Abstract
The purpose of this article was to identify significant differences in the hunting management process in Poland and selected European countries in the context of their impact on the preservation of biodiversity and the implementation of the idea of sustainable development. The goal was achieved through the analysis of hunting management in selected European countries through the prism of the assumptions made by Aldo Leopold in 1933. Based on the analysis carried out, it was found that hunting management in relation to Leopold’s postulates has best been undertaken by France. Moreover, the wild game management process should be actively implemented and based on the still up-to-date, universal postulates of Leopold, which can be treated as a model approach.
1. Introduction
As defined in the Oxford Advanced Learner’s Dictionary 7th edition dictionary, hunting is chasing, catching wild game or birds to kill them, for specific benefits such as gaining food, sports entertainment, and selling for money [1]. The reference to this definition is the starting point for the considerations undertaken in this work. This dictionary was chosen because of its popularity not only in Poland, but also worldwide. The definition in the Oxford dictionary is undoubtedly compatible with the currently prevailing beliefs of consumers. A critical analysis of the literature shows that the concept of hunting is defined differently in different countries [2], which would make it challenging to compare identical sounding concepts that actually differ in scope. For example, in Poland, hunting includes both those elements identified in the Oxford Advanced Learner’s Dictionary 7th edition dictionary and activities related to keeping the population in the best possible condition and health [3]. For this work, we will limit ourselves to the activities described in the cited definition.
Leopold defined the wildlife management process as an activity that allows for the economical use of the potential of wild animals. An accurate definition of wildlife management indicates that it is an art to produce a given area, each year, a certain number of animals intended to use functional (e.g., meat, antlers, skin). At the same time, it should be emphasised that the very act of hunting (killing) cannot be perceived as a pleasure [4]. The approach proposed by Aldo Leopold was the paradigm adopted in this work. The concept of hunting management, understood as the given above-quoted definition, was formulated in 1933 by Aldo Leopold and identifies the need to undertake game management measures aimed at restoring the wildlife population.
Although this definition was formulated almost a hundred years ago, it is still valid. Therefore, it should be constituted as a reference point in considerations regarding the identification of differences in hunting management in different countries. The approach to hunting management proposed by Aldo Leopold seems to be more proper than the popular but general concept of management formulated by Griffin [5], which states that management should be perceived as a particular set of activities aimed at achieving a specific goal related to the interest of a given subject of management, carried out in the following sequence: planning (selecting goals and methods of achieving them as well as specifying relevant tasks and deadlines for their implementation); organising (allocating and providing resources necessary for the implementation of planned activities, in a way that guarantees effectiveness and efficiency of management); leadership (managing and motivating cooperation during the implementation of tasks); and controlling (constant monitoring of progress and making corrective decisions). This canon of management described by Griffin [5] can be a good tool in the comparative assessment of evolutionary changes in hunting management in a specific area, but from a broad time perspective, which was reflected in the already published work [6]. Nevertheless, Aldo Leopold’s approach seems more appropriate, assuming that the comparative analysis presented in this work will cover a narrow time-space but a vast territorial space. It aims to assess the rationality of solutions to organisational and legal functioning in different countries in the context of concepts of sustainable development, emphasising sustainable consumption.
The annual increase in the wild animal population in a given area is independent of man. Therefore, it cannot be considered as a management feature until man controls at least one or more factors. This control should be aimed at increasing the productivity of the game, perceived primarily through the prism of meatiness. Hunting management has been defined as a deliberate process, invariably adapted to the dynamically changing situation and has been systematically improved. The dynamically changing situation of game animals in various European countries including Poland has prompted the promotion of the concept of active management in hunting, which may constitute the basis for social acceptance and long-term, effective protection of wild animals, as pointed out by Gula et al. [7].
Undoubtedly, hunting in Poland has a very long tradition. Hunting laws in Poland are regulated by relevant laws and regulations. However, assuming that, just as achieving perfection is impossible, striving for perfection is necessary. For improvement, every time it is appropriate, in the field of hunting in Poland, it is necessary and justified to take actions aimed at eliminating the shortcomings of the current system or improving its functioning.
Managing wildlife populations varies greatly depending on the country, climatic zone, or even broadly understood tradition. The use of the hunting management paradigm presented by Leopold allows us to demonstrate: (1) diversity of legal regulations related to hunting management; (2) differentiation in the advancement of the management process in different European countries; and (3) diversity of hunting traditions in different countries. For example, France, with a high percentage of hunters, has a highly sophisticated wildlife population management system. Almost all factors constituting the pillars of Leopold’s concept have been regulated. Corrective measures covered factors that were not fully regulated.
An example may be the census of the game using various methods, which leads to the selection of the most appropriate method [8]. In Belgium, on the other hand, the management of wild animal populations is organised in a more chaotic manner. At the same time, Belgium is not characterised by an abundant wild animal population. Furthermore, in Belgium, the percentage of hunters is also low [9]. A detailed analysis of this area is discussed later in the article.
At this point, it should be emphasised that it is undoubtedly not only the management of wild animals that affects the image of the hunter and the meat obtained by them but also many other factors described in the article by Niewiadomska et al. [10].
The concept of hunting management proposed by Aldo Leopold should be further developed and subjected to further refinement, as shown by the experience of the last 90 years. Specific deficits in the hunting management process in different countries result from the general nature of the assumptions of Leopold’s concept, which did not take the form of a specific procedure. Examples of such deficits are: (1) hunting dates, which are not determined by animal welfare and the quality of the obtained meat [11,12]; (2) too restrictive control of the abundance of predators, which led to the abundance of wild animals [13]; (3) inadequate (excessive or insufficient) protection of wild game conditioned by the extreme diversity of the areas used for its implementation in Europe [8,14]; (4) wild game population size assessment, which is the biggest problem in the hunting management process due to the lack of a specific and uniform method for all countries [15]; and (5) lack of proper care for the environmental conditions of the game in terms of regulating the control of complementary feeding, ensuring access to water, and controlling disease factors [3,15].
Summing up, hunting management in the selected European countries is carried out in very different ways. Therefore, further research and a critical analysis of the effects of the solutions adopted are necessary. Above all, more tremendous efforts should be made by lawmakers, foresters, and hunters, and society as a whole to achieve the welfare of the game.
Hence, the purpose of this article is to identify significant differences in the hunting management process in Poland and selected European countries in the context of their impact on the preservation of biodiversity and the implementation of the idea of sustainable development.
2. Materials and Methods
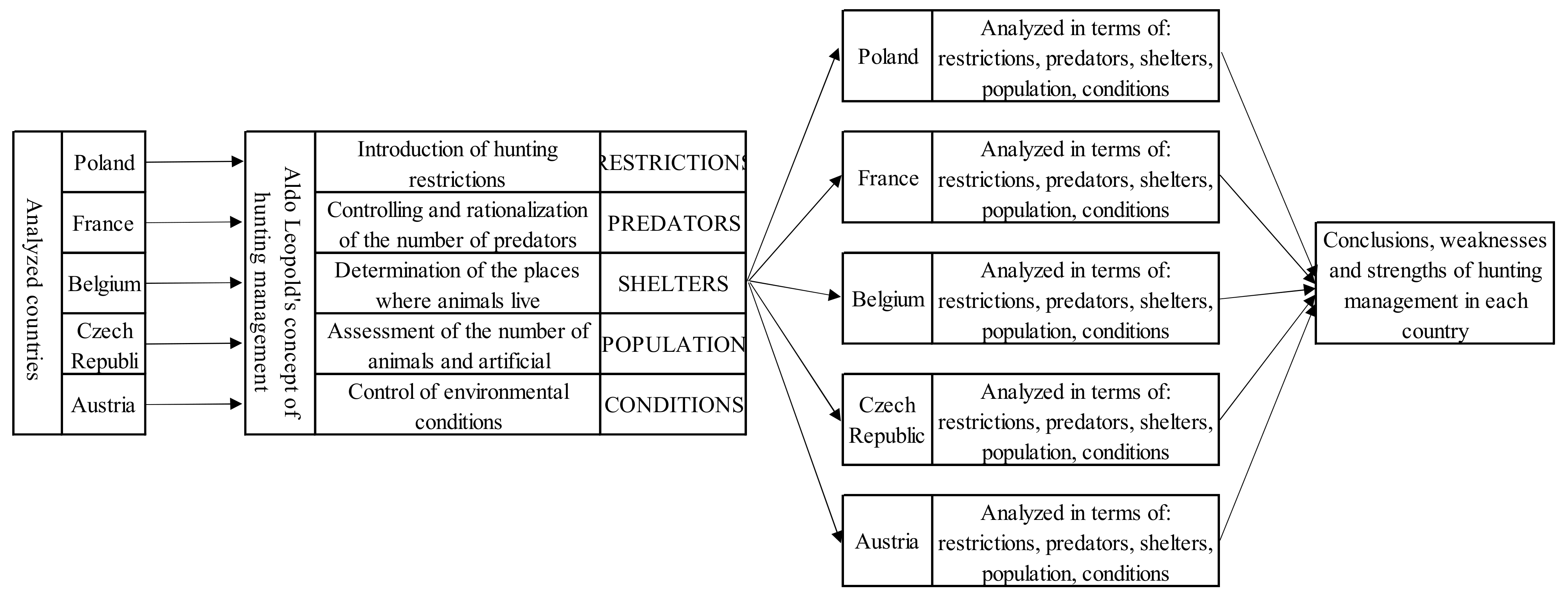
The paradigm that is the starting point for the analysis presented in this work is the management concept proposed by Aldo Leopold. This researcher found that the benefits of wild game harvesting decreased as the number of controlled environmental factors increased. It should be emphasised that in order to achieve the set goal, not only the number of controlled factors is essential, but also the order in which they are taken. This list, in the initial period of introducing this concept, included [4]:
- Introduction of hunting restrictions;
- Controlling and possible rationalisation of the number of predators, determination of the places of the existence of the game;
- Determination of the places where animals live;
- Assessment of the number of animals and artificial replenishment of the population;
- Control of environmental conditions (i.e., the quantity and quality of food, the possibility of shelter, the presence of diseases in the population).
The factors indicated by Leopold are so universal that each of them should be a critical element in the process of wildlife management, regardless of where the process takes place. Since the fundamental spatial and organisational units are countries that differ, to a greater or lesser degree, in terms of the population of wild animals, the environment, climate, and, consequently, the related differences in the course of the life cycle of animals, their implementation will result in significant differences in the provisions constituting the basis for legal regulations in force in individual countries. For this reason, the provisions existing in individual countries should not be compared directly, but analysed through the prism of the implementation of the factors identified by the pioneer of hunting management, Aldo Leopold.
According to the methodology described by Leopold, hunting management almost always starts with the control of five factors related to the wild animal population. Other factors and regulations not indicated by Leopold result from the specificity of individual countries. The factors and their order proposed by Leopold will be the basis for discussing the differences in wildlife management in Poland and other European countries in this article.
2.1. Study Area
This task was carried out as a result of comparing how the regulations in force in selected European countries allowed for hunting management, which should be understood as striving to make rational decisions based on up-to-date and reliable information.
Hence, this article aimed to critically analyse the solutions in force in the Polish hunting management system in recent years with the solutions in Austria, Belgium, the Czech Republic, and France, aimed at optimising the existing legislative solutions in Poland. The assumption was made that legal regulations allowing for the control of significant areas determining the implementation of wildlife management will allow both the preservation of biodiversity and sustainable development expressed in sustainable consumption. The selection of the countries indicated for the comparative analysis was not accidental. Each of these countries has struggled with different hunting problems over the last century, and in each of them, different solutions have been introduced [8,14,15,16].
The hunting laws currently in effect in Poland entered into force on 13 October 1995, and has been successively amended. This Act defines the concept of hunting and the goals of its individual activities, ownership of wild game, a list of animal species occurring in Poland, the method of obtaining the right to hunt, the organisational structure of Polish hunting, obligations in the field of hunting management (i.e., preparation of annual hunting plans, annual inventory of the game, rules for reducing and sanitary shooting, methods of obtaining game in the hunting ground, the obligation to mark the carcasses and evaluate the hunting, methods of game protection, methods of predator population control, the law on breeding hunting dogs, and feeding the game). Moreover, the Act sets out the conditions under which economic activity in the field of hunting may be conducted (i.e., under which conditions tourist services including hunting may be provided). In Poland, specific damage caused by game are recorded each year, and the method of compensation (e.g., in agriculture) is also strictly defined in the Act. The Polish Hunting Association (PHA), which is the foremost unit in the control of polish hunting, right after the Minister responsible for the environment, is also regulated in the cited Act. It should be emphasised that information management at the national level is inaccurate because the latest version of the Act [3] shows that hunting is the Minister’s responsibility for the environment. All activities and bodies (i.e., hunting clubs, financing methods, disciplinary liability, hunting offenses, and penalties applied to the PHA) are specified in the Act. The Act also regulates the scope of activities of the State Hunting Guard and other vital issues.
The Czech Republic is the only country that has direct borders with Poland. At the same time, Poland lies in a warm temperate climate zone, similar to France, Belgium, the Czech Republic, and Austria. However, the temperature amplitude and biotopes, and thus the living conditions of animals, are slightly different. These factors determine the possibility of the occurrence of various species of wild animals in these areas. It should be emphasised that a moderately warm climate zone may have three types of climate: continental, maritime, and transitional, which will determine the existence of noticeable differences.
Another factor determining this choice of countries was the data on the number of hunters per 1000 inhabitants, conducted by Statista in 2018. The values of this indicator were as follows: Belgium 2.0; Poland 2.8; the Czech Republic 10.3; Austria 13.4; and France 19.7. Therefore, the comparison of these values does not make it possible to state unequivocally whether the popularity of hunting is related to the size of the country [9].
2.2. Methodology
The research methodology is presented by the authors in the form of a conceptual map (Figure 1).
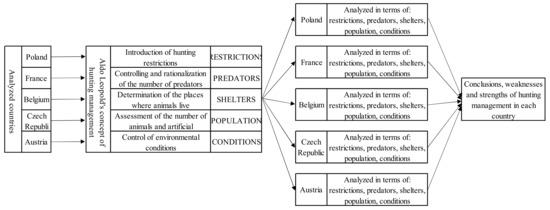
Figure 1.
Conceptual map of the research methodology. Source: Own study.
3. Results and Discussion
3.1. Restriction of Hunting
Pursuant to Leopold’s postulate, game management rules must be defined based on the hunting control regulations to limit the acquisition of game of a given species in a given area within a specified time. These activities are aimed at preventing the possibility of exceeding the production capacity of this area. As the productive capacity of the area may increase as a result of both hunting and other wildlife control, it may be acceptable over time to gradually increase the amount of game harvested. This indicates that the concept of management in hunting should be based on active management, which has been postulated in numerous publications by Okarma [7,17,18,19].
Increasing the game collection may also become necessary under certain circumstances to adapt the size of the game population to the size of forest land or crops. Such action becomes justified in the context of the need to limit the damage caused by wild game. In addition, increased harvesting may be necessary due to the need to regulate the species and distribution of animals to optimize and prevent the extermination of individual species. Hence, legislation should also extend to private landlords if the property is associated with the right to hunt. In the countries analysed in this article, this situation is regulated differently.
Leopold ([4], Chapter IX) defined the three most basic premises for hunting control as:
- Determination of the time, place, purpose, and volume of harvesting, the permitted hunting method, the number of hunters in the area, and the harvested species; these regulations should be formulated in the form of internal rules of hunting associations or legal regulations in the state;
- Creating incentives (e.g., material or fiscal) for the hunter, landowner, or landowner to reduce hunting by voluntarily applying self-interest restraint; and
- The synergy of both previously described solutions as a third source of control resulting from both the applicable legal regulations and individual ethics or the hunter’s attitude.
When analysing the above indications, it should be stated that in Poland, they are reflected in the provisions of the Act on Hunting Law of 13 October 1995 [3], and have been additionally developed in the following documents:
- Specification of hunting periods for game animals, Journal of Laws 2005.48.459 [20];
- Hunting authorisation, Journal of Laws 2010.3.19 [21]; and
- Detailed conditions for hunting and carcass marking, Journal of Laws 2005.61.548 [22].
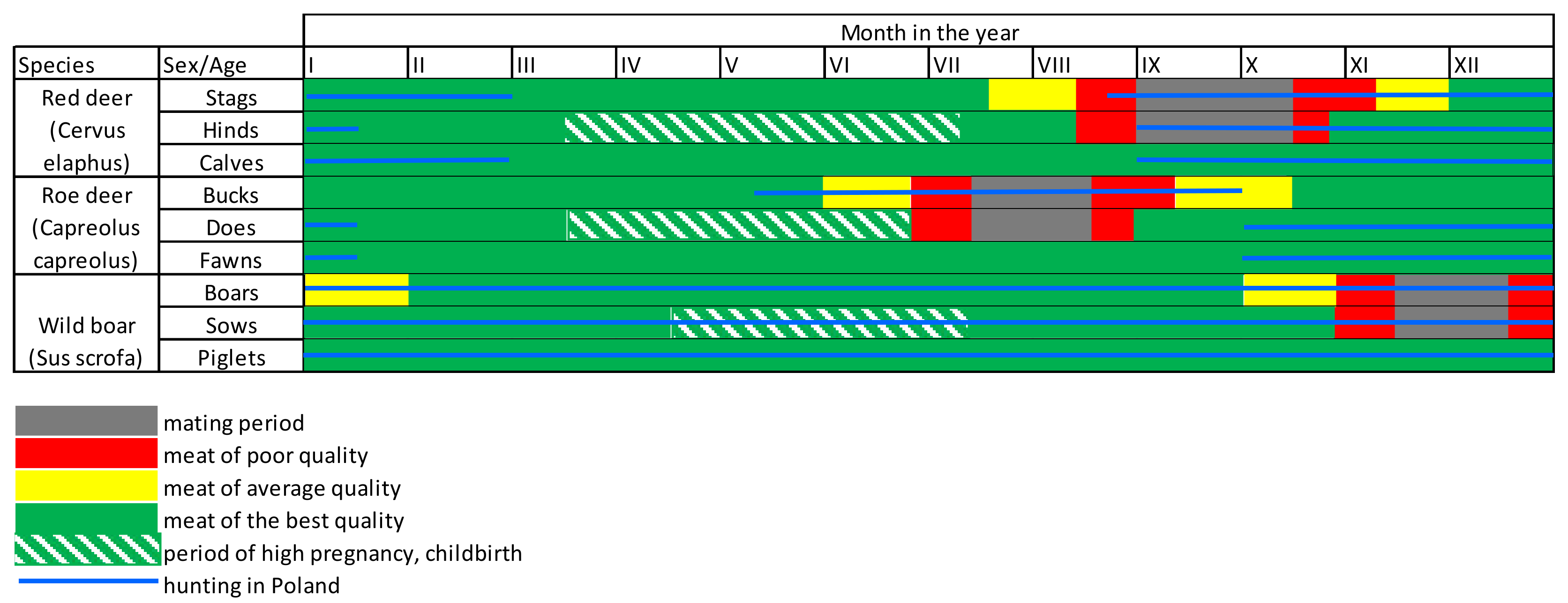
The hunting schedule in force in Poland is based, in opinion of the authors, mainly on the mating season of the game. The hunting season focuses on excluding hunting when young are being raised and in the difficult (for animals) time at the end of winter. The mating season should be excluded because the quality of the game meat is worse during that time.
The Polish system of determining the hunting schedule does not result from official decisions, but is based on a premise resulting from the specificity of game reproduction. The data presented in Table 1 shows that this system should be considered highly imperfect as it is based on only one element. At this point, the question arises as to why a rational determination of the hunting schedule is so important.

Table 1.
Schedule of hunting for selected species of big game in Poland.
Depending on the period in which the meat is obtained, it will be characterised by a different level of sensory quality conditioned by its chemical composition. For example, meat obtained from males in the second half of the rut is characterised by a meagre amount of fat and may have a highly unappetising smell due to the presence of a large number of hormones at that time. In turn, the male accumulates fat immediately before the mating season (perioral, peri-muscular, intramuscular), which also has a negative impact on the quality of the obtained meat. Changes in the composition of game meat depending on the season have been widely described in the literature [24,25,26,27].
The red deer hunting period begins almost simultaneously with the roaring season. At this point, it should be noted that these dates are indicative, and in favourable weather conditions, females’ oestrus may begin a little sooner. The most rationally regulated schedule for roe deer hunting is that the hunting season begins in May and ends in September. The mating season takes place at the turn of July and August. Therefore, at the beginning of the hunting season, goat meat has the desired quality, which changes over time (Table 1, Figure 2). It should be emphasised that the product obtained from the same species of animal, in the same hunting season and in the same area may radically differ.
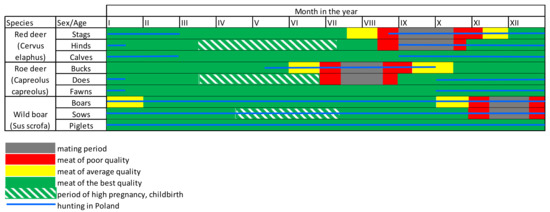
Figure 2.
Chart showing the hunting season and meat quality of selected species of large game during the year in Poland. Source: Own study.
A modern consumer following trends, looking for healthy and lean food (so-called “fit”) can reach for venison, as encouraged by its pro-health properties and low-fat content [28]. At the same time, the consumer may be satisfied or highly disappointed with the sensory quality of the game. A meat of low sensory quality certainly has not gained acceptance among consumers, as reflected in the extremely low level of its consumption in Poland (0.08 kg/person/year) [29]. Therefore, other measures can be taken to develop it. One way is to export and sell this meat at a low price for processing [30,31,32]. However, it should be emphasised that exports also force the necessity to preserve the meat by freezing it. In combination with transport, this process contributes to the burden on the natural environment, which makes wild animal meat a product that does not fit in with the concept of sustainable production [33]. This is why it is rational and purposeful to correctly set a hunting schedule that allows for meat of the sensory quality desired by the consumer to be obtained, which will allow a reduction in the unnecessary environmental pollution. This approach is fundamental as venison is naturally produced meat.
In the context of the initial identification of reservations related to the organisation and management of hunting in Poland, the characteristics of these activities in selected European countries were made.
The hunting season in Austria is determined based on different criteria than in Poland. First, the game division distinguishes a broader spectrum of game including hind without calves, hind with calves, does with fawns, or yearlings. Table 2 shows the hunting schedule for selected big game in Austria.

Table 2.
Schedule of hunting for selected species of big game in Austria.
Second, the hunting period in Austria for roe deer does, for example, is longer, almost 1.5 months, than that in Poland.
Third, it should be emphasised that the data for Austria were generalised as each of the nine provinces of this country is free to set the rules governing the hunting management process [35].
In Poland, the entire hunting legislation is supervised by the government and the Polish Hunting Association, so the situation is more stabilised.
The main content of the regulations in force in all Austrian provinces covers defined species of game that can be hunted, the hunting seasons, the compensation procedure, and possibly creating nature protection areas. The right to hunt directly related to the ownership of land, on one hand, allows hunters to create an emotional bond with the area. On the other hand, it may lead to excessive exploitation resulting from the ownership and associated rights or the desire to earn money by renting hunting grounds to other hunters [36]. Therefore, theoretically, all the requirements according to Leopold’s classification are met, but to a minimal extent.
In Belgium, the situation is very different to that in Poland because the hunting schedule is defined for the entire country, or separately for two regions: Flanders and Wallonia. Table 3 shows the hunting schedule for selected big game in Belgium. The hunting period depends on the hunting method (pulpit hunting, tracking, hunting with dogs) and the age, sex, and sometimes even size of the animal.

Table 3.
Schedule of hunting for selected species of big game in Belgium.
In Belgium, hunting is regulated regionally (i.e., both of these regions have their hunting organisations, rights, and hunting seasons). There is no overarching legislation at the national level, but these systems are still relatively similar due to the country’s relatively recent regional division. In Wallonia, large game management is focused on red deer, as hunting plans are large in numbers. In Flanders, the most significant emphasis is on obtaining roe deer. In Wallonia, a hunting license is subject to membership of a specific game management unit. There are no professional foresters in Flanders, and private hunters are associated with game management units covering the area of a min. 1000 ha (5000 ha in Wallonia). The forest administration must approve shooting plans in both regions [16]. Despite many simplifications and the lack of professional hunters, Belgium is very good at managing their wild game, according to Leopold’s concept.
In the Czech Republic, hunting laws have been in effect since 2001, almost unchanged. Hunting periods are determined by the species of animal, practically regardless of age and sex. Table 4 shows the hunting schedule for selected big game in Czech Republic.

Table 4.
Schedule of hunting for selected species of big game in the Czech Republic.
The most excellent differentiation in the Polish and Czech schedules is visible in the case of wild boars. Piglets have been hunted in the Czech Republic for as long as they have been in Poland. In the Czech Republic, the so-called Game Management Act was issued in 2003, establishing game management objectives at the state level (e.g., maintaining numbers, preventing damage, maintaining genetic purity, and quality of game). Wild game management in the Czech Republic is carried out on three levels, with much of it delegated to regional authorities within each of the 14 individual administrative districts. The shooting of game may only occur in recognised hunting areas, which local authorities determine. Within these areas, landowners can set up a hunting guild if they themselves or with their neighbours have at least 500 ha of continuous land and thus have the right to establish a fishery. As part of a guild, similar to Austria, the landowner can hunt for their own use or rent the land. In Poland, there is no relationship between land ownership and the right to hunt as everything is determined by the hunting clubs and the parent organisation—PHA.
Additionally, apart from a hunting permit and firearms, in the Czech Republic, people also need to have insurance to hunt [40]. To sum up, the Czech Republic lacks many detailed regulations that exist in Polish legislation. However, a three-tier hunting management system in a country as small as the Czech Republic could have both positive and negative effects.
In France, hunting dates and species that can be hunted are defined at the provincial level. In France, there are four biogeographical regions: Atlantic, Continental, Alpine, and the Mediterranean. Table 5 shows the hunting schedule for selected big game in France.

Table 5.
Schedule of hunting for selected species of big game in France.
In France, only the dates and methods of hunting are regulated. Each province has a private association, the Department Hunters Federation (FDC), dedicated to improving hunting conditions as well as conserving and managing wildlife and their habitats. The right to hunt is related to the ownership of the land. Therefore, a hunter who does not own the land must either lease it or be a member of an Approved Communal Hunting Association (ACCA). Another system (covering 1/3 of the country) requires hunters to join one association from the municipality, which theoretically covers all areas belonging to the municipality, which means that legally, all landowners are ACCA members. The hunting laws in France evolve practically all the time, and this is a manifestation of active management. For example, the law adopted on 24 July 2019 established the French Biodiversity Authority, modified the federation’s missions, and strengthened the environmental police [41]. The French government considers hunting activities very broadly and continues to improve them. When assessing the French actions through the prism of Leopold’s concept, it should be concluded that the adopted solutions meet the conditions he specified.
Hunting in selected countries is very diverse in acquiring hunting rights and their relationship to land ownership, which is very popular outside of Poland. An essential element of hunting regulation is the rational definition of hunting seasons by considering animal welfare, animal peace during the mating season, quality of the obtained meat, sex, age, and physiological condition of the animals. The necessity of having a hunting license is also a frequent element of hunting regulations. Polish legislation could benefit significantly from analysing and implementing some aspects of the legal regulations in force in other countries. However, the most important change that would be worth introducing in Polish legislation is making hunting independent of the mating season.
3.2. Predator Control
Predator control should be considered as the second most important issue after hunting restrictions. Leopold [4] characterised four groups of people who had the most extreme views on the control of predator numbers: tourists (+), hunters and foresters (+), natural history students (−), and the fur industry (−). Nevertheless, this division is likely to be somewhat different today.
In addition, Leopold [4] stated that the knowledge of modern man on the relationship between the coexistence of different species of wild animals in a given area, regardless of whether they are predatory animals or not, is incomplete. Hence, the interpretation of trophic relationships occurring in the population of wild animals is flawed.
According to Leopold [4], the person managing the fishery, guided by complete and up-to-date knowledge, must take care of a predator population control policy to ensure the welfare of the rest of the game. This approach, leading to the eradication of predatory animal species, has led to the situation today. The control of the number of predators has become necessary, not for their extermination, but their reintroduction.
The excessive number of predators may lead to the extermination of non-predatory animals threatened with extinction in a given region. This is due to the fact that “wild food” (e.g., roe deer for wolves), is more attractive to wolves than livestock [42].
However, in his considerations, Leopold [4] primarily discussed the losses of the game caused by predators and the determinants of this state (i.e., the annual mortality of a given species in a given area) directly related to predators. Leopold characterised five main factors: (1) game population density and condition; (2) predator population density; (3) predators’ food preferences; (4) physical fitness of game and possible escape; and (5) the abundance of alternative sources of food for predators. The standards for the measurement of game losses caused by predators are regulated as follows: (1) the number of animals killed directly by one predator at a given time; (2) total collection of the game by a given predatory species within a fixed period of time; and (3) the percentage of the game population obtained by a given predatory species in a fixed time. The management activities so far have meant that instead of regulating the populations of predators according to Leopold’s concept, we must now focus on their protection. The necessity of this type of action is demonstrated by the examples described below. These were limited only to Poland and the Czech Republic due to the immediate vicinity and similarity in terms of the current ecosystems.
The grey wolf and brown bear are protected at the European Union level through the so-called Habitats Directive No. 92/43/EEC (Annex II and IV) [43]. These species are an essential element of the provisions of the Bern Convention (Annex II) [44]. In the territory of the Czech Republic, they are protected in the Beskids area. According to Czech legislation, the bear is a specially protected species and highly threatened with extinction [45]. Additionally, in Poland, following the Act of 16 April 2004 on nature protection [46] and the Regulation of the Minister of the Environment of 16 December 2016 on the protection of animal species [47], the brown bear is a strictly protected species requiring active protection. There was also an obligation to create protection zones within 500 m around the lairs from November 1 to April 30.
The threat of a bear as a predator depends on its omnivorous nature, which means that these animals eat both plant food and hunt wild game. Thus, including the bear among the protected species means that it could possibly be perceived by some people as potential competition when acquiring game such as roe deer. Thus, episodes of poaching may occur in an improperly conducted harvest management and forest management process because people with limited knowledge, convinced that bears eat only wild ungulates, could surmise that the bears are taking away their potential trophies.
In the case of the lynx, it was not covered by complete species protection until 1995. Through the Regulation of the Minister of Environmental Protection, Natural Resources and Forestry on 6 January 1995 regarding the protection of animal species [48], the lynx was entered on the list of strictly protected species. Through a separate Ordinance on 30 January 1995 [49], the lynx was excluded from the list of wild game animals. After updating the Ordinance on wild species under protection in 2004 [50], the lynx is now under strict protection as a species requiring active protection. On 16 December 2016, the Minister of the Environment, regarding the protection of animal species [47], additionally imposed an obligation to designate protection zones to 500 m around the identified breeding sites for lynx that was valid from 1 April to 31 August. The lynx is listed in Annexes II and IV of the Habitats Directive [48] and Annex III of the Bern Convention [44]. It has been assigned to the NT threat category in the Polish Red Book—a lower risk species, but close to threat. In the Czech Republic, the lynx is protected in a unique habitat area of Beskydy, Šumava, Blansky les, and Boletice.
The size of the wolf population in eastern Poland in the 20th century ranged from 0 to 9.1 individuals per 100 km2. The last time-intensive fight against wolves took place in Poland from 1955 to 1975, and it almost ended with the extermination of this species. In 1975, when restrictions related to hunting wolves were introduced, the estimated number of this species in Poland was 100 individuals distributed in the north-eastern and south-eastern regions of the country. As a result of protective measures, by 2018, the number of wolves on the western side of the Vistula increased to about 800–1000 individuals, and the IUCN changed its status from CE (critically endangered) to VU (vulnerable) [7]. According to the official data presented by the Chief Inspectorate for Environmental Protection, the size of the wolf population varies considerably across the country. It is estimated that on average it is from 1.5 to 6 individuals per 100 km2. Thus, it can be seen that the wolf population in the western territories is lower than in the eastern territories, since the average quoted earlier was so lowered after adding western Poland [51].
The work of Gula et al. [7] indicated that in order to manage the wolf population actively, it is necessary to have up-to-date information, particularly, in terms of the wolf population structure, range, habitats used, and the species’ demographic history. Furthermore, it is also important to systematically follow the process of recolonising the species in the areas covered by management, and in justified cases, to reduce the population size.
Summarising the topic of predator control, it should be stated that wild predatory animals have always been exterminated by humans, which led to the partial or complete extinction of some species. Therefore, it is now necessary to rebuild the population of wild predatory animals and bring the numbers of these species to a stable state that allows for a natural existence. Thus, the control of the number of predators postulated by Leopold should occur through active hunting management, which has been indicated in numerous publications [7,52,53,54].
The first stage of active management must be to include animals that are food for predators in hunting plans by counting the number of game obtained by predators. In addition, wild game plans for the area should be reduced by the amount of wild game intended for food by the predators. The second stage should take place only after the predator population size has stabilised. It should consider the sanitary shooting of predators and the possible reduction of too large a population of predators disturbing the ecological balance.
3.3. Reservation of Game Lands as Parks, Forests, Refuges
The game refuge is a closed element of a hunting ground, which is excluded from hunting to create conditions conducive to restoring wild animal populations and their diffusion into neighbouring areas. The adjacent area is understood as a hunting area against which the refuge acts as a game source for the hunting grounds. Another use of these closed habitats is to protect wild game species prohibited or threatened with extinction. The name refuge is most often used because it best describes the fundamental functions of this area across the entire wildlife conservation mechanism. A refuge is an area that only fishery managers have access to. This is due to the recommendations described by Leopold [4], according to which refuges are intended for the intensive restoration of endangered populations, and the presence of people could disturb it.
On the other hand, parks should be understood as game protection or breeding sites and tourist attractions. The function of parks is recreation and education. In a favourable situation, when the number of wild animals increases in the parks, its spontaneous diffusion toward hunting grounds occurs. Consequently, the number of wild game in the hunting grounds is replenished [4].
To sum up, the primary purpose of creating any habitat is to ensure free reproduction of game and its natural release to hunting grounds. However, a significant factor that should be considered is the mobility of animals related to their ability to migrate to hunting areas per day, per month, and/or one year. This approach is designed to control the number of wild animals in the immediate vicinity of the refuge. This approach also points to the need to create protected areas throughout the country, not only selected enclaves but also to maintain biodiversity and support endangered species [55].
The surface and terrain of these protective habitats must be adapted to the game species’ requirements to ensure optimal living conditions. The creation of habitats must be deliberate and result from rational premises for maintaining the welfare of the game. In areas designated as refuges, the occurrence of natural hiding places of a given species of game should be considered, so that the sense of safety of wild game and ensuring its freedom and welfare result not only from eliminating the possibility of hunting and the presence of people [55].
Leopold [4] also considered the cost of a given habitat. This concept covers the cost of maintaining the habitat in one hunting season, the length of the habitat’s useful life under the assumption of constant costs as well as the increase in these costs associated with the time of its use. Such a comprehensive approach to the cost-consumption of the habitat allows for the estimation of the maximum period of habitat use, resulting from the pursuit of economic optimisation of the conservation activities carried out. Refuges protect animals from hunters, help protect against predators, and even against hunger, but unfortunately not against diseases, although the protection of the game may accelerate its recovery.
The situation in Poland in terms of animal shelters is currently quite favourable. Namely, areas where one can hunt are referred to as hunting districts [3]. For the area to be included in the hunting district, it must constitute an area of land with a total area of more than 3000 ha, closed by borders. Additionally, favourable conditions for hunting must be established in its area. Hunting areas can be forests and fields; these are designated throughout the country with some exceptions. The following cannot be counted towards hunting districts:
- National parks and nature reserves (except their parts where hunting is allowed in the conservation or hunting plan);
- Areas within the administrative borders of cities;
- Areas occupied by localities not classified as cities, but including residential and farm buildings with yards, squares, streets, roads; and
- Buildings, plants, devices, and areas intended for specific purposes—social, religious, commercial, transport, storage, industrial—moreover, monuments.
Entering the hunting grounds does not give people the right to hunt. A hunting club of the PHA must lease the circuit, and the amount of harvest included in the annual hunting plans is assigned to a specific hunting area [3].
The allocation of a given area to a hunting circuit is not a final decision. Because the minister responsible for the environment, after consulting the PHA or receiving disturbing messages from the PHA, has the right to exclude certain hunting districts from a lease through a decision and transfer them to the management for a minimum period of 10 years for the GBC (game breeding centres). At the GBC, hunting is a task carried out to a minimal extent, mainly for sanitary purposes, and the tasks are mainly related to [56]:
- Developing the management and conducting an exemplary manner of fisheries;
- Conducting scientific research;
- Restoring populations of disappearing species of wild game;
- Breeding native game species in order to populate fisheries;
- Breeding valuable game in forest biocenoses; and
- Conducting hunting training.
Undoubtedly, the idea of the GBC is part of the creation of habitats described by Leopold [4]. National parks and nature reserves can be viewed similarly. Although they are hunting grounds, they are not included in hunting districts. As of 2021, there are 23 national parks in Poland. Each of them is characterised by a different type of flora and fauna. Therefore, all of them can be considered a specific refuge. However, their area is insufficient as it constitutes 3151.00 km2, and thus approximately 1% of the country’s area [57].
In Poland, 1498 nature reserves have been distinguished, covering approximately 169,200 ha, so 0.54% of the country’s area. For example, 737 objects are forest reserves covering 66,773 ha (0.21% of the country area), and 139 objects are fauna reserves covering 43,037 ha (0.13% of the country area). As in national parks, protection within the reserves is strictly defined based on the species of both fauna and flora typical of the reserve [58].
Protected areas in the Czech Republic are defined by the Nature and Landscape Protection Act [50]. The situation of national parks in the Czech Republic has been stable since 1999. Since then, there have been four national parks there, covering 119,500 ha or 1.5% of the country’s area. There are 26 (14.43% of the country’s area) protected landscape areas in the Czech Republic, and 110 national nature reserves (0.38% of the country’s area). As far as nature reserves are concerned, 817 sites represent 0.54% of the country’s area, and the situation has been stable for many years. The size of protected areas in the Czech Republic is not defined in the regulations. However, it can be concluded that the number of sites for protecting wild animals present in the Czech Republic is sufficient. For example, parks defined by Leopold in the Czech Republic are represented by four sites (1.5% of the country’s area), while the refuge by 26 sites (14.43% of the country’s area) [59].
In Belgium, PAs are represented by one national park, covering 57.5 km2, although there are also several non-human-managed natural parks and a cross-border park between Belgium and Germany. There are also small nature reserves in Belgium. According to Leopold’s concept, refuges should be located in places that ensure the diffusion of wild animals throughout the country. Despite the fact that Belgium is a very small country, for example, 2.5 times smaller than the Czech Republic, the percentage of protected areas cannot be considered sufficient. In the Czech Republic, protected areas cover about 15% of the country’s area, and in Belgium, it is less than 1% [60]. Hence, it can be assumed that the system of organisation of protected areas in Belgium requires elaboration.
There are six national parks in Austria covering 2380.35 km2, the smallest of which is only 13.3 km2 [61]. There are 453 nature reserves in Austria [as of 2011], 48 nature parks with a total area of approximately 500,000 hectares [as of 2013], and 249 landscape protection areas [as of 2011]. Overall, around 24% of Austria’s territory is protected land, which can be considered as a significant proportion compared to Belgium and even the Czech Republic. In national parks, there is a system for managing populations of ungulates, just like in Poland, only in specific places and times, considering the harvesting plan. Austria is perfectly meeting the assumptions made by Leopold [4].
The history of nature conservation in France dates back to the 19th century. In 1861, the first protected area in France was established on an area of 1097 ha. Contemporary French national parks base their activities on the law from 1960, which was amended in 2006. There are currently 11 national parks in France, three of which are located in overseas territories (as of 2019). Parks cover 60,728 km2—9.5% of the territory of France. In France, there is a price list for the illegal harvesting of wild game, but the penalties are much stricter. For example, red deer stag—1700 euro, Corsican deer stag—3200 euro, and wild boar—500 euro [62]. In Poland, there are also penalties for poaching. According to Polish law, poaching is a violation of the hunting law, punishable by up to five years in prison. There are also financial penalties for killing animals. The poacher should pay 900 euro for the illegally harvested elk and deer, for wild boar and roe deer—450 euro, for other game—225 euro. However, these are indicative amounts. Data analysis shows that the French approach to the implementation of Leopold’s third postulate is insufficient, especially in the area of the diversity of land functions. There is also a need to increase the area occupied by protection areas in France, similar to that in Austria.
Summarising the postulate regarding the determination of the habitats of animals, it can be stated that numerous issues require improvement in most of the analysed countries. Protected areas are of great ecological, social, social, cultural, economic, and scientific importance. Therefore, their percentage on the scale of the country’s surface area should be sufficiently high. Austria (25%) is a model for such action. In this context, Poland and Belgium differ unfavourably from other countries (approx. 1%), which should be considered when improving and striving for the active management of hunting.
Providing game shelter is undoubtedly a vital management element. Proper placement and sufficient refuge in terms of size will reduce the need for the artificial reintroduction of game and industrialised farming.
3.4. Artificial Replenishment (e.g., Restocking and Game Farming)
Artificial replenishment of the population of wild animals is necessary in some cases. It consists of systematically checking whether the species requires protection, artificial farm breeding, or reintroduction from another country. Then, depending on the population density of this species in a given area and the introduction of corrective measures, the population is replenished [63].
Leopold [4] described the conditions indicating the need for artificial replenishment of the wild game population, consisting of four successive stages: (1) abundance assessment; (2) evaluation of the efficiency and productivity of game in a given area (compared to standards); (3) diagnosis—assessment of the factors of the animal’s habitat (e.g., the availability of food and water) and then testing the possible remedial measures on a small scale; and (4) ongoing oversight of problematic factors diagnosed in stage 3 on a large scale. Only such a comprehensive approach will ultimately be able to influence the wildlife population.
Determining the abundance of wildlife populations is a highly complex exercise. Leopold [4] distinguished three types of techniques used to assess the size of the population: (1) sampling consisting in recording the spotted individuals in the entire study area or its part (as a sample); (2) determining the percentage of animals with the use of traps or catching individual specimens (samples), marking them, and then releasing them; and (3) indirect observation of the condition of individuals and population density with the use of specific indicators essential for the site manager.
It should be said that neither of these concepts is perfect and practical because in a given place, one animal may appear many times or never, and the overall observation of the area is highly cost-intensive and time-consuming.
In Poland, the number of animals is referred to as inventory and is a requirement laid down in the Hunting Law of 1995 [3]. An inventory (i.e., a count of the number of animals) must be carried out by leaseholders or managers of hunting districts by 10 March of each year. Pursuant to the Act, the number of game animals is to be “estimated”, which means that there are no defined requirements regarding the methodology of carrying out these activities. The inventory is prepared using the form provided in the Act.
Bobek et al. estimated four methods of estimating wild ungulate populations. Results of the assessment of the size of populations showed that low density and/or large size of animal group made estimation difficult and expensive with the use of taxation areas as it requires taking a large number of samples. The total census method reduces the variability of the collected samples. Bobek et al. stated that it requires additional work and calculations related to the method errors. The verification of the results obtained by means of the total census can be performed once using parallel appraisal areas or on the basis of the acquisition and growth of the realised population. The achieved increase in population should be calculated by assessing the size of the population in the taxation areas between two years, assuming that the error of assessment is not higher than ±10% of the number after the end of the hunting season, and its value is equal to the number of individuals obtained in the hunting season between the two compared years [64].
Wild game supervision is a very important element of the management process. Neglect in this regard led to the extinction of the tarpan (Equus gmelini) and the aurochs (Bos primigenius) in Poland [65,66], where “The attractiveness of the wild horse as hunting trophy can be proved by the information on the measures undertaken to protect these animals, similar to protection of the aurochs, which was almost completely killed off in that period” [65]. An equally important aspect is also the problem with the reproduction of these animals. This is why scientists in the country have been creating breeding centres for years, but unfortunately, this has not always been successful. However, such drastic measures have never been taken to destroy the population of the remaining large game living in Poland (i.e., red deer, roe deer, fallow deer, sika deer, elk, wild boar, mouflon). However, some populations of wild animals are definitely less numerous than others, therefore, for example, elks are under year-round protection, and few animals are obtained from mouflons. The assessment of the number of animals is extremely important because its neglect may eventually lead to the extinction of the species. An example of neglect in Poland is wild chamois, which are not included in any registers related to game. Therefore, wild chamois have been considered as wild animals and not game since 1868. According to the Red Book of Endangered and Endangered Animals, the Tatra chamois is an extremely endangered species (CR) in Poland [67].
Sika deer were introduced to Poland in 1895 [68], while fallow-deer as far back as the 13th century [69]. In turn, as a native species, elk have been protected in Poland since 1925 in the Biebrza Marshes, thanks to which it survived the Second World War. As a result of conservation measures in many regions, it was possible to restore the size of individual populations to the level that guaranteed the existence of biodiversity. For example, in the Kampinos Forest, elk were reintroduced in 1951 with several individuals from Belarus.
In the case of the Czech Republic, the situation is entirely different. Up until the 10th century, the distribution of animals was even and related to the density of afforestation. Later, facilities (castles) were built for hunting and managing wild populations. Only in the 20th century, during wars, intense poaching activity, and significant deforestation, did the number and distribution of wild animals change drastically. Currently, there are 11 species of ungulates in the Czech Republic: red deer, roe deer, fallow deer, sika deer, wild boar, mouflon, northern chamois, wild goat, white-tailed mule deer, elk, and Barbara sheep. Only four of them are native to the Czech Republic: roe deer, red deer, elk, and wild boar. The rest were either introduced or ended up there by accident. Examples of game extermination in the Czech Republic [70] are:
- Moose—exterminated in the 14th century and then reintroduced from Poland in the second half of the 20th century. Currently, it is found only in the south of the country, and its population is small;
- Red deer—in the 17th century, it was almost exterminated in the Czech Republic; therefore, to improve the population size, deer kept in parks were released, and subspecies (i.e., Carpathian deer) were introduced. Moreover, the appearance of sika deer, maral, and wapiti caused the loss in the genetic purity of this species in the Czech Republic;
- Roe deer—in the Czech Republic, it has never been seriously affected by human activity. However, attempts have been made to cross native populations with Siberian roe deer in order to improve the quality of the trophy, but to no avail;
- Boar—died out in the wild in the Czech Republic in the 18th century, but it was possible to recolonize it naturally by releasing animals kept in farms;
- European bison—a species out of the Czech Republic.
In accordance with the regulations in force in the Czech Republic [40], a person with the right to hunt is obliged to carry out a census of the number of wild game and all its species occurring in the managed fishery each year. The annual inventory is usually carried out by visual inspection at a specific time (e.g., spring) in a specific area. There are no legal regulations regarding the applicable methodology in this respect. Looking through the prism of Leopold’s guidelines, the Czech pragmatist deviates from the recommendations, and this has resulted in the extermination of many species of wild animals. The counting methods used are insufficient and are likely to be the cause of this.
Belgium is not a country rich in a variety of wild game species. The only wild ungulate species in Flanders are roe deer, besides a few fallow deer, deer, and wild boar. In the Walloon region, however, wild boar, deer, and roe deer populations are widespread, and there are also mouflons, but only in the Semois Valley. Until the end of the 18th century, deer, wild boars, roe deer, and even fallow deer were present in Belgium in high densities, especially in heavily forested areas. At the end of the 18th century, a significant number of wild boars were killed due to the damage they caused to crops. Then, revolutions and wars, as in the case of the Czech Republic, led to the extermination of some animals. After 1830, attempts were made to increase the population of Belgian game. Red deer, fallow deer, and roe deer were reintroduced in several places in the country. Unfortunately, the improvement in the situation did not last long because two consecutive world wars in the 20th century led to deforestation in Belgium and the destruction of part of the game population. After the end of the war, both deer and deer populations were only a fraction of the 18th century. Nevertheless, remedial action after the war led to an improvement in the state of the population [71].
Determining the abundance of animals in Belgium is carried out differently depending on the region. In Flanders, it is obligatory to estimate the size of the roe deer population to justify the planned harvest. However, there are no guidelines on the counting methodology. Attempts were made to impose detailed kilometre indexes, which, however, were not legally regulated. In Wallonia, on the other hand, there is no legal obligation for tenants to count game as the regional forestry administration handles this. However, some tenants either develop abundance estimation methods or use existing ones to complete administrative estimates. The most frequently used methods are counting from hunting pulpits, counting from a car while traversing hunting territory, and tracking game. Hence, the conclusion that the implementation of Leopold’s postulate regarding replenishment of the wild game population in Belgium is necessary. A crucial task in the field is to develop a methodology for counting game [16].
There are currently nine species of ungulates in Austria: roe deer, red deer, northern ibex, wild boar, mouflon, sika deer, ibex, fallow deer, and elk. The moose is a native species of Austria. In the 10th century, it disappeared from its territory, and now has a small population. The natural recolonisation of the moose takes place due to the migration of individuals from the Czech Republic. Fallow deer, in turn, were introduced in Austria in the 15th and 16th centuries. On the other hand, the populations of roe deer, chamois, deer, and wild boar suffered little as a result of legal and illegal hunting activities, as indicated by retrospective data [14].
The abundance of individual wild game species in Austria has not been systematically recorded. Only the results of the annual harvest are collected and reported by the chief hunter in each hunting district. These data are taken as an indicator of changes in the size of individual populations and include the number of animals killed by hunters, road accidents, and other deaths. More direct methods of estimating the abundance of individual species are only used locally. In this case, game tracks or faeces are assessed. However, there are no state registers for data on the abundance of the big game population in Austria.
Consequently, the harvesting plans established are not based on wild animal population estimates. Referring to this approach to Leopold’s recommendations, it can be stated that the method used in Austria for estimating the size of individual populations based on the number of dead individuals does not allow for the correct forecasting of changes in the number of animals. This conclusion is also supported by the fact that various species of wild animals were exterminated in Austria (e.g., elk, European bison) [14].
France is a country with as many as nine species of big game including six native species (red deer [including Corsican deer], roe deer, wild boar, northern chamois, mouflon, and alpine ibex) and three introduced species—fallow deer, sika deer, and Chinese water deer. Chinese water deer and the alpine ibex are species that cannot be hunted [8]. In France, roe deer were never threatened with extinction, but in order to stabilize their population density, translocations were made. On the other hand, red deer almost disappeared from the south of France in the mid-19th century due to intensive deforestation. After World War II, animals were released from protected areas and were even reintroduced from abroad (Germany, Austria, and Hungary) [72]. Wild boars are numerous in France, and problems with their shortage, but rather their excessive occurrence, have not been noted.
Counting the number of individual species of wild animals in France is the most advanced. It is because almost all known methods have been tried (e.g., counting from a hunter [73], tracking, counting at pastures and in baits [74], pointage flash methods, and capture-mark-recapture methods [75]). Despite the efforts made, an underestimation of the number of up to 50% was found. At the same time, it was found that the applied methods of calculating the population size allow for estimating only the minimum size and not the real one. It was also found that underestimating increased with the increase in the number of individual species of wild animals. Due to the fact that the management of the wild animal population cannot be rational if only the minimum size is known, efforts have been made in France to develop new techniques to quantify numbers. For this purpose, the concept of ecological change indicators was used, based on the observation that well-established populations of large mammals have a specific impact on the habitat. When the population density is too high, the deterioration of the population was noted, manifested by unfavourable changes in the individual characteristics of animals (phenotypic quality, reproductive performance), which is related to the availability of natural resources. With an appropriate population density, in relation to the availability of natural resources in a given area, neither deterioration nor improvement in the population’s quality was observed. These indicators to monitor changes in the dynamics of numbers of individual species of wild animals were described as early as 1973 [76]. By analysing changes in population density, from the time of colonisation to the saturation of the area, it can be concluded that there are several indicators helpful in tracking ecological changes (e.g., population size—the kilometre index of animal movement; animal condition—male winter weight and length of the hind leg; productivity population—birth during the year; the intensity of plucking of stands—site visits). Such an approach can be considered a refinement of the third method of assessing the state of the population indicated by Leopold in 1933 (indirect observation of the condition or density of population through the use of indices). Therefore, it can be concluded that France stands out among the countries covered by this analysis in terms of the implementation of Leopold’s fourth postulate. Table 6 summarizes the presence or introductions of selected animal species in the analyzed countries.

Table 6.
Selected species of wild animals occurring in the discussed countries.
Failure to manage wild animal populations may lead to the extermination of native game species. This, in turn, is one of the reasons for reintroduction. At the same time, it should be emphasised that there is no justification for introducing exotic species to a given area. The totality of these activities is usually highly ineffective and cannot be justified by the pursuit of biodiversity. For example, in Poland, fallow deer were introduced instead of maintaining native species such as tarpan and aurochs. To sum up, introductions are not always in the interests of the state, hunters, and fisheries.
Proper assessment of the number of animals and, consequently, the knowledge of the actual condition of individual populations is the basis for their management. The conducted review showed that in most of the countries discussed, there is a requirement to assess the number of wild animals of individual species, but it does not indicate how to do it properly. Therefore, there is a need to refine the methods for this purpose. Otherwise, hunting plans may be overestimated, which may lead to the extermination of certain species of wild animals. In this light, the implementation of the fourth postulate of Leopold [4] requires elaboration of the methodical approach to the assessment of the state of abundance, which would be universally applicable. For this purpose, modern technical solutions can be used (e.g., game chip chipping). Initially, this method would generate significant costs and require the significant involvement of foresters. However, in the long run, it would bring tangible benefits.
In the opinion of the authors, Leopold’s fourth postulate should be considered the most important. Lack of supervision over the population size may result in extermination of the game, and consequently, the remaining demands will lose any meaning. Nevertheless, it should be noted that an improperly conducted hunting management process may also lead to an overpopulation of a given species. Hence, it can be seen how important this aspect is.
3.5. Environmental Controls (e.g., Control of Food, Cover, Special Factors, and Diseases)
The last of the postulates indicated by Leopold [4] regarding the concept of wild game management is the control of environmental factors that directly impact the existence of animals. This postulate includes activities such as feeding the game, providing shelter, protecting against diseases, and meeting any special requirements specific to the species. These factors are intertwined and are strongly correlated with each other. Hence, the necessity of a comprehensive approach to this postulate. Leopold emphasised that problems with the access of food and game hideouts are seasonal rather than all-year-round. Hence, determining the type, size, and distribution of food are most important in seasons that are critical for the game.
Leopold [4] indicated that the knowledge of the physiology of wild game is not complete. At that time, it was only possible to find out what an animal ate, not necessarily how much or why. However, there is now well-established knowledge about the quantitative and qualitative food requirements of wildlife. Therefore, complementary feeding type depends somewhat on the game’s preferences and its specific species characteristics (e.g., specialised gastrointestinal tract).
Wild game food is any matter that will be ingested by the game for the sake of its welfare. Food control covers the amount, type, and quality of food that a given species of animal needs, depending on sex, age, and season in a particular environment. The control of feeding the game should be based on the knowledge of foods consumed by wild game preferences (e.g., different leaves of trees, different shrubs, herbs, etc.). Since plant control is possible only on feeding plots and not in the whole forest, plant selection on these plots must be purposely selected. Therefore, plants should be selected based on such factors such as which crops can be obtained (purchased or grown) without much difficulty during feeding; which is tasty for game; which will ensure that the game meets its physiological needs (growth, lactation, pregnancy); and what the game are used to eating. Food that meets the requirements described above is the most optimal for feeding. It should be emphasised that there is no ideal food. Its diversity is the most important. It should be noted that nowhere is full-year supplementation used, usually only winter feeding [4].
Currently, in the literature, winter feeding of animals has been very widely criticised. Winter feeding does not affect the bodyweight of the game and may cause some problems. The game should obtain food by itself. Game feeding, even justified by striving to avoid damage to the natural environment such as plucking young stands or stripping the bark is inappropriate. Feeding may be counterproductive. Experience shows that game, instead of only feeding itself from feeders, uses them as the fundamental source of food, which makes it largely dependent on human activities [77].
Leopold [4] also described the four most common methods of winter feeding: (1) feeding plots; (2) “self-feeding stations”—feeders with fodder requiring some involvement on the part of the game (e.g., with whole bundles of unprocessed cereals, grains of unhusked cereals, etc.); (3) feeding stations—feeders with feed ready for consumption; and (4) emergency feeding (e.g., feeding the piglets after the sow’s death).
The animals should also have access to water, although not necessarily and not always in the form of a liquid, but also plants with high water content. Providing animals with access to the optimal amount of water should depend on the environmental conditions. Leopold [4] found that providing water for wildlife was not always associated with costs. He illustrated this by saying that game should not be seen only through the prism of its fleshiness. Wild animals are an element of the ecosystem that, under optimal conditions, does not require human resources and resources to ensure their existence. Hence, wild animal meat is actually a by-product of the environment, which is an added value and does not burden it.
In forest ecosystems, there are water reservoirs sufficient to provide animals with an optimal amount of water. Sometimes natural reservoirs may dry up, or are absent in a specific area, which will result in the need to create artificial reservoirs. These, in turn, may positively or negatively affect agriculture, forestry, etc. It is worth noting that in natural conditions, game migrates in order to ensure access to water and more food. Therefore, it is not a problem that requires more attention.
A shelter is defined by Leopold [4] as a hiding place for game that enables its vegetation (preservation of fundamental life processes) or a place allowing for survival, hiding from predators. Thus, a cover can either be a bush or a burrow in the ground. It follows that vegetation is not only food for animals, but also provides the possibility of hiding and shelter. Therefore, appropriate vegetation is one of the most important factors that should be taken care of. However, each biotope is constantly changing. The vegetation cover changes as a result of changes in the soil composition, and thus the habitat of a given species changes (over time). This is the justification for the protection of habitats (refuge), described in Section 3.
Leopold [4] distinguished between the methods of supervision over the number and quality of wild game shelters:
- accelerating plant succession through planting, fencing against game, protection against fire; and
- delaying plant succession by ploughing, burning, grassing game, cutting plants.
These methods can be called natural tools. However, it should be emphasised that the entire supervision process is complex and time-consuming. Leopold [4] also suggests the possibility of creating artificial shelters using brushwood, branches, and tree trunks. However, building artificial shelters, although faster, is ineffective in the long run.
The cover allows the game to hide, rest, sleep, play, raise young, and eat. Therefore, when managing wild game populations, one should consider all factors specific to a given species of this game. In addition, when managing the habitats of wild animals, one should consider their level of tolerance to the variability of individual environmental conditions. For example, the size of the wild game population may be a function of the number of shelters available. Hence, it is necessary to ensure that there is a minimum number of habitats that meet the requirements of a given species.
Leopold [4] distinguished five types of shelters, and this division is still valid today: (1) winter shelters (providing invisibility and mechanical protection against snow); (2) refuge cover (areas where it is forbidden to hunt); (3) loafing cover (a place, not necessarily large, usually not too far from 1 or 2, providing shade in summer and protection against the wind in winter); (4) nesting cover (a habitat for raising young); and (5) nocturnal habitat. The methods of game protection are discussed in detail in point 3.
Leopold [4] only outlined a general framework for managing wild game populations regarding their diseases. Of course, it is impossible to eliminate all pathogens in wildlife habitats, but every effort should be made to limit them. It should also be emphasised that the treatment of diseases is not subject to the management of wild animal populations. On the other hand, population management should focus on creating an idea of the mechanisms, ranges, and strength of game diseases so that the manager can easily fight them in managed populations. Leopold put enormous pressure on the development of this management department.
At the beginning of the 20th century, the importance of disease in managing game populations was underestimated. The disease was not considered as an essential factor in wildlife management. However, the disease-related extinction of some wild animal species indicates the importance of this factor in the management process [78,79,80]. In particular, predators can be vectors of diseases because they feed on small rodents, and can become infected with their diseases [81]. Therefore, diseases are also an essential factor that should be considered when planning the acquisition of game. In the epidemic situation, the possibility of losing a significant part of the population has to be considered. Fluctuations in the population density in a given area may, to some extent, depend on the dynamics of the spread, virulence, and resistance of the pathogenic microorganisms. It should be emphasised that diseases can only attack animals of a certain sex or age, which affects the proportionality of the population [82].
Disease symptoms in the case of wild game are extremely difficult or even impossible to observe. This is due to the lack of tagging of the game, the speed of its migration, duration of the disease, but also the susceptibility of the game weakened by the disease to succumb to natural enemies [82].
Surveillance of all factors leading to disease in wildlife is negligible. Many interesting ideas for this purpose have been described in the literature, but are challenging to implement on a large scale. There are eight fundamental factors causing diseases in game, and the knowledge of the characteristics of each individual allows for the selection of an appropriate method of population management. In nature, we most often deal with the coexistence of pathogens such as (1) viruses; (2) bacteria; (3) protozoa; (4) fungi; (5) malnutrition; (6) parasites; (7) chemical poisoning; and (8) mechanical injuries.
Estimating disease mortality in animals is extremely important in the population management process. The high population density greatly accelerates the spread of infectious diseases. In addition, the reduced condition of the game caused by, for example, malnutrition or extreme environmental conditions also increases its susceptibility to disease. Disease control in wildlife is also important from the perspective of domestic animals and humans as foraging deer or wild boar in crops can transfer pathogens. Similarly, faeces left behind by animals as well as dead individuals can be a vector of diseases [83,84].
Therefore, in the context of the active management of wild game, it is necessary to carry out ongoing health checks of individuals based on regular observation of herds and possible sanitary hunting. In the literature, one can also find quite drastic methods of fighting diseases (i.e., shooting the entire population in the area potentially affected by the disease and the local animals’ gradual settlement). Another method is the selective removal of potentially susceptible groups of individuals (e.g., females, malnourished, or young individuals) [4].
Leopold [4], describing his concept of wild game management, referred to a quite controversial method of artificially immunising the game. This method relies on the controlled spread of mild strains of the disease. Numerous literature studies have indicated the use of this method of immunisation, for example, of rats against Trichinella spiralis [85]; mice against Dwarf Tapeworm, Hymenolepis nana var. fraterna [86]; and even farmed elk against Brucinellosis [87,88]. Thorne’s 1978 research has been indirectly continued in the U.S. by attempting to vaccinate single moose against Brucella abortus [89]. Unfortunately, the results of these works should be considered as a premise for use in closed breeding and not a method appropriate for wild game. This is indicated by the lack of control over the spread of the risk factor. Moreover, there is a debatable humanitarian nature of this approach.
The actions suggested by Leopold almost a century ago may constitute a premise justifying the artificial immunisation of wild game in its natural environment. However, only additional research in this area could significantly enrich population management methods in the context of surveillance of the emergence and spread of diseases. Currently, management methods are mainly limited to killing entire herds or destroying habitats to disperse the animal population.
According to Leopold, there are only two sentences regarding the activities described in point 5 of the wild game management paradigm in Polish legislation. Namely, the Hunting Law of 13 October 1995 [3] in Art. 13 on the feeding of game states that tenants and land administrators may feed the game if it does not pose an epizootic risk. This provision is characterised by a high level of generality. It can be interpreted differently, not always in line with the concept of wild game management. The Act’s provisions on the Protection of Animal Health indicate that a powiat veterinarian may temporarily prohibit the feeding of game animals. This is done by way of an ordinance, which is an act of local law.
Nevertheless, the obligatory prohibition of feeding wild boars in the areas at risk (red, blue, and yellow zones) results from the provisions of the Regulation of the Minister of Agriculture and Rural Development of 24 January 2018. Additionally, in 2018, the “Program for the early detection of infections with the virus that causes African swine fever and for increasing the knowledge about this disease and its control” was introduced in Poland [90]. These records show that it is impossible to feed only wild boars when they are the only disease vector because it is impossible to control which animal eats from any pasture. This is why in the areas covered by those above-mentioned, the program limits the feeding of animals in general. In addition, the most difficult period for game (i.e., winter) is milder in Poland every year, and often, snow cover appears in only a few places so the game can obtain food on its own.
In turn, Art. 14 of the Hunting Law Act [3] refers to animal diseases, namely the need to notify the State Veterinary Inspection of any noticed disease symptoms of wild game.
In Poland, the control of environmental factors is limited only to the regulations above-mentioned. There is no information about providing the game with watering places, and when it comes to habitats, only the protected areas discussed in Section 3 are mentioned. There may be provisions regarding these issues in the internal regulations of individual hunting clubs. However, this approach is characterised by a high level of individualisation and indicates the need to improve the legislation in line with the concept of Leopold [4].
Due to the multitude of factors specified by Leopold in the fifth point, only references to complementary feeding will be presented in the following discussion.
Complementary feeding of game in the Czech Republic is compulsory by law [39]. This practice has been in use for almost 500 years. In the light of the law, the user of the hunting grounds (lessee) is obliged to take care of the game’s habitats, feeding areas, pastures, salt licks, and, importantly, watering places. In addition, the leaseholder should use supplementary feeding if necessary. Although the climatic conditions in the Czech Republic are similar to those in Poland, the winters are not as severe. Therefore, despite the dubious need for additional feeding, they are used because of a centuries-old tradition. In addition, land users prefer to overfeed animals to avoid punishment. Czech legislation states that the state game management authority may control the living conditions of the game and call on the fishery user to rectify the situation immediately. If these conditions are not met, the animals are fed by the state at the expense of the land user, which is usually more expensive. It can be stated that the system of feeding animals in force in the Czech Republic is irrational from the point of view of Leopold’s concept. Feeding wild animals should be a matter of necessity, not tradition.
In Belgium, due to the division of the country into two separate legislations, the care of game is also diversified. In Flanders, feeding the game is neither prohibited nor obligatory. The winters in this region are mild and agriculture is dispersed, which means that the game has access to many types of natural food resources. Complementary feeding in Flanders is rarely used, and is usually to keep deer in wooded areas away from roads. However, it is forbidden to hunt for game in the baiting area. Such conduct is considered inhumane. In Wallonia, on the other hand, a distinction is made between side-feeding and diversification. Supplementary feeding is intended for cervids, usually in the winter. Naturally available plants are used for this purpose, while the use of industrial by-products is prohibited. The diversification feeding is for wild boar and is based on barley, corn, and peas. The purpose of feeding wild boars is to keep them in forests, away from crops. However, diversification feeding is not always carried out rationally with appropriate moderation [16]. Land users are tempted to achieve a rapid weight gain of adults and increase their fertility, which should not be the goal of this feeding. Therefore, it can be stated that in Belgium, the diversification feeding of wild boars should be rationalised in order to fulfil Leopold’s postulate.
In Austria, supplementary feeding is often done daily. This is used for deer, roe deer, and mouflon from November to May (fall–spring) in many districts. Depending on the province, the law varies greatly and can be mandatory, permitted in an emergency, or voluntary. Animals near feeders and feeding stations are not allowed to be shot. Chamois and ibex are not fed. On the other hand, in wild boar, a lure with corn is used. Additionally, the lure can be used all year round and is supposed to help in shooting it [14]. This procedure is prohibited in Belgium (Flanders) whereas in Austria, it standard practice. In Austria, it is even used to enclose deer forage sites [10–50 ha] and keep them there throughout the winter, hoping to provide them with the best conditions to survive the winter [91]. In light of Leopold’s concept, the practice used in Austria does not comply with the guideline expressed in Leopold’s fifth point.
In France, additional feeding is used as in the previously discussed countries, for example, in order to limit the amount of damage caused by wild boars. However, this is undertaken in a completely different way. Wild boars are fed with cereals only during the period of crop sensitivity (i.e., in the milky-waxy period of maize (April–October) and during the period of intensive activity of the vineyards (July–September)) [92,93]. Cereal grains are scattered in the forest, not closer than 300 m from the edge of the cultivated fields. Another way it is used in France is through the use of winter feeding as a supplement to the natural food base. However, naturally occurring plants and the easiest to breed plants are not used for this purpose, but only those that help to improve the population efficiency. This procedure is unique to France. Due to the diversity of game habitats in France, additional feeding may be used only in cases of poor-quality habitat or extreme environmental conditions such as drought, etc. The regulations prohibit feeding animals during the hunting season to limit unfair practices of luring animals to their areas by tenants or users as well as avoid shooting at animals while baiting, which has been strictly forbidden in France since 1 April 1986 [8]. Complementary feeding in France is carried out in a manner close to that recommended by Leopold.
As the analysis shows, the practices of feeding animals are very widespread. On the other hand, legal regulations concerning providing animals with access to water or managing disease agents are not as common anymore. They are usually expressed primarily in sub-laws. Each district, hunting unit, or other administrative unit involved in the management of wild game is characterised by specificity (e.g., vegetation, climate, pathogens). Therefore, it is necessary to adapt detailed recommendations to the current situation.
4. Conclusions
This article formulates questions on important issues such as how important is hunting for humanity? Should hunting management be implemented systematically, based on a universal model? What is the management of hunting and wild game populations in Poland, and how is it understood in the world? Why are hunters perceived as murderers in Poland? How can this perception be changed? The answers to these and other questions have been systematically presented in detail in this article. However, the synthetic statements resulting from the analysis carried out have already been formulated in the following paragraphs.
The perception of hunters in Poland is probably conditioned by stereotypical thinking, lack of knowledge, negative attitude towards diversity, and neglect in the environmental education of the society. Changing the perception of hunters due to various factors is extremely difficult because it is grassroots work that requires people’s involvement, time, money, and effort to conduct training and implement properly prepared educational programs [10]. Nevertheless, a comprehensive approach to the problem may, in the first stage, overcome the resistance resulting from negative attitudes towards hunting and hunters, and consequently encourage consumers to try game meat. Only comprehensive measures can create the conditions allowing potential consumers to see the benefits of hunting management and sustainable consumption including the consumption of wild animal meat.
- Hunting is an integral element of human functioning, striving to implement the idea of sustainable development and consumption.
- The wild game management process should be actively implemented and based on the still up-to-date, universal postulates of Leopold, which can be treated as a model approach.
- When analysing the wild game management process based on Leopold’s postulates, it can be stated that France is a country that stands out in terms of its proper implementation.
- The wildlife management framework needs to be improved in all the countries concerned, and the severity of identified shortcomings varies.
- The hunting schedule in all countries requires improvement. It should be a product of factors such as sex, age, species, mating season, physiological condition, animal condition, and a season of the year.
- The management of predator populations has been focused more on one-way activities, mainly related to protecting animals, than on active management and rational regulation of the population.
- The postulate concerning the provision of habitats for wild animals is implemented in various ways, with Poland and Belgium having the worst results in this respect.
- To date, no reliable method of counting animals has been developed to allow for the surveillance of the population size in any of the analysed countries. However, in France, many methods of determining the abundance of wild game have been tested, which indicates that this is where the problem of the lack of a reliable method will be solved the fastest. In Poland tests were also conducted, but in a smaller range.
- Feeding wild animals is carried out most rationally and effectively in France because the adopted solutions are based on the principle of humanitarianism and result from natural premises. Nevertheless, Belgium also has good solutions for wild animal feeding.
Author Contributions
Both authors have contributed equally to this research and both authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by PhD Students Self-Government Fund of Gdynia Maritime University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wehmeier, S. (Ed.) Oxford Advanced Learner’s Dictionary of Current English; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Putman, R.; Apollonio, M.; Andersen, R. (Eds.) Ungulate Management in Europe: Problems and Practices; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Dz.U.2020.1683 t.j. z dnia 2020.09.30—Ustawa prawo łowieckie. Available online: https://mdpi-res.com/data/mdpi_references_guide_v5.pdf (accessed on 5 October 2021).
- Leopold, A. Game Management; Charles Scribner’s Sons: New York, NY, USA, 1933. [Google Scholar]
- Griffin, R. Fundamentals of Management; Cengage learning: Boston, MA, USA, 2016. [Google Scholar]
- Mesinger, D.; Ocieczek, A. Evolution of the hunting management process in Poland. In Management in Commodity Science—Theoretical and Practical Aspects; Wydawnictwo Uniwersytetu Morskiego w Gdyni: Gdynia, Poland, 2020; pp. 122–140. [Google Scholar]
- Gula, R.; Bojarska, K.; Theuerkauf, J.; Król, W.; Okarma, H. Re-evaluation of the wolf population management units in central Europe. Wildl. Biol. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Maillard, D.; Gaillard, J.-M.; Hewison, A.J.M.; Ballon, P.; Duncan, P.; Loison, A.; Toigo, C.; Baubet, E.; Bonenfant, C.; Garel, M.; et al. Chapter 20. Ungulates and their management in France. In Ungulate Management in Europe: Problems and Practices; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Hunting: Ireland Ahead of the Pack. Available online: https://www.statista.com/chart/19732/number-of-hunters-per-1000-inhabitants (accessed on 26 July 2021).
- Niewiadomska, K.; Kosicka-Gębska, M.; Gębski, J.; Gutkowska, K.; Jeżewska-Zychowicz, M.; Sułek, M. Game meat consumption—Conscious choice or just a game? Foods 2020, 9, 1357. [Google Scholar] [CrossRef] [PubMed]
- ARRETE n° 20EB0287-DDTM relatif à l’ouverture et à la clôture de la chasse pour la campagne cynégétique 2020-2021 dans le département de la Charente-Maritime; LE PREFET Officier de l’Ordre National du Mérite Chevalier de la Légion d’Honneur. Available online: https://www.chasseurs17.com/pdf/reglementation/document144_1.pdf (accessed on 5 October 2021).
- Dz.U.2005.48.459 Rozporządzenie Ministra Środowiska z dnia 16 marca 2005 r. w sprawie określenia okresów polowań na zwierzęta łowne (Dz. U. Nr 48, poz. 459 z późn. zm.). Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/okreslenie-okresow-polowan-na-zwierzeta-lowne-17175098 (accessed on 5 October 2021).
- Capeans, R.; Sabuco, J.; Sanjuan, M.A.F. When less is more: Partial control to avoid extinction of predators in an ecological model. Ecol. Complex. 2014, 19, 1–8. [Google Scholar] [CrossRef]
- Reimoser, F.; Reimoser, S. Chapter 16. Ungulates and their management in Austria. In Ungulate Management in Europe: Problems and Practices; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Bartos, L.; Kotrba, R.; Pintir, J. Chapter 12. Ungulates and their management in Czech Republic. In Ungulate Management in Europe: Problems and Practices; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Casaer, J.; Licoppe, A. Chapter 9. Ungulates and their management in Belgium. In Ungulate Management in Europe: Problems and Practices; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Zalewski, D.; Okarma, H.; Panek, M. Monitoring Liczebności i Jakości Populacji Dzikich Zwierząt; University of Warmia and Mazury in Olsztyn: Olsztyn, Poland, 2018; ISBN 978-83-950935-5-5. [Google Scholar]
- Berezowska-Cnota, T.; Luque-Márquez, I.; Elguero-Claramunt, I.; Bojarska, K.; Okarma, H.; Selva, N. Effectiveness of different types of hair traps for brown bear research and monitoring. PLoS ONE 2017, 12, e0186605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojarska, K.; Kwiatkowska, M.; Skórka, P.; Gula, R.; Theuerkauf, J.; Okarma, H. Anthropogenic environmental traps: Where do wolves kill their prey in a commercial forest? For. Ecol. Manag. 2017, 397, 117–125. [Google Scholar] [CrossRef]
- Określenie okresów polowań na zwierzęta łowne Dz.U.2005.48.459. Available online: http://www.zbikprzemysl.pl/upl_doc/Rozporzadzenie_w_sprawie_okre%C5%9Blenia_okresow_polowan_na_zwierzeta_lowne.pdf (accessed on 5 October 2021).
- Uprawnienia do wykonywania polowania Dz.U.2010.3.19. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/uprawnienia-do-wykonywania-polowania-17588933?unitId=par(19) (accessed on 5 October 2021).
- Szczegółowe warunki wykonywania polowania i znakowania tusz Dz.U.2005.61.548. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/szczegolowe-warunki-wykonywania-polowania-i-znakowania-tusz-17180386 (accessed on 5 October 2021).
- Janiszewski, P.; Daszkiewicz, T. Zwierzęta łowne: Zasady prawidłowego pozyskiwania i zagospodarowania; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Poland, 2010. [Google Scholar]
- Stevenson, J.M.; Seman, D.L.; Littlejohn, R.P. Seasonal variation in venison quality of mature, farmed red deer stags in New Zealand. J. Anim. Sci. 1992, 70, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Dryden, G.M. Venison in the human diet-is venison a low-fat meat? Proc. Nutr. Soc. Aust. 1997, 21, 44–51. [Google Scholar]
- Dahlan, I.; Norfarizan Hanoon, N.A. Chemical composition, palatability and physical characteristics of venison from farmed deer. Anim. Sci. J. 2008, 79, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Piaskowska, N.; Daszkiewicz, T.; Kubiak, D.; Janiszewski, P. The effect of gender on meat (Longissimus Lumborum Muscle) quality characteristics in the fallow deer Dama Dama L. Italian J. Anim. Sci. 2015, 14, 3845. [Google Scholar] [CrossRef]
- Daszkiewicz, T.; Mesinger, D. Fatty acid profile of meat (Longissimus lumborum) from female roe deer (Capreolus capreolus L.) and red deer (Cervus elaphus L.). Int. J. Food Prop. 2018, 21, 2276–2282. [Google Scholar] [CrossRef] [Green Version]
- Schulp, C.J.; Thuiller, W.; Verburg, P.H. Wild food in Europe: A synthesis of knowledge and data of terrestrial wild food as an ecosystem service. Ecol. Econ. 2014, 105, 292–305. [Google Scholar] [CrossRef]
- Game Meat from Poland. Available online: https://polfood.de/en/assortment/game/ (accessed on 25 April 2021).
- Ostrich and Exotic Products & Game. Available online: https://www.raverco.com/en/exotic-products.php (accessed on 25 April 2021).
- Rocznik Statystyczny Leśnictwa 2020, Główny Urząd Statystyczny. Available online: https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-statystyczny-lesnictwa-2020,13,3.html (accessed on 5 October 2021).
- Mesinger, D.; Ocieczek, A. Consumer education as an important condition for increasing wild animal meat consumption in the context of promoting the idea of sustainable development in Poland. Pol. J. Environ. Stud. 2020, 29, 3485–3492. [Google Scholar] [CrossRef]
- Red Stag Hunting in Austria. Available online: http://www.huntaustria.com/offer/red-deer-stag-hunting-in-austria/ (accessed on 25 April 2021).
- Summary of National Hunting Regulations: Austria. Available online: http://datazone.birdlife.org/userfiles/file/hunting/HuntingRegulations_Austria.pdf (accessed on 25 April 2021).
- GP XXVII RV 942/2013 AB 993/2013 LT 38 Upper Austria Hunting Law, 1964 z późn. zm. Available online: http://extwprlegs1.fao.org/docs/pdf/aut81719.pdf (accessed on 5 October 2021).
- M.B. du 05/10/2005, p. 42998 (Numac: 2005202621). Available online: https://wallex.wallonie.be/contents/acts/7/7419/5.html (accessed on 25 April 2021).
- Het Jachtvoorwaardenbesluit van 25 April 2014. Available online: https://codex.vlaanderen.be/Zoeken/Document.aspx?DID=1024157¶m=inhoud (accessed on 25 April 2021).
- Vyhláška, Č. 403/2013 Sb. Available online: https://www.zakonyprolidi.cz/cs/2013-403?text=480%2F2002 (accessed on 25 April 2021).
- Vyhláška, Č. 244/2002 Sb. Available online: https://www.zakonyprolidi.cz/cs/2002-244/zneni-20180101 (accessed on 25 April 2021).
- LOI n° 2019-773 du 24 juillet 2019 portant création de l’Office français de la biodiversité, modifiant les missions des fédérations des chasseurs et renforçant la police de l’environnement. Available online: https://www.legifrance.gouv.fr/dossierlegislatif/JORFDOLE000037611465/ (accessed on 5 October 2021).
- Meriggi, A.; Lovari, S. A review of wolf predation in southern Europe: Does the wolf prefer wild prey to livestock? J. Appl. Ecol. 1996, 33, 1561–1571. [Google Scholar] [CrossRef]
- Dyrektywa Rady 92/43/EWG z dnia 21 maja 1992 r. w sprawie ochrony siedlisk przyrodniczych oraz dzikiej fauny i flory (Dz. U. UE. L. z 1992 r. Nr 206, str. 7 z późn. zm.). Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/?uri=celex%3A31992L0043 (accessed on 5 October 2021).
- KONWENCJA o ochronie gatunków dzikiej flory i fauny europejskiej oraz ich siedlisk, sporządzona w Bernie dnia 19 września 1979 r. (Dz. U. z 1996 r. Nr 58, poz. 263 z późn. zm.). Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU19960580263/O/D19960263.pdf (accessed on 5 October 2021).
- Ustawa nr 114/1992 Sb. o ochronie przyrody i krajobrazu, rozporządzenie nr 395/1992 Sb. Available online: https://www.zakonyprolidi.cz/cs/1992-114 (accessed on 5 October 2021).
- Ustawa z dnia 16 kwietnia 2004 r. o ochronie przyrody (t.j. Dz. U. z 2020 r. poz. 55 z późn. zm.). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20040920880/T/D20040880L.pdf (accessed on 5 October 2021).
- Rozporządzenie Ministra Środowiska z dnia 16 grudnia 2016 r. w sprawie ochrony gatunkowej zwierząt (Dz. U. poz. 2183 z późn. zm.). Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20160002183/O/D20162183.pdf (accessed on 5 October 2021).
- Rozporządzenie Ministra Ochrony Środowiska, Zasobów Naturalnych i Leśnictwa z dnia 6 stycznia 1995 r. w sprawie ochrony gatunkowej zwierząt. Dz.U. 1995 nr 13 poz. 61. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/ochrona-gatunkowa-zwierzat-16796569 (accessed on 5 October 2021).
- Rozporządzenie Ministra Ochrony Środowiska, Zasobów Naturalnych i Leśnictwa z dnia 30 stycznia 1995 r. w sprawie uznania niektórych gatunków dzikich zwierząt za łowne oraz wyłączenia niektórych gatunków ze spisu dzikich zwierząt łownych. Dz.U. 1995 nr 11 poz. 50. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU19950110050/O/D19950050.pdf (accessed on 5 October 2021).
- Rozporządzenie Ministra Środowiska z dnia 28 września 2004 r. w sprawie gatunków dziko występujących zwierząt objętych ochroną Dz.U. 2004 nr 220 poz. 2237. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20042202237/O/D20042237.pdf (accessed on 5 October 2021).
- Polski Wilk. Available online: https://www.polskiwilk.org.pl/wilk/liczebnosc-i-rozmieszczenie (accessed on 14 September 2021).
- Hindrikson, M.; Remm, J.; Pilot, M.; Godinho, R.; Stronen, A.V.; Baltrunaite, L.; Czarnomska, S.; Leonard, J.; Randi, E.; Nowak, C.; et al. Wolf population genetics in Europe: A systematic review, meta-analysis and suggestions for conservation and management. Biol. Rev. 2017, 92, 1601–1629. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.S.; Ritchie, E.G. Stop jumping the gun: A call for evidence-based invasive predator management. Conserv. Lett. 2017, 10, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Clark, T.J.; Hebblewhite, M. Predator control may not increase ungulate populations in the future: A formal meta-analysis. J. Appl. Ecol. 2021, 58, 812–824. [Google Scholar] [CrossRef]
- Bobek, B.; Merta, D.; Furtek, J. Winter food and cover refuges of large ungulates in lowland forests of south-western Poland. For. Ecol. Manag. 2016, 359, 247–255. [Google Scholar] [CrossRef]
- Wiszniewska-Łaszczych, A.; Wysok, B.; Wojtacka, J.; Szteyn, J.; Michalski, M.; Sołtysiuk, M. High seroprevalence of Toxoplasma gondii antibodies in wild boars hunted in the game breeding center in north-eastern Poland. Med. Weter. 2019, 75, 759–762. [Google Scholar] [CrossRef]
- Parki Narodowe. Available online: https://zpppn.pl/parki-narodowe (accessed on 7 May 2021).
- Centralny Rejestr Form Ochrony Przyrody. Available online: http://crfop.gdos.gov.pl/CRFOP/search.jsf (accessed on 7 May 2021).
- Územní Ochrana. Available online: https://www.ochranaprirody.cz/uzemni-ochrana/ (accessed on 7 May 2021).
- Natuurpunt. Available online: https://www.natuurpunt.be/ (accessed on 7 May 2021).
- National Parks in Austria. Available online: https://www.austria.info/en/things-to-do/lakes-and-nature/national-parks (accessed on 7 May 2021).
- Conseil d’administration, 19.06.2012, decyzja nr 12/17. Available online: http://data.over-blog-kiwi.com/0/41/64/58/201301/ob_f2ec2b_bareme-valeur-gibier-19062012.pdf (accessed on 5 October 2021).
- Perea, R.; Perea-Garcia-Calvo, R.; Diaz-Ambrona, C.; San Miguel, A. The reintroduction of a flagship ungulate Capra pyrenaica: Assessing sustainability by surveying woody vegetation. Biol. Conserv. 2015, 181, 9–17. [Google Scholar] [CrossRef]
- Bobek, B.; Merta, D.; Furtek, J.; Wojciuch-Ploskonka, M.; Kopec, K.; Maslanka, J.; Ziobrowski, M. Ocena dynamiki liczebności i zagęszczenia populacji dzikich kopytnych przy użyciu różnych metod w czterech regionach Polski. Stud. Mater. Cent. Edukac. Przyr.-Leśnej 2013, 15, 88–101. [Google Scholar]
- Pasicka, E. Polish Konik Horse–Characteristics and historical background of native descendants of tarpan. Acta Sci. Pol. Med. Vet. 2013, 12, 25–38. [Google Scholar]
- Rokosz, M. History of the aurochs (Bos taurus primigenius) in Poland. Anim. Genet. Resour. 1995, 16, 5–12. [Google Scholar] [CrossRef]
- The IUCN Red List of Threated Species. Available online: https://www.iucnredlist.org/ (accessed on 7 May 2021).
- Eick, E.; König, R.; Willett, J. (Eds.) Sika, Cervus Nippon Temminck; International Sika Society: Möhnesee, Germany, 1838. [Google Scholar]
- Obidziński, A.; Kiełtyk, P.; Borkowski, J.; Bolibok, L.; Remuszko, K. Autumn-winter diet overlap of fallow, red, and roe deer in forest ecosystems, Southern Poland. Open Life Sci. 2013, 8, 8–17. [Google Scholar] [CrossRef]
- Cevreny, J. Biologie zvere srstnate. In Encyklopedie Myslivosti; Cerveny, J., Kamler, J., Kholova, H., Koubek, P., Martinkova, N., Eds.; Ottovo nakladatelstvi: Prague, Czech Republic, 2003; pp. 249–345. [Google Scholar]
- Hayez, F. Le grand gibier en Belgique; Exposition Nationale de Trophees de Grands Gibier: Laveaux-Sainte-Anne, Belgium, 1999; pp. 25–34. [Google Scholar]
- Pfaff, E.; Klein, F.; Saint-Andrieux, C.; Guibert, B. La situation du cerf élaphe en France. Rés. Inventaire 2005, 280, 40–50. [Google Scholar]
- Denis, M. Quelques méthodes pratiques pour l’estimation de l’effectif d’une population de chevreuils (Capreolus capreolus L.). In Proceedings of the XVII IUGB Congress, Brussels, Belgium, 17–21 September 1985; pp. 979–989. [Google Scholar]
- Vassant, J.; Brandt, S.; Julien, J.M. Essai de denombrement d’une population de sangliers par observation sur places d’affouragements. Bull. Mens. Office Nat. Chasse 1990, 147, 21–26. [Google Scholar]
- Houssin, H.; Loison, A.; Jullien, J.M.; Gaillard, J.M. Validite de la methode du pointage-flash pour l’estimation des effectifs de chamois (Rupicapra rupicapra). Gibier Faune Sauvage 1994, 11, 287–298. [Google Scholar]
- Gilpin, M.E.; Ayala, F.J. Global models of growth and competition. Proc. Natl. Acad. Soc. USA 1973, 70, 3590–3593. [Google Scholar] [CrossRef] [Green Version]
- Putman, R.J.; Staines, B.W. Supplementary winter feeding of wild red deer Cervus elaphus in Europe and North America: Justifications, feeding practice and effectiveness. Mammal. Rev. 2004, 34, 285–306. [Google Scholar] [CrossRef]
- Gulland, F.M.D. The impact of infectious diseases on wild animal populations: A review. Ecol. Infect. Dis. Nat. Popul. 1995, 1, 20–51. [Google Scholar]
- Hudson, P.J.; Rizzoli, A.P.; Grenfell, B.T.; Heesterbeek, J.A.P.; Dobson, A.P. Ecology of wildlife diseases; Oxford University Press: Oxford, UK, 2002; ISBN 9780198506195. [Google Scholar]
- Gortázar, C.; Ferroglio, E.; Höfle, U.; Frölich, K.; Vicente, J. Diseases shared between wildlife and livestock: A European perspective. Eur. J. Wildl. Res. 2007, 53, 241–256. [Google Scholar] [CrossRef]
- Stronen, A.V.; Brook, R.K.; Paquet, P.C.; McLachlan, S. Farmer attitudes toward wolves: Implications for the role of predators in managing disease. Biol. Conserv. 2007, 135, 1–10. [Google Scholar] [CrossRef]
- Artois, M. Wildlife infectious disease control in Europe. J. Mt. Ecology 2003, 7, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wacheck, S.; Fredriksson-Ahomaa, M.; König, M.; Stolle, A.; Stephan, R. Wild boars as an important reservoir for foodborne pathogens. Foodborne Pathog. Dis. 2010, 7, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson-Ahomaa, M. Wild boar: A reservoir of foodborne zoonoses. Foodborne Pathog. Dis. 2019, 16, 153–165. [Google Scholar] [CrossRef]
- McCoy, O.R. Artificial immunization of rats against Trichinella spiralis. Am. J. Hyg. 1935, 21, 200–213. [Google Scholar] [CrossRef]
- Larsh, J.E., Jr. Studies on the artificial immunization of mice against infection with the dwarf tapeworm, hymenolepis nana var. fraterna. Am. J. Hyg. 1944, 39, 129–132. [Google Scholar]
- Thorne, E.T.; Morton, J.K.; Thomas, G.M. Brucellosis in elk I. Serologic and bacteriologic survey in Wyoming. J. Wildl. Dis. 1978, 14, 74–81. [Google Scholar] [CrossRef]
- Thorne, E.T.; Morton, J.K.; Blunt, F.M.; Dawson, H.A. Brucellosis in elk. II. Clinical effects and means of transmission as determined through artificial infections. J. Wildl. Dis. 1978, 14, 280–291. [Google Scholar] [CrossRef]
- Roffe, T.; Jones, L.; Coffin, K.; Drew, M.; Sweeney, S.; Hagius, S.; Elzer, P.; Davis, D. Efficacy of single calfhood vaccination of elk with Brucella Abortus strain 19. J. Wildl. Manag. 2004, 68, 830–836. [Google Scholar] [CrossRef]
- Rozporządzenie Ministra Rolnictwa i Rozwoju Wsi z dnia 24 stycznia 2018 r. w sprawie wprowadzenia w 2018 r. na terytorium Rzeczypospolitej Polskiej "Programu mającego na celu wczesne wykrycie zakażeń wirusem wywołującym afrykański pomór świń i poszerzenie wiedzy na temat tej choroby oraz jej zwalczanie" (Dz. U. poz. 316 z późn. zm.). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20180000316/O/D20180316.pdf (accessed on 5 October 2021).
- Sackl, K. Erfahrungen mit der Kommissionierung von Rotwildfutterungen und Rotwildwintergattern. Master Thesis, Agricultural University of Vienna, 1992. Available online: https://boku.ac.at/fileadmin/data/H03000/H83000/H83200/Publikationen/BOKU-Berichte/BOKU_Berichte_zur_Wildtierforschung_04.pdf (accessed on 5 October 2021).
- Vassant, J.; Brandt, S.; Jullien, J.M. Reducing wild boar damage to wheat and oat in summer: Study of the effectiveness of maize distribution in the forest. In Proceedings of the 18th IUGB Congress, Global Trends in Wildlife Management; Bobek, B., Perzanowski, K., Regelin, W.L., Eds.; Jagiellonian University: Krakow, Poland, 1987. [Google Scholar]
- Calenge, C.; Maillard, D.; Fournier, P.; Fouque, C. Efficiency of spreading maize in the garrigues to reduce wild boar (Sus scrofa) damage to Mediterrenean vineyards. Eur. J. Wildl. Res. 2004, 50, 112–120. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).