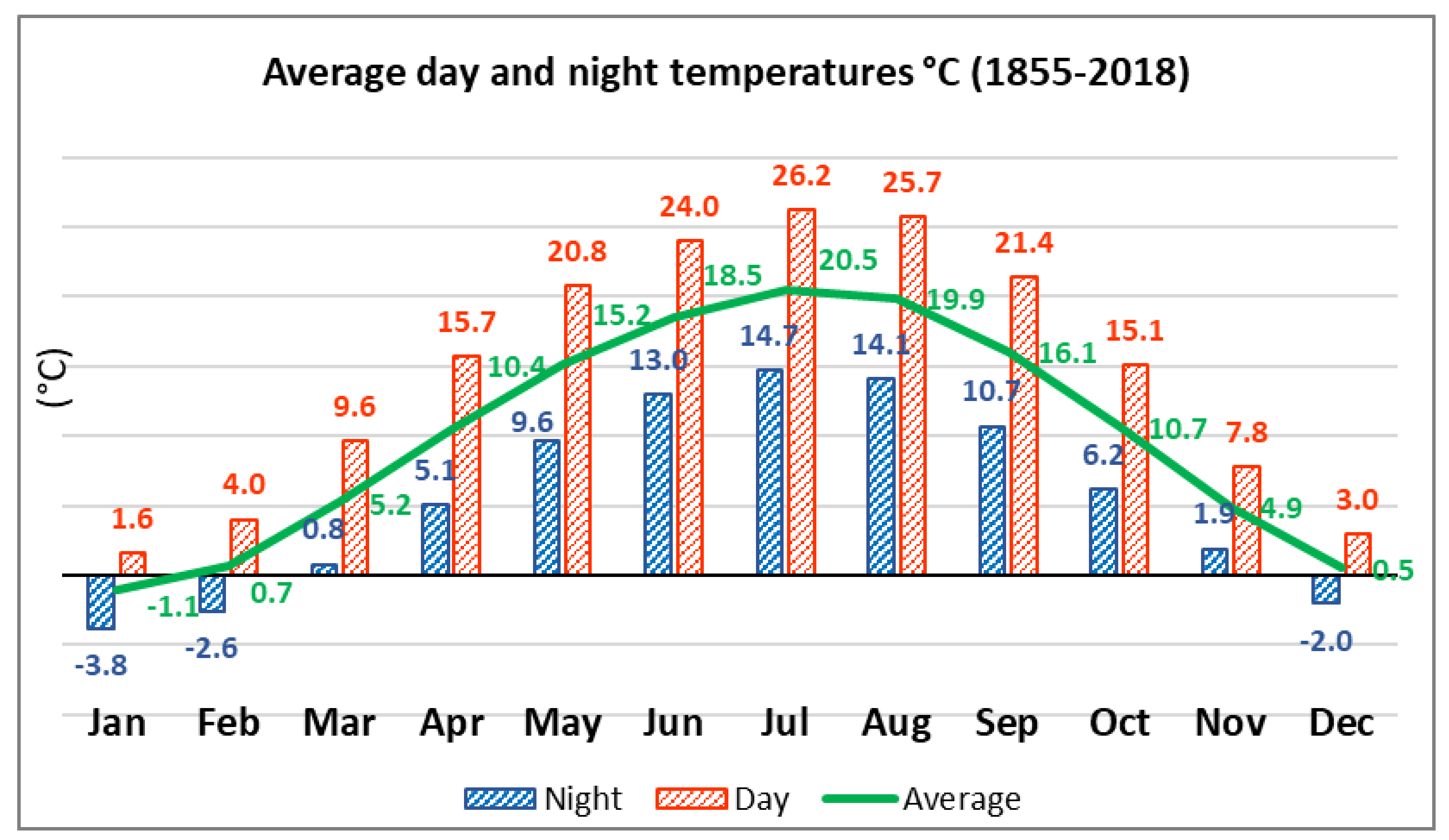
2.1. Characteristics of the Territory
2.2. Sites of Urban Lawns
- (1)
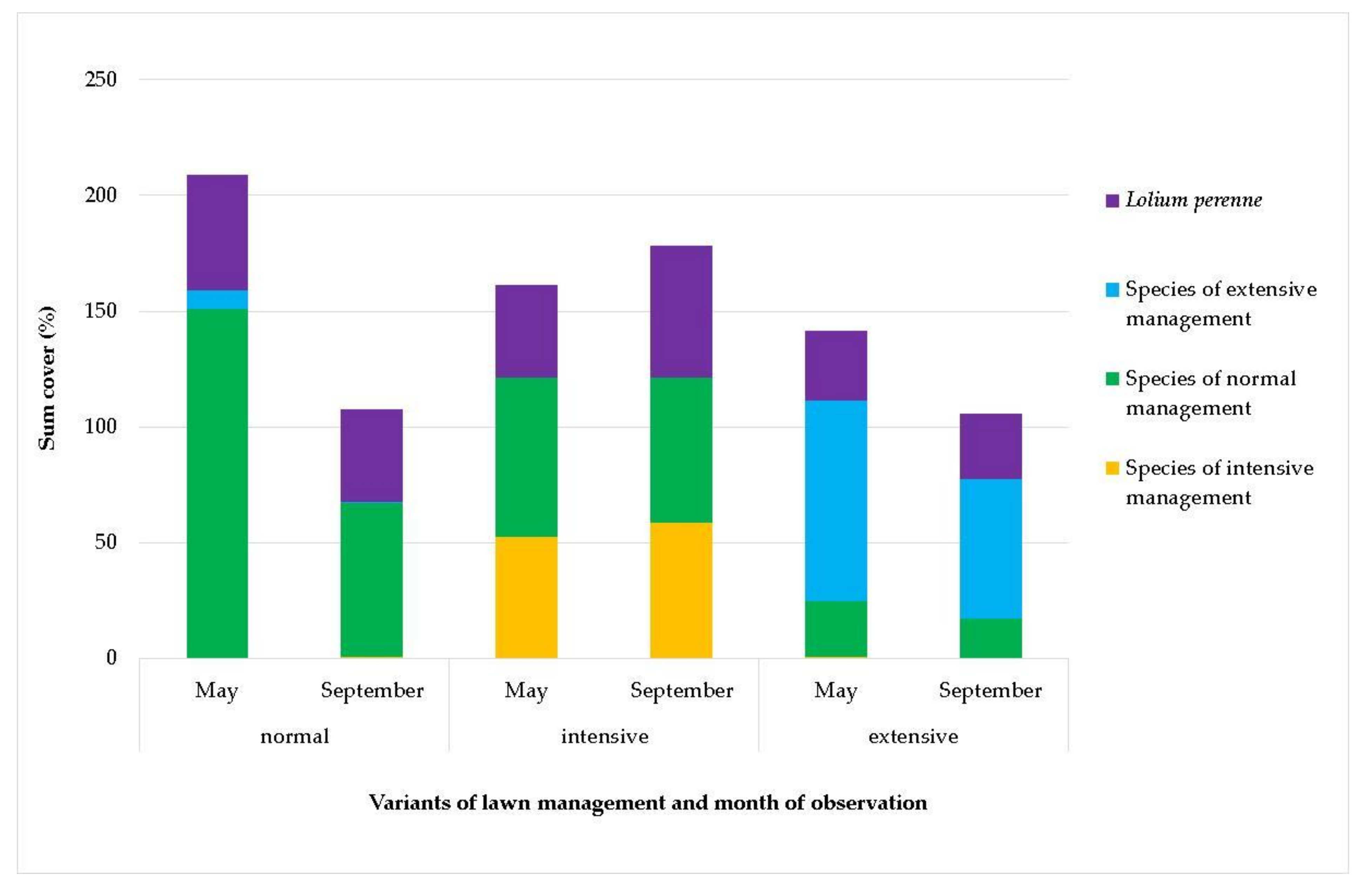
- Normal management: Grass areas are mowed twice a year, and biomass is taken away after the first mowing and mulched and left on the site after the second mowing. The site is part of the Na Hlíny housing estate, the most recent and smallest in Trnava City. Its construction began in the 1980s and ended in the 1990s. The housing estate covers an area of 1.1 ha (GPS, 48.3883103N, 17.5972586E) (Figure 4).
- (2)
- Intensive management: The lawn is mowed several times a year and biomass are removed. The Calvary Park site is a place of reverence, situated on an original cemetery established in 1747. The Park has interesting historical monuments, memorials of important persons, the Trnava Calvary (built on the site of the first historical Station of the Cross in the Austro-Hungarian Empire), and two massive lime trees, whose age is estimated at 260 years (GPS 48.3851289N, 17.5774719E) The housing estate covers an area of 2.1 ha (Figure 5).
- (3)
- Extensive management: The lawn is mowed irregularly, once a year at maximum, and biomass is left unevenly on the site, which is part of a land property with a road behind the Tesco shopping centre and in the vicinity of the Trnava water reservoir protection zone, representing an area of 5.4 ha (GPS 48.3865733N, 17.6052408E) (Figure 6).
2.3. Methodology of Vegetation Assessment
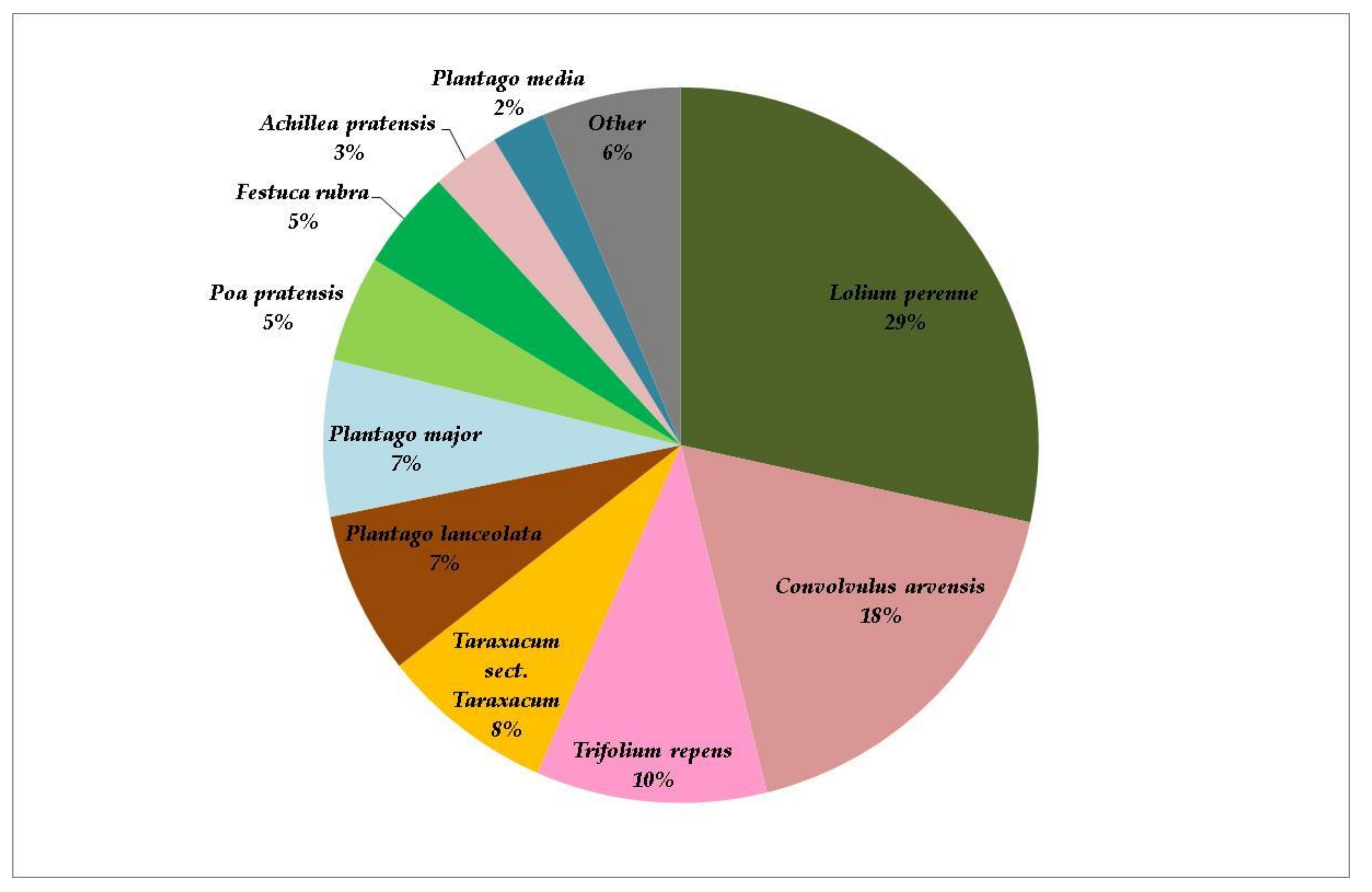
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, Y. Urban Flooding Mitigation Techniques: A Systematic Review and Future Studies. Water 2020, 12, 3579. [Google Scholar] [CrossRef]
- Francoeur, X.W.; Dagenais, D.; Paquette, A.; Dupras, J.; Messier, C. Complexifying the Urban Lawn Improves Heat Mitigation and Arthropod Biodiversity. Urban For. Urban Green. 2021, 60, 127007. [Google Scholar] [CrossRef]
- Lehmann, I.; Mathey, J.; Rößler, S.; Bräuer, A.; Goldberg, V. Urban Vegetation Structure Types as a Methodological Approach for Identifying Ecosystem Services – Application to the Analysis of Micro-Climatic Effects. Ecol. Indic. 2014, 42, 58–72. [Google Scholar] [CrossRef]
- Bolund, P.; Hunhamma, S. Ecosystem Services in Urban Areas. Ecol. Econ. 1999, 29, 293–301. [Google Scholar] [CrossRef]
- Knot, P.; Hrabe, F.; Hejduk, S.; Skladanka, J.; Kvasnovsky, M.; Hodulikova, L.; Caslavova, I.; Horky, P. The Impacts of Different Management Practices on Botanical Composition, Quality, Colour and Growth of Urban Lawns. Urban For. Urban Green. 2017, 26, 178–183. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.-H. Environmental Co-Benefits of Urban Greening for Mitigating Heat and Carbon Emissions. J. Environ. Manag. 2021, 293, 112963. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Zhao, X.; Yang, J.; Song, J. Cooling and Energy Saving Potentials of Shade Trees and Urban Lawns in a Desert City. Appl. Energy 2016, 161, 437–444. [Google Scholar] [CrossRef]
- Chollet, S.; Brabant, C.; Tessier, S.; Jung, V. From Urban Lawns to Urban Meadows: Reduction of Mowing Frequency Increases Plant Taxonomic, Functional and Phylogenetic Diversity. Landsc. Urban Plan. 2018, 180, 121–124. [Google Scholar] [CrossRef]
- Gillner, S.; Vogt, J.; Tharang, A.; Dettmann, S.; Roloff, A. Role of Street Trees in Mitigating Effects of Heat and Drought at Highly Sealed Urban Sites. Landsc. Urban Plan. 2015, 143, 33–42. [Google Scholar] [CrossRef]
- Hedblom, M.; Lindberg, F.; Vogel, E.; Wissman, J.; Ahrné, K. Estimating Urban Lawn Cover in Space and Time: Case Studies in Three Swedish Cities. Urban Ecosyst. 2017, 20, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.M.; Neill, C.; Groffman, P.M.; Avolio, M.; Bettez, N.; Cavender-Bares, J.; Chowdhury, R.R.; Darling, L.; Grove, J.M.; Hall, S.; et al. Continental-Scale Homogenization of Residential Lawn Plant Communities. Landsc. Urban Plan. 2017, 165, 54–63. [Google Scholar] [CrossRef]
- Norton, B.A.; Bending, G.; Clark, R.; Corstanje, R.; Dunnett, N.; Evans, K.L.; Grafius, D.; Gravestock, E.; Grice, S.M.; Harris, J.; et al. Urban Meadows as an Alternative to Short Mown Grassland: Effects of Composition and Height on Biodiversity. Ecol. Appl. 2019, 29, e01946. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Ignatieva, M.; Larsson, A.; Zhang, S.; Ni, N. Public Perceptions and Preferences Regarding Lawns and Their Alternatives in China: A Case Study of Xi’an. Urban For. Urban Green. 2019, 46, 126478. [Google Scholar] [CrossRef]
- Ramer, H.; Nelson, K.C. Applying ‘action situation’ Concepts to Public Land managers’ Perceptions of Flowering Bee Lawns in Urban Parks. Urban For. Urban Green. 2020, 53, 126711. [Google Scholar] [CrossRef]
- Hugie, K.L.; Watkins, E. Performance of Low-Input Turfgrass Species As Affected by Mowing and Nitrogen Fertilization in Minnesota. HortScience 2016, 51, 1278–1286. [Google Scholar] [CrossRef] [Green Version]
- Lerman, S.; Milam, J. Bee Fauna and Floral Abundance Within Lawn-Dominated Suburban Yards in Springfield, MA. Ann. Entomol. Soc. Am. 2016, 109, 713–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronson, M.F.J.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the City: Key Challenges for Urban Green Space Management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.J.; Carignan-Guillemette, L.; Turcotte, C.; Maire, V.; Proulx, R. Ecological and Economic Benefits of low-intensity Urban Lawn Management. J. Appl. Ecol. 2019, 57, 436–446. [Google Scholar] [CrossRef]
- Lampinen, J.; Tuomi, M.; Fischer, L.K.; Neuenkamp, L.; Alday, J.G.; Bucharova, A.; Cancellieri, L.; Casado-Arzuaga, I.; Čeplová, N.; Cerveró, L.; et al. Acceptance of Near-Natural Greenspace Management Relates to Ecological and Socio-Cultural Assigned Values Among European Urbanites. Basic Appl. Ecol. 2021, 50, 119–131. [Google Scholar] [CrossRef]
- Lotfata, A. Using Remote Sensing in Monitoring the Urban Green Spaces: A Case Study in Qorveh, Iran. Eur. J. Environ. Earth Sci. 2021, 2, 11–15. [Google Scholar] [CrossRef]
- Sikorska, D.; Ciężkowski, W.; Babańczyk, P.; Chormański, J.; Sikorski, P. Intended Wilderness as a Nature-Based Solution: Status, Identification and Management of Urban Spontaneous Vegetation in Cities. Urban For. Urban Green. 2021, 62, 127155. [Google Scholar] [CrossRef]
- Wang, J.; Rich, P.M.; Price, K.P.; Kettle, W.D. Relations Between NDVI and Tree Productivity in the Central Great Plains. Int. J. Remote Sens. 2004, 25, 3127–3138. [Google Scholar] [CrossRef]
- Fernandez-Anez, N.; Krasovskiy, A.; Müller, M.; Vacik, H.; Baetens, J.; Hukić, E.; Solomun, M.K.; Atanassova, I.; Glushkova, M.; Bogunović, I.; et al. Current Wildland Fire Patterns and Challenges in Europe: A Synthesis of National Perspectives. Air Soil Water Res. 2021, 14, 1–19. [Google Scholar] [CrossRef]
- Lazarus, B.E.; Germino, M.J.; Brabec, M.; Peterson, L.; Walker, R.N.; Moser, A. Post-Fire Management-Scale Trials of Bacterial Soil Amendment MB906 Show Inconsistent Control of Invasive Annual Grasses. Rangel. Ecol. Manag. 2020, 73, 741–748. [Google Scholar] [CrossRef]
- Campos, I.; Abrantes, N. Forest Fires as Drivers of Contamination of Polycyclic Aromatic Hydrocarbons to the Terrestrial and Aquatic Ecosystems. Curr. Opin. Environ. Sci. Heal. 2021, 24, 100293. [Google Scholar] [CrossRef]
- Ubysz, B.; Szczygieł, R. A Study on the Natural and Social Causes of Forest Fires in Poland. For. Ecol. Manag. 2006, 234, S13. [Google Scholar] [CrossRef]
- Piwnicki, J.; Szczygieł, R.; Ubysz, B.; Kwiatkowski, M. Economic Analysis of the Functioning of the Forest Fire Protection System in Poland. For. Ecol. Manag. 2006, 234, S209. [Google Scholar] [CrossRef]
- Marcisz, K.; Lamentowicz, M.; Galka, M.; Colombaroli, D.; Adolf, C.; Tinner, W. Responses of Vegetation and Testate Amoeba Trait Composition to Fire Disturbances in and Around a Bog in Central European Lowlands (northern Poland). Quat. Sci. Rev. 2019, 208, 129–139. [Google Scholar] [CrossRef]
- Denník, N. Mapy Zelene Miest Slovenska: Objavte Fliačky Zelene v Betónovej Džungli. Available online: https://dennikn.sk/189258/Mapy-Zelene-Miest-Slovenska-Objavte-Fliacky-Zelene-V-Betonovej-dzungli/ (accessed on 10 June 2021). [in Slovak].
- Hikersbay. Available online: http://hikersbay.com/climate-conditions/slovakia/trnava/klimaticke-Podminky-V-trnava.html?Lang=cs (accessed on 10 June 2021). [in Slovak].
- PLADIAS. PLADIAS, 2020: Department of Botany and Zoology Faculty of Science Masaryk University. Database of the Czech Flora and Vegetation. 2018. Available online: https://pladias.cz/En/ (accessed on 10 June 2021).
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and user’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Neary, D.G.; Ryan, K.C.; DeBano, L.F. Wildland Fire in Ecosystems: Effects of Fire on Soils and Water; General Technical Report RMRSGTR-42-Vol.4; USDA, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2005; p. 250. [Google Scholar]
- Socher, S.A.; Prati, D.; Boch, S.; Müller, J.; Klaus, V.H.; Hölzel, N.; Fischer, M. Direct and Productivity-Mediated Indirect Effects of Fertilization, Mowing and Grazing on Grassland Species Richness. J. Ecol. 2012, 100, 1391–1399. [Google Scholar] [CrossRef]
- Salemme, R.K.; Fraterrigo, J.M. Grass Invasion Reduces the Resilience of Tree Regeneration to Fire in the Central Hardwoods Region. For. Ecol. Manag. 2021, 491, 119202. [Google Scholar] [CrossRef]
- D’Antonio, C.; Tunison, J.; Loh, R. Variation in the Impact of Exotic Grass Fueled Fires on Species Composition across an Elevation Gradient in Hawaii. Austral. Ecol. 2000, 25, 507–522. [Google Scholar] [CrossRef]
- Grigulis, K.; Lavorel, S.; Davies, I.D.; Dossantos, A.; Lloret, F.; Vila, M. Landscape-Scale Positive Feedbacks Between Fire and Expansion of the Large Tussock Grass, Ampelodesmos Mauritanica in Catalan Shrublands. Glob. Chang. Biol. 2005, 11, 1042–1053. [Google Scholar] [CrossRef]
- Stavi, I. Wildfires in Grasslands and Shrublands: A Review of Impacts on Vegetation, Soil, Hydrology, and Geomorphology. Water 2019, 11, 1042. [Google Scholar] [CrossRef] [Green Version]
- Bond, W. Fires, Ecological Effects of. In Encyclopedia of Biodiversity; Academic Press: Cambridge, MA, USA, 2001; Volume 2, pp. 745–753. [Google Scholar]
- Miller, R.G.; Tangney, R.; Enright, N.J.; Fontaine, J.B.; Merritt, D.J.; Ooi, M.K.; Ruthrof, K.X.; Miller, B.P. Mechanisms of Fire Seasonality Effects on Plant Populations. Trends Ecol. Evol. 2019, 34, 1104–1117. [Google Scholar] [CrossRef]
- Zaller, J.G.; Frank, T.; Drapela, T. Soil Sand Content Can Alter Effects of Different Taxa of Mycorrhizal Fungi on Plant Biomass Production of Grassland Species. Eur. J. Soil Biol. 2011, 47, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofer, D.; Suter, M.; Buchmann, N.; Lüscher, A. Severe Water Deficit Restricts Biomass Production of Lolium Perenne L. and Trifolium Repens L. And Causes Foliar Nitrogen But Not Carbohydrate Limitation. Plant Soil 2017, 421, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Ringselle, B.; De Cauwer, B.; Salonen, J.; Soukup, J. A Review of Non-Chemical Management of Couch Grass (Elymus repens). Agronomy 2020, 10, 1178. [Google Scholar] [CrossRef]
- Winkler, J.; Koda, E.; Skutnik, Z.; Černý, M.; Adamcová, D.; Podlasek, A.; Vaverková, M.D. Trends in the Succession of Synanthropic Vegetation on a Reclaimed Landfill in Poland. Anthropocene 2021, 35, 100299. [Google Scholar] [CrossRef]
- Dirks, I.; Streit, J.; Meinen, C. Above and Belowground Relative Yield Total of Clover–Ryegrass Mixtures Exceed One in Wet and Dry Years. Agriculture 2021, 11, 206. [Google Scholar] [CrossRef]
- Wilson, M.V.; Clark, D.L. Controlling Invasive Arrhenatherum Elatius and Promoting Native Prairie Grasses through Mowing. Appl. Veg. Sci. 2001, 4, 129–138. [Google Scholar] [CrossRef]
- Pooya, E.S.; Tehranifar, A.; Shoor, M.; Selahvarzi, Y.; Ansari, H. The Use of Native Turf Mixtures to Approach Sustainable Lawn in Urban Landscapes. Urban For. Urban Green. 2013, 12, 532–536. [Google Scholar] [CrossRef]
- Weston, L.A.; Barney, J.N.; DiTommaso, A. A Review of the Biology and Ecology of Three Invasive Perennials in New York State: Japanese Knotweed (Polygonum cuspidatum), Mugwort (Artemisia Vulgaris) and Pale Swallow-Wort (Vincetoxicum Rossicum). Plant Soil 2005, 277, 53–69. [Google Scholar] [CrossRef]
- Sikorska, D.; Sikorski, P.; Archiciński, P.; Chormański, J.; Hopkins, R.J. You Can’t See the Woods for the Trees: Invasive Acer Negundo L. In Urban Riparian Forests Harms Biodiversity and Limits Recreation Activity. Sustainability 2019, 11, 5838. [Google Scholar] [CrossRef] [Green Version]
- Rovná, K.; Ivanišová, E.; Žiarovská, J.; Ferus, P.; Terentjeva, M.; Kowalczewski, P.; Kačániová, M. Characterization of Rosa Canina Fruits Collected in Urban Areas of Slovakia. Genome Size, IPBS Profiles and Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, N.; Sheng, H.; Ip, C.; Yang, L.; Chen, Y.; Sang, Z.; Tadesse, T.; Lim, T.P.Y.; Rajabifard, A.; et al. Urban Drought Challenge to 2030 Sustainable Development Goals. Sci. Total. Environ. 2019, 693, 133536. [Google Scholar] [CrossRef]
- Bo, M.; Mercalli, L.; Pognant, F.; Berro, D.C.; Clerico, M. Urban Air Pollution, Climate Change and Wildfires: The Case Study of an Extended Forest Fire Episode in Northern Italy Favoured by Drought and Warm Weather Conditions. Energy Rep. 2020, 6, 781–786. [Google Scholar] [CrossRef]
- Ganteaume, A.; Barbero, R.; Jappiot, M.; Maillé, E. Understanding Future Changes to Fires in Southern Europe and Their Impacts on the Wildland-Urban Interface. J. Saf. Sci. Resil. 2021, 2, 20–29. [Google Scholar] [CrossRef]
- Calheiros, T.; Nunes, J.P.; Pereira, M. Recent Evolution of Spatial and Temporal Patterns of Burnt Areas and Fire Weather Risk in the Iberian Peninsula. Agric. For. Meteorol. 2020, 287, 107923. [Google Scholar] [CrossRef]
- Banerjee, S.; Das, D.; John, R. Grassland Vegetation and Roads Have Dominant Influence on Decadal-Scale Spatial-Temporal Patterns of Fires in a Species-Rich Protected Terai Habitat in Northeastern India. Agric. For. Meteorol. 2021, 304–305, 108411. [Google Scholar] [CrossRef]
- Calheiros, T.; Pereira, M.; Nunes, J. Assessing Impacts of Future Climate Change on Extreme Fire Weather and Pyro-Regions in Iberian Peninsula. Sci. Total. Environ. 2021, 754, 142233. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, J.; Malovcová, M.; Adamcová, D.; Ogrodnik, P.; Pasternak, G.; Zumr, D.; Kosmala, M.; Koda, E.; Vaverková, M.D. Significance of Urban Vegetation on Lawns Regarding the Risk of Fire. Sustainability 2021, 13, 11027. https://doi.org/10.3390/su131911027
Winkler J, Malovcová M, Adamcová D, Ogrodnik P, Pasternak G, Zumr D, Kosmala M, Koda E, Vaverková MD. Significance of Urban Vegetation on Lawns Regarding the Risk of Fire. Sustainability. 2021; 13(19):11027. https://doi.org/10.3390/su131911027
Chicago/Turabian StyleWinkler, Jan, Monika Malovcová, Dana Adamcová, Paweł Ogrodnik, Grzegorz Pasternak, David Zumr, Marek Kosmala, Eugeniusz Koda, and Magdalena Daria Vaverková. 2021. "Significance of Urban Vegetation on Lawns Regarding the Risk of Fire" Sustainability 13, no. 19: 11027. https://doi.org/10.3390/su131911027
APA StyleWinkler, J., Malovcová, M., Adamcová, D., Ogrodnik, P., Pasternak, G., Zumr, D., Kosmala, M., Koda, E., & Vaverková, M. D. (2021). Significance of Urban Vegetation on Lawns Regarding the Risk of Fire. Sustainability, 13(19), 11027. https://doi.org/10.3390/su131911027










