Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing
Abstract
:1. Introduction
- 1.
- It is a simpler and relatively cheaper conversion process.
- 2.
- Pyrolysis is suitable for a wider variety of feedstock.
- 3.
- It reduces the landfill requirements and greenhouse gas (GHG) emissions.
- 4.
- It has very little water pollution potential.
- 5.
- Pyrolysis reactor construction is relatively rapid process.
2. Conversion Mechanism of Biomass by Pyrolysis
2.1. Pyrolysis Primary Conversion Mechanisms
2.1.1. Mechanism of Char Formation
2.1.2. Mechanism of Depolymerization
2.1.3. Mechanism of Fragmentation
2.2. Pyrolysis Secondary Conversion Mechanisms
2.3. Principle of Pyrolysis and Product Distribution
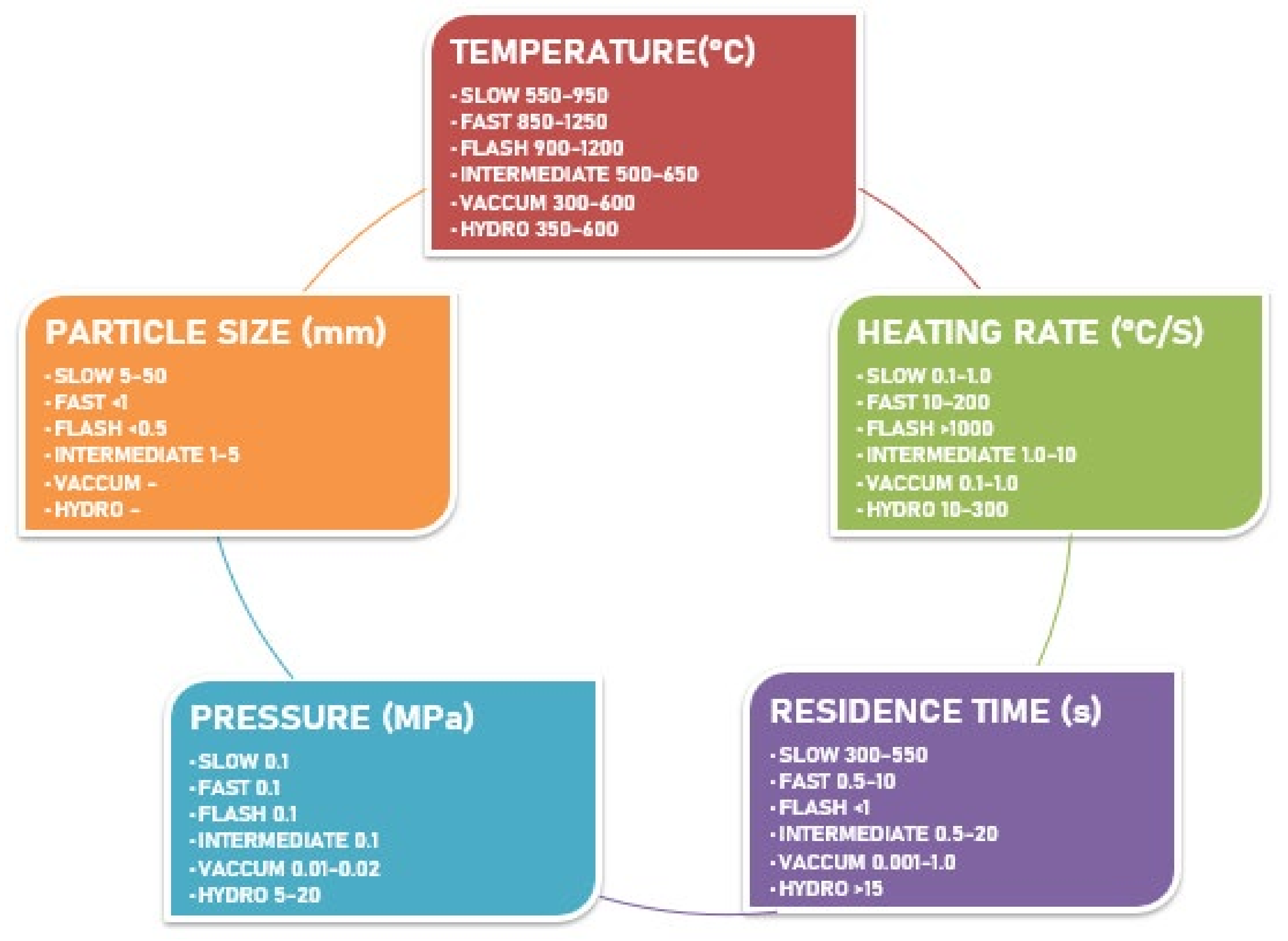
- 1.
- Effect of the biomass particle size
- 2.
- Effect of the operating temperature
- 3.
- Effect of the heating rate
- 4.
- Effect of the residence time
- 5.
- Effect of pressure
- 6.
- Effect of the catalyst
- 7.
- Effect of the pyrolysis bed-height
- 8.
- Effect of the carrier gas flow rate
3. Biomass Feedstock Availability and Economic Analysis
- Feedstock particle shape and size;
- Bulk density;
- Elemental and chemical composition;
- Energy content (MJ/Kg);
- Protein, lipid, extractives, and ash content.
4. Recent Progress in the Biomass Pyrolysis Process
4.1. Fast Pyrolysis Process
- 1.
- The phenomenon takes place with high heat and heat transfer rates. Therefore, biomass materials need to be very small.
- 2.
- The controlled temperature range is 450–550 °C in the vapor phase.
- 3.
- The vapor residence times are as short as two seconds.
- 4.
- The vapors are converted to bio-oil by instantaneous cooling.
- ➢
- Fast Pyrolysis of Biomass via a Bubbling Fluidized-Bed Reactor,
- ➢
- Fast Pyrolysis of Biomass via a Circulating Fluidized-bed Reactor,
- ➢
- Fast Pyrolysis of Biomass via a Fixed Bed Reactor,
- ➢
- Fast Pyrolysis of Biomass via an Ablative Reactor,
- ➢
- Pyrolysis of Biomass via an Entrained Flow Reactor, and
- ➢
- Catalytic Fast Pyrolysis of Biomass
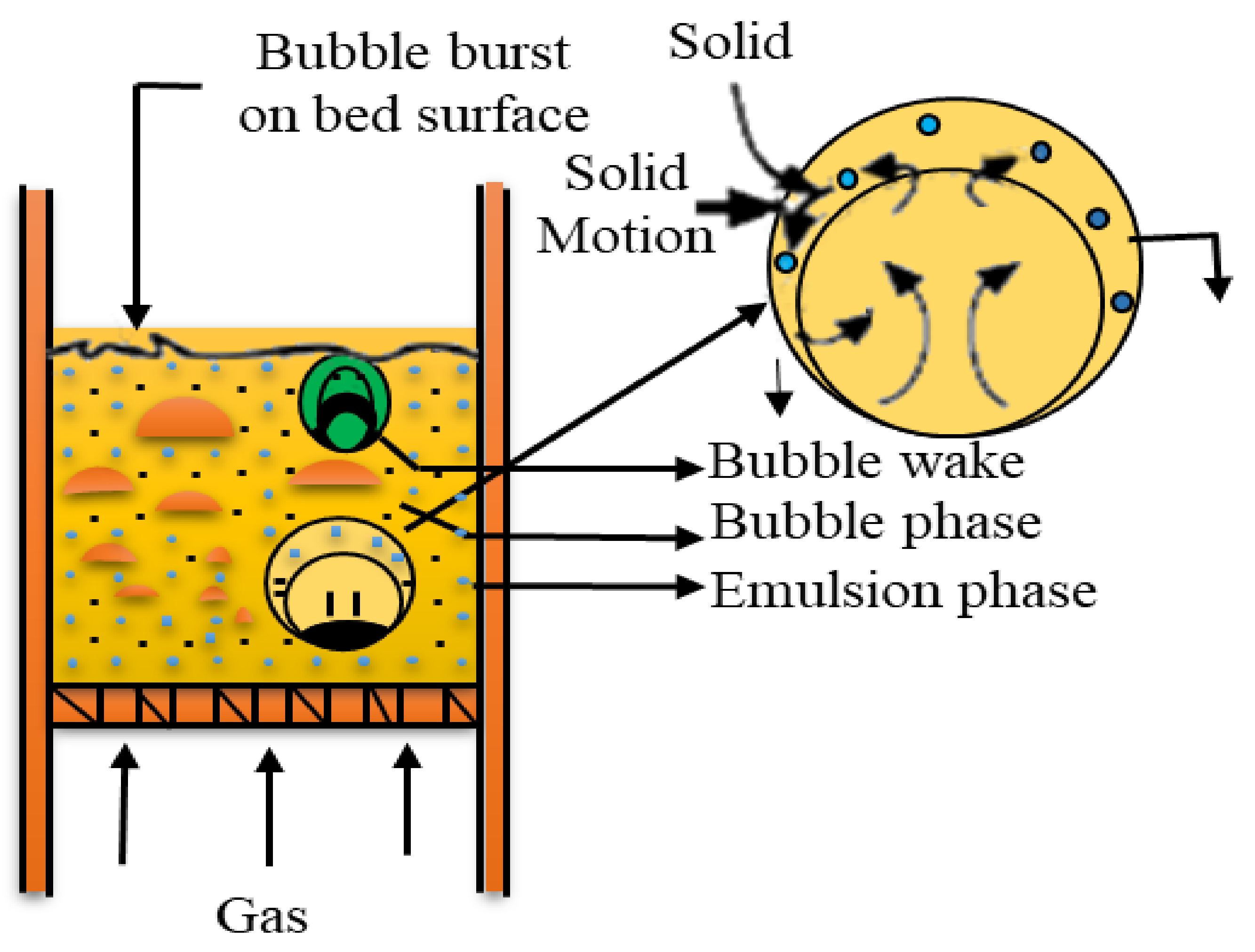
4.1.1. Fast Pyrolysis of Biomass via Bubbling Fluidized-Bed Reactor
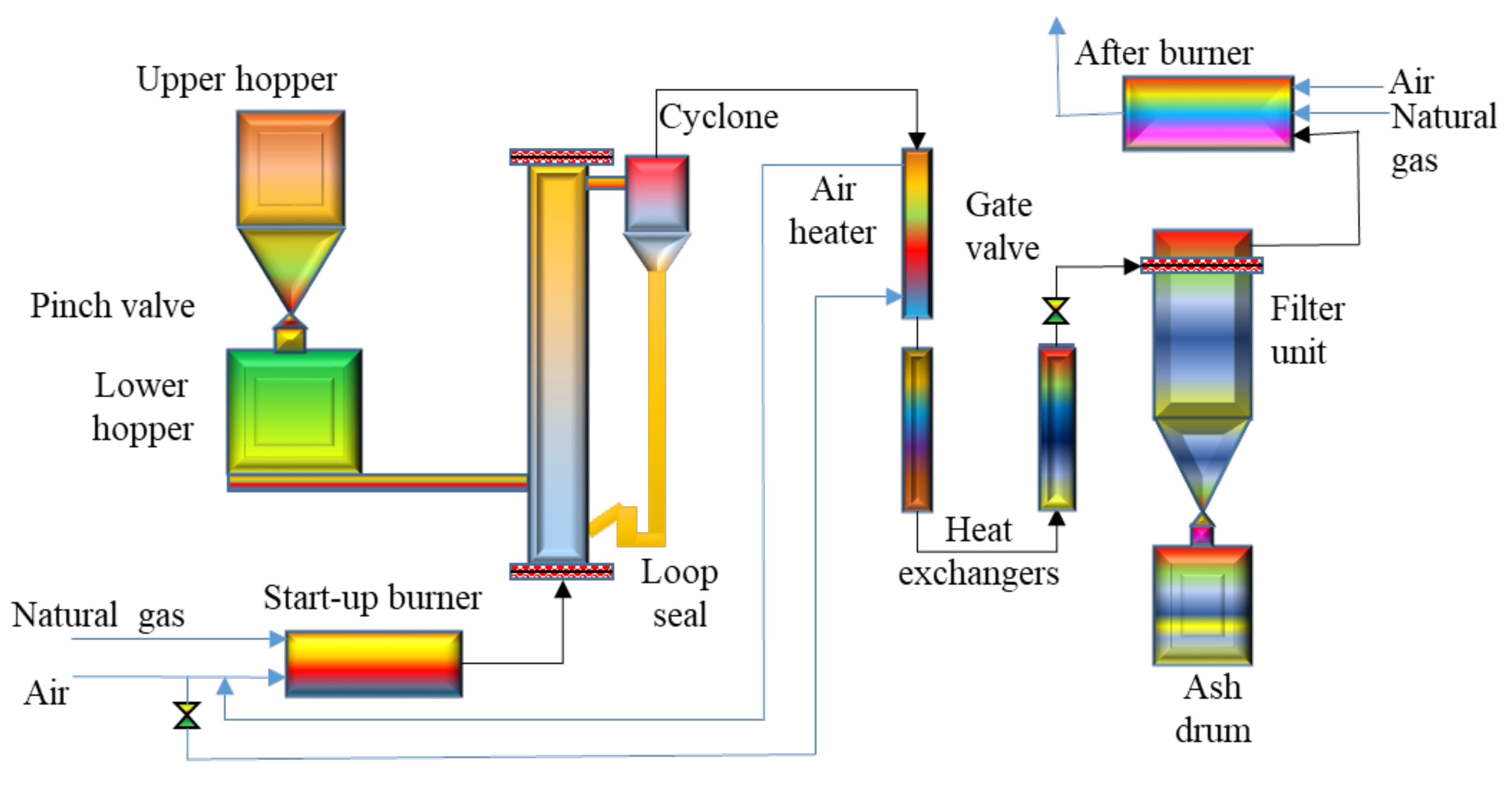
4.1.2. Fast Pyrolysis of Biomass via Circulating Fluidized-Bed Reactor
4.1.3. Fast Pyrolysis of Biomass via Fixed-Bed Reactors
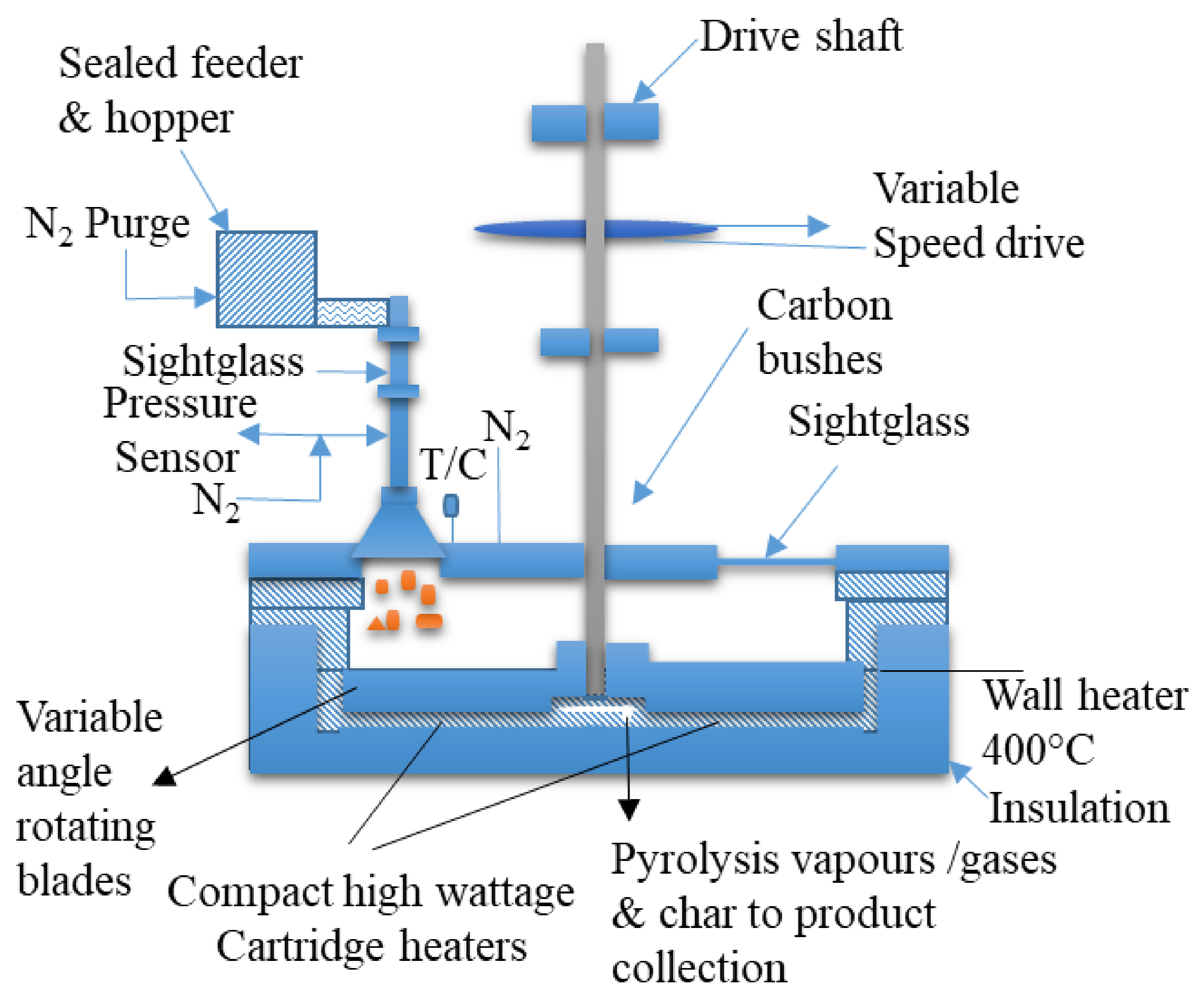
4.1.4. Fast Pyrolysis of Biomass via Ablative Reactor
4.1.5. Fast Pyrolysis of Biomass via Entrained Flow Reactor
4.1.6. Catalytic Fast Pyrolysis of Biomass
4.2. Slow Pyrolysis Process
- ➢
- Slow Pyrolysis of Biomass via Fixed-Bed Reactor,
- ➢
- Slow Pyrolysis of Biomass via Augers Reactor,
- ➢
- Slow Pyrolysis of Biomass via Rotary-Kiln Reactor, and
- ➢
- Catalytic Slow Pyrolysis of Biomass
4.2.1. Slow Pyrolysis of Biomass via Fixed-Bed Reactor
4.2.2. Slow Pyrolysis of Biomass Using the Augers Reactor
4.2.3. Slow Pyrolysis of Biomass via Rotary Kiln Reactor
4.2.4. Catalytic Slow Pyrolysis of Biomass
5. Advanced Pyrolysis Processes
- 1.
- Vacuum pyrolysis of biomass,
- 2.
- Microwave pyrolysis of biomass,
- 3.
- Flash pyrolysis of biomass,
- 4.
- Biomass pyrolysis via plasma technology, and
- 5.
- Biomass pyrolysis via solar energy.
5.1. Vacuum Pyrolysis of Biomass
5.2. Microwave Pyrolysis of Biomass
5.3. Flash Pyrolysis of Biomass
5.4. Pyrolysis of Biomass via Plasma Technology
5.5. Pyrolysis of Biomass via Solar Energy
6. Future Perspective and Commercialization of Pyrolysis Technology
- 1.
- Pyrolysis reactors should be efficient and effective in heat transfer,
- 2.
- Should speed up the reactivity of pyrolysis,
- 3.
- Produce bio-oil with a lower molecular weight,
- 4.
- Pyrolysis products should have zero toxicity,
- 5.
- Thermally stable pyrolysis reactors,
- 6.
- Less ash agglomeration in reactor beds, and
- 7.
- Should have good control over temperature and heating rates.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirkels, A.F.; Verbong, G.P.J. Biomass gasification: Still promising? A 30-year global overview. Renew. Sustain. Energy Rev. 2011, 15, 471–481. [Google Scholar] [CrossRef]
- Onsree, T.; Tippayawong, N.; Zheng, A.; Li, H. Pyrolysis behavior and kinetics of corn residue pellets and eucalyptus wood chips in a macro thermogravimetric analyzer. Case Stud. Therm. Eng. 2018, 12, 546–556. [Google Scholar] [CrossRef]
- Farooq, A.; Moogi, S.; Jang, S.H.; Ahmed, A.; Kim, Y.M.; Reddy Kannapu, H.P.; Valizadeh, S.; Jung, S.C.; Lam, S.S.; Rhee, G.H.; et al. Biohydrogen synthesis from catalytic steam gasification of furniture waste using nickel catalysts supported on modified CeO2. Int. J. Hydrogen Energy 2021, 46, 16603–16611. [Google Scholar] [CrossRef]
- Schneider, D.; Escala, M.; Supawittayayothin, K.; Tippayawong, N. Characterization of biochar from hydrothermal carbonization of bamboo. Int. J. Energy Environ. 2011, 2, 647–652. [Google Scholar]
- Asghar, U.; Rafiq, S.; Anwar, A.; Iqbal, T.; Ahmed, A.; Jamil, F.; Khurram, M.S.; Akbar, M.M.; Farooq, A.; Shah, N.S.; et al. Review on the progress in emission control technologies for the abatement of CO2, SOx and NOx from fuel combustion. J. Environ. Chem. Eng. 2021, 9, 106064. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Silva, V.; Rouboa, A. Co-gasification and recent developments on waste-to-energy conversion: A review. Renew. Sustain. Energy Rev. 2018, 81, 380–398. [Google Scholar] [CrossRef]
- Suleman, M.; Zafar, M.; Ahmed, A.; Rashid, M.U.; Hussain, S.; Razzaq, A.; Mohidem, N.A.; Fazal, T.; Haider, B.; Park, Y. Castor Leaves-Based Biochar for Adsorption of Safranin from Textile Wastewater. Sustainability 2021, 13, 6926. [Google Scholar] [CrossRef]
- Lopamudra, D.; Krzysztof, J.P.; Frans, J.J.G.J. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar]
- Link, S.; Arvelakis, S.; Paist, A.; Liliedahl, T.; Rosén, C. Effect of leaching pretreatment on the gasification of wine and vine (residue) biomass. Renew. Energy 2018, 115, 1–5. [Google Scholar] [CrossRef]
- Francois, J.; Abdelouahed, L.; Mauviel, G.; Patisson, F.; Mirgaux, O.; Rogaume, C.; Rogaume, Y.; Feidt, M.; Dufour, A. Detailed process modeling of a wood gasification combined heat and power plant. Biomass Bioenergy 2013, 51, 68–82. [Google Scholar] [CrossRef]
- Atabani, A.; Shobana, S.; Mohammed, M.; Uğuz, G.; Kumar, G.; Arvindnarayan, S.; Aslam, M.; Ala′a, H. Integrated valorization of waste cooking oil and spent coffee grounds for biodiesel production: Blending with higher alcohols, FT–IR, TGA, DSC and NMR characterizations. Fuel 2019, 244, 419–430. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global challenges in the sustainable development of biomass gasification: An overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Yang, P.; Tan, G.-Y.A.; Aslam, M.; Kim, J.; Lee, P.-H. Metatranscriptomic evidence for classical and RuBisCO-mediated CO2 reduction to methane facilitated by direct interspecies electron transfer in a methanogenic system. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Khalid, A.; Aslam, M.; Qyyum, M.A.; Faisal, A.; Khan, A.L.; Ahmed, F.; Lee, M.; Kim, J.; Jang, N.; Chang, I.S. Membrane separation processes for dehydration of bioethanol from fermentation broths: Recent developments, challenges, and prospects. Renew. Sustain. Energy Rev. 2019, 105, 427–443. [Google Scholar] [CrossRef]
- Fernando, N.; Narayana, M. A comprehensive two dimensional Computational Fluid Dynamics model for an updraft biomass gasifier. Renew. Energy 2016, 99, 698–710. [Google Scholar] [CrossRef]
- Jaroenkhasemmeesuk, C.; Diego, M.E.; Tippayawong, N.; Ingham, D.B.; Pourkashanian, M. Simulation analysis of the catalytic cracking process of biomass pyrolysis oil with mixed catalysts: Optimization using the simplex lattice design. Int. J. Energy Res. 2018, 42, 2983–2996. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Juárez, M.C.; Morales, M.P.; Muñoz, P.; Mendívil, M.A. Biomass gasification for electricity generation: Review of current technology barriers. Renew. Sustain. Energy Rev. 2013, 18, 174–183. [Google Scholar] [CrossRef]
- Hidayat, S.; Bakar, M.S.A.; Ahmed, A.; Iryani, D.A.; Hussain, M.; Jamil, F.; Park, Y.K. Comprehensive kinetic study of Imperata Cylindrica pyrolysis via Asym2sig deconvolution and combined kinetics. J. Anal. Appl. Pyrolysis 2021, 156, 105133. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Adibah, W.; Azwar, E.; Fong, S.; Ahmed, A.; Peng, W.; Tabatabaei, M.; Aghbashlo, M.; Park, Y.; Lam, S.S. Valorization of municipal wastes using co-pyrolysis for green energy production, energy security, and environmental sustainability: A review. Chem. Eng. J. 2021, 129749. [Google Scholar]
- What Is Pyrolysis? Available online: https://www.azocleantech.com/article.aspx?ArticleID=336 (accessed on 17 January 2021).
- Weldekidan, H.; Strezov, V.; He, J.; Kumar, R.; Asumadu-Sarkodie, S.; Doyi, I.N.; Jahan, S.; Kan, T.; Town, G. Energy conversion efficiency of pyrolysis of chicken litter and rice husk biomass. Energy Fuels 2019, 33, 6509–6514. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; He, C.; Ruan, R.; Yu, Z.; Jiang, L.; Zeng, Z.; Wu, Q. A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass. Sci. Total. Environ. 2020, 749, 142386. [Google Scholar] [CrossRef]
- Garcia-Nunez, J.A.; Pelaez-Samaniego, M.R.; Garcia-Perez, M.E.; Fonts, I.; Abrego, J.; Westerhof, R.J.M.; Garcia-Perez, M. Historical Developments of Pyrolysis Reactors: A Review. Energy Fuels 2017, 31, 5751–5775. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Pyrolysis behaviors of wood and its constituent polymers at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 78, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Elyounssi, K.; Collard, F.-X.; Mateke, J.-A.N.; Blin, J. Improvement of charcoal yield by two-step pyrolysis on eucalyptus wood: A thermogravimetric study. Fuel 2012, 96, 161–167. [Google Scholar] [CrossRef]
- Glenn, B.R.A. Division of Fuel Chemistry Preprints-Advantages and Problems. J. Chem. Doc. 1963, 3, 64–65. [Google Scholar] [CrossRef]
- Vernaglia, B.A.; Wornat, M.; Li, C.-h.; Nelson, P.F. The effects of pyrolysis temperature and ion-exchanged metals on the composition of brown coal tars produced in a fluidized-bed reactor. Symp. Int. Combust. 1996, 26, 3287–3294. [Google Scholar] [CrossRef]
- Banyasz, J.L.; Li, S.; Lyons-Hart, J.; Shafer, K.H. Gas evolution and the mechanism of cellulose pyrolysis. Fuel 2001, 80, 1757–1763. [Google Scholar] [CrossRef]
- Azeez, A.M.; Meier, D.; Odermatt, J. Temperature dependence of fast pyrolysis volatile products from European and African biomasses. J. Anal. Appl. Pyrolysis 2011, 90, 81–92. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, Q.; You, T.; Zhang, X.; Xu, F. Microwave-assisted efficient depolymerization of alkaline lignin in methanol/formic acid media. Bioresour Technol. 2018, 264, 238–243. [Google Scholar] [CrossRef]
- Costa, F.F.; Costa, M. Particle fragmentation of raw and torrefied biomass during combustion in a drop tube furnace. Fuel 2015, 159, 530–537. [Google Scholar] [CrossRef]
- Costa, F.F.; Wang, G.; Costa, M. Combustion kinetics and particle fragmentation of raw and torrified pine shells and olive stones in a drop tube furnace. Proc. Combust. Inst. 2015, 35, 3591–3599. [Google Scholar] [CrossRef]
- Evans, R.J.; Milne, T.A. Molecular Characterization of the Pyrolysis of Biomass 1. Fundamentals. Energy Fuels Am. Chem. Soc. J. 1989, 1, 124–136. [Google Scholar]
- López, M.B.; Blanco, C.G.; Martínez-Alonso, A.; Tascón, J.M.D. Composition of gases released during olive stones pyrolysis. J. Anal. Appl. Pyrolysis 2002, 65, 313–322. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Mubarak, N.; Kundu, A.; Sahu, J.; Abdullah, E.; Jayakumar, N. Synthesis of palm oil empty fruit bunch magnetic pyrolytic char impregnating with FeCl3 by microwave heating technique. Biomass Bioenergy 2014, 61, 265–275. [Google Scholar] [CrossRef]
- Conesa, J.; Marcilla, A.; Moral, R.; Moreno-Caselles, J.; Perez-Espinosa, A. Evolution of gases in the primary pyrolysis of different sewage sludges. Thermochim. Acta 1998, 313, 63–73. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Dalluge, D.L.; Shanks, B.H.; Brown, R.C. Distinguishing primary and secondary reactions of cellulose pyrolysis. Bioresour. Technol. 2011, 102, 5265–5269. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Canabarro, N.; Soares, J.F.; Anchieta, C.G.; Kelling, C.S.; Mazutti, M.A. Thermochemical processes for biofuels production from biomass. Sustain. Chem. Process. 2013, 1, 22. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.S.; Brammer, J.G.; Hornung, A.; Steele, A.; Poulston, S. The intermediate pyrolysis and catalytic steam reforming of Brewers spent grain. J. Anal. Appl. Pyrolysis 2013, 103, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Melligan, F.; Hayes, M.; Kwapinski, W.; Leahy, J. Hydro-pyrolysis of biomass and online catalytic vapor upgrading with Ni-ZSM-5 and Ni-MCM-41. Energy Fuels 2012, 26, 6080–6090. [Google Scholar] [CrossRef]
- Benallal, B.; Roy, C.; Pakdel, H.; Chabot, S.; Poirier, M. Characterization of pyrolytic light naphtha from vacuum pyrolysis of used tyres comparison with petroleum naphtha. Fuel 1995, 74, 1589–1594. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Encinar, J.; Gonzalez, J.; Gonzalez, J. Fixed-bed pyrolysis of Cynara cardunculus L. Product yields and compositions. Fuel Process. Technol. 2000, 68, 209–222. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S. Effect of particle size on pyrolysis of single-component municipal solid waste in fixed bed reactor. Int. J. Hydrog. Energy 2010, 35, 93–97. [Google Scholar] [CrossRef]
- Pütün, A.; Özcan, A.; Pütün, E. Pyrolysis of hazelnut shells in a fixed-bed tubular reactor: Yields and structural analysis of bio-oil. J. Anal. Appl. Pyrolysis 1999, 52, 33–49. [Google Scholar] [CrossRef]
- Sensoz, S.; Angin, D. Pyrolysis of safflower (Charthamus tinctorius L.) seed press cake: Part 1. The effects of pyrolysis parameters on the product yields. Bioresour. Technol. 2008, 99, 5492–5497. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Y.; Cen, K.; Luo, M.; Li, H.; Lu, B. Pyrolysis polygeneration of poplar wood: Effect of heating rate and pyrolysis temperature. Bioresour. Technol. 2016, 218, 780–788. [Google Scholar] [CrossRef]
- Park, H.J.; Park, Y.-K.; Kim, J.S. Influence of reaction conditions and the char separation system on the production of bio-oil from radiata pine sawdust by fast pyrolysis. Fuel Process. Technol. 2008, 89, 797–802. [Google Scholar] [CrossRef]
- Antal, M.J., Jr.; Allen, S.G.; Dai, X.; Shimizu, B.; Tam, M.S.; Grønli, M. Attainment of the theoretical yield of carbon from biomass. Ind. Eng. Chem. Res. 2000, 39, 4024–4031. [Google Scholar] [CrossRef]
- Zhao, B.; O′Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Ertaş, M.; Alma, M.H. Pyrolysis of laurel (Laurus nobilis L.) extraction residues in a fixed-bed reactor: Characterization of bio-oil and bio-char. J. Anal. Appl. Pyrolysis 2010, 88, 22–29. [Google Scholar] [CrossRef]
- Ahmed, A.; Abu Bakar, M.S.; Hamdani, R.; Park, Y.K.; Lam, S.S.; Sukri, R.S.; Hussain, M.; Majeed, K.; Phusunti, N.; Jamil, F.; et al. Valorization of underutilized waste biomass from invasive species to produce biochar for energy and other value-added applications. Environ. Res. 2020, 186, 109596. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Stahl, R.; Younesi, H.; Rashidi, A.; Troeger, N.; Ghoreyshi, A.A. Effect of process conditions on product yield and composition of fast pyrolysis of Eucalyptus grandis in fluidized bed reactor. J. Ind. Eng. Chem. 2014, 20, 2594–2602. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Ladanai, S.; Vinterbäck, J. Global Potential of Sustainable Biomass for Energy; Institutionen för Energi och Teknik, SLU: Uppsala, Sweden, 2009; ISSN 1654-9406. [Google Scholar]
- Mushrush, G.W.; Beal, E.J.; Hughes, J.M.; Wynne, J.H.; Sakran, J.V.; Hardy, D.R. Biodiesel fuels: Use of soy oil as a blending stock for middle distillate petroleum fuels. Ind. Eng. Chem. Res. 2000, 39, 3945–3948. [Google Scholar] [CrossRef]
- Cheng, F.; Bayat, H.; Jena, U.; Brewer, C.E. Impact of feedstock composition on pyrolysis of low-cost, protein and lignin-rich biomass: A review. J. Anal. Appl. Pyrolysis 2020, 147, 104780. [Google Scholar] [CrossRef]
- Spelter, H.; Toth, D. North America′s Wood Pellet Sector; Research Paper FPL-RP-656; US Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2009; Volume 656, 21p. [Google Scholar]
- Oasmaa, A.; Kuoppala, E.; Solantausta, Y. Fast pyrolysis of forestry residue. 2. Physicochemical composition of product liquid. Energy Fuels 2003, 17, 433–443. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A. Chemical composition of bio-oils produced by fast pyrolysis of two energy crops. Energy Fuels 2008, 22, 2104–2109. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Delong, M.; Swanberg, D.; Oelke, E. Sustainable Biomass Energy Production and Rural Economic Development Using Alfalfa as Feedstock; National Renewable Energy Laboratory: Golden, CO, USA, 1995. [Google Scholar]
- Sheaffer, C.C.; Martin, N.P.; Lamb, J.F.; Cuomo, G.R.; Jewett, J.G.; Quering, S.R. Leaf and stem properties of alfalfa entries. Agron. J. 2000, 92, 733–739. [Google Scholar] [CrossRef]
- Oudenhoven, S.; van der Ham, A.G.; van den Berg, H.; Westerhof, R.J.M.; Kersten, S.R. Using pyrolytic acid leaching as a pretreatment step in a biomass fast pyrolysis plant: Process design and economic evaluation. Biomass Bioenergy 2016, 95, 388–404. [Google Scholar] [CrossRef] [Green Version]
- Ateş, F.; Miskolczi, N.; Saricaoğlu, B. Pressurized pyrolysis of dried distillers grains with solubles and canola seed press cake in a fixed-bed reactor. Bioresour. Technol. 2015, 177, 149–158. [Google Scholar] [CrossRef]
- Smets, K.; Schreurs, S.; Carleer, R.; Yperman, J. Valorization of raspberry seed cake by flash and slow pyrolysis: Product yield and characterization of the liquid and solid fraction. J. Anal. Appl. Pyrolysis 2014, 107, 289–297. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Co-pyrolysis of biodiesel-derived glycerol with Greek lignite: A laboratory study. J. Anal. Appl. Pyrolysis 2013, 100, 166–172. [Google Scholar] [CrossRef]
- Song, R. Lipid Peroxidation in Corn Dried Distillers Grains with Solubles (DDGS) and Effects of Feeding a Highly Oxidized DDGS Source to Swine. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2013. [Google Scholar]
- Pasangulapati, V.; Ramachandriya, K.D.; Kumar, A.; Wilkins, M.R.; Jones, C.L.; Huhnke, R.L. Effects of cellulose, hemicellulose and lignin on thermochemical conversion characteristics of the selected biomass. Bioresour. Technol. 2012, 114, 663–669. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage sludge combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- EPA. Advancing Sustainable Materials Management: Facts and Figures, 2016; Office of Land and Emergency Management (5306P): Washington, DC, USA, 2014; p. 20460. [Google Scholar]
- EPA. Overview of Greenhouse Gases. 2017. Available online: http://learning-cleanairasia.org/lms/library/ga3/99-Overview-of-Greenhouse-Gases.pdf (accessed on 2 February 2021).
- Cao, J.-P.; Li, L.-Y.; Morishita, K.; Xiao, X.-B.; Zhao, X.-Y.; Wei, X.-Y.; Takarada, T. Nitrogen transformations during fast pyrolysis of sewage sludge. Fuel 2013, 104, 1–6. [Google Scholar] [CrossRef]
- De Vrieze, J. The next frontier of the anaerobic digestion microbiome: From ecology to process control. Environ. Sci. Ecotechnology 2020, 3, 100032. [Google Scholar] [CrossRef]
- Inguanzo, M.; Domınguez, A.; Menéndez, J.; Blanco, C.; Pis, J. On the pyrolysis of sewage sludge: The influence of pyrolysis conditions on solid, liquid and gas fractions. J. Anal. Appl. Pyrolysis 2002, 63, 209–222. [Google Scholar] [CrossRef]
- Fonts, I.; Kuoppala, E.; Oasmaa, A. Physicochemical properties of product liquid from pyrolysis of sewage sludge. Energy Fuels 2009, 23, 4121–4128. [Google Scholar] [CrossRef]
- Ahmad, A.; Yasin, N.M.; Derek, C.; Lim, J. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Acikgoz, C.; Kockar, O. Flash pyrolysis of linseed (Linum usitatissimum L.) for production of liquid fuels. J. Anal. Appl. Pyrolysis 2007, 78, 406–412. [Google Scholar] [CrossRef]
- Scott, D.S.; Majerski, P.; Piskorz, J.; Radlein, D. A second look at fast pyrolysis of biomass—The RTI process. J. Anal. Appl. Pyrolysis 1999, 51, 23–37. [Google Scholar] [CrossRef]
- Fonts, I.; Azuara, M.; Gea, G.; Murillo, M. Study of the pyrolysis liquids obtained from different sewage sludge. J. Anal. Appl. Pyrolysis 2009, 85, 184–191. [Google Scholar] [CrossRef]
- Duku, M.H. Bio-oil Production from Lignocellulosic Biomass Using Fast Pyrolysis in a Fluidized-bed Reactor. Ph.D. Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2015. [Google Scholar]
- Kim, S.-S.; Agblevor, F.A. Thermogravimetric analysis and fast pyrolysis of Milkweed. Bioresour. Technol. 2014, 169, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-P.; Xiao, X.-B.; Zhang, S.-Y.; Zhao, X.-Y.; Sato, K.; Ogawa, Y.; Wei, X.-Y.; Takarada, T. Preparation and characterization of bio-oils from internally circulating fluidized-bed pyrolyses of municipal, livestock, and wood waste. Bioresour. Technol. 2011, 102, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Lopez, G.; Amutio, M.; Artetxe, M.; Barbarias, I.; Arregi, A.; Bilbao, J.; Olazar, M. Characterization of the bio-oil obtained by fast pyrolysis of sewage sludge in a conical spouted bed reactor. Fuel Process. Technol. 2016, 149, 169–175. [Google Scholar] [CrossRef]
- Önal, E.P.; Uzun, B.B.; Pütün, A.E. Steam pyrolysis of an industrial waste for bio-oil production. Fuel Process. Technol. 2011, 92, 879–885. [Google Scholar] [CrossRef]
- García, A.; Alriols, M.G.; Labidi, J. Evaluation of different lignocellulosic raw materials as potential alternative feedstocks in biorefinery processes. Ind. Crop. Prod. 2014, 53, 102–110. [Google Scholar] [CrossRef]
- Lee, H.; Hamid, S.B.A.; Zain, S. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Alrumman, S.A. Enzymatic saccharification and fermentation of cellulosic date palm wastes to glucose and lactic acid. Braz. J. Microbiol. 2016, 47, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Galiwango, E.; Al-Marzouqi, A.H.; Abu-Omar, M.M.; Khaleel, A.A.; Rahman, N.A. Estimating combustion kinetics of UAE date palm tree biomass using thermogravimetric analysis. J. Nat. Sci. Res. 2017, 7, 106–120. [Google Scholar]
- Kung, C.-C.; McCarl, B.A.; Cao, X. Economics of pyrolysis-based energy production and biochar utilization: A case study in Taiwan. Energy Policy 2013, 60, 317–323. [Google Scholar] [CrossRef]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 2010, 89, S2–S10. [Google Scholar] [CrossRef] [Green Version]
- Thilakaratne, R.; Brown, T.; Li, Y.; Hu, G.; Brown, R. Mild catalytic pyrolysis of biomass for production of transportation fuels: A techno-economic analysis. Green Chem. 2014, 16, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Gebreslassie, B.H.; Slivinsky, M.; Wang, B.; You, F. Life cycle optimization for sustainable design and operations of hydrocarbon biorefinery via fast pyrolysis, hydrotreating and hydrocracking. Comput. Chem. Eng. 2013, 50, 71–91. [Google Scholar] [CrossRef]
- Jones, S.B.; Valkenburt, C.; Walton, C.W.; Elliott, D.C.; Holladay, J.E.; Stevens, D.J.; Kinchin, C.; Czernik, S. Production of Gasoline and Diesel from Biomass via Fast Pyrolysis, Hydrotreating and Hydrocracking: A Design Case; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2009. [Google Scholar]
- Li, Q.; Zhang, Y.; Hu, G. Techno-economic analysis of advanced biofuel production based on bio-oil gasification. Bioresour. Technol. 2015, 191, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Cleary, J.; Caspersen, J.P. Comparing the life cycle impacts of using harvest residue as feedstock for small-and large-scale bioenergy systems (part I). Energy 2015, 88, 917–926. [Google Scholar] [CrossRef]
- Kung, C.-C.; Zhang, N. Renewable energy from pyrolysis using crops and agricultural residuals: An economic and environmental evaluation. Energy 2015, 90, 1532–1544. [Google Scholar] [CrossRef]
- Popp, J.; Lakner, Z.; Harangi-Rakos, M.; Fari, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef] [Green Version]
- Kuppens, T.; Van Dael, M.; Vanreppelen, K.; Thewys, T.; Yperman, J.; Carleer, R.; Schreurs, S.; Van Passel, S. Techno-economic assessment of fast pyrolysis for the valorization of short rotation coppice cultivated for phytoextraction. J. Clean. Prod. 2015, 88, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Bridgwater, A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Ahmed, A.; Abu Bakar, M.S.; Azad, A.K.; Sukri, R.S.; Phusunti, N. Intermediate pyrolysis of Acacia cincinnata and Acacia holosericea species for bio-oil and biochar production. Energy Convers. Manag. 2018, 176, 393–408. [Google Scholar] [CrossRef]
- Radmanesh, R.; Chaouki, J.; Guy, C. Biomass gasification in a bubbling fluidized bed reactor: Experiments and modeling. AIChE J. 2006, 52, 4258–4272. [Google Scholar] [CrossRef]
- Zenz, F.A. The Fluid Mechanics of Bubbling Beds. Fibonacci Q. 1978, 16, 171–189. [Google Scholar]
- Basu, P. Combustion and Gasification in Fluidized Beds; Taylor and Francis: Didcot, UK, 2006. [Google Scholar]
- Warnecke, R. Gasification of biomass: Comparison of fixed bed and fluidized bed gasifier. Biomass Bioenergy 2000, 18, 489–497. [Google Scholar] [CrossRef]
- Mellin, P.; Kantarelis, E.; Yang, W. Computational fluid dynamics modeling of biomass fast pyrolysis in a fluidized bed reactor, using a comprehensive chemistry scheme. Fuel 2014, 117, 704–715. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Huang, H.; Xiao, G. Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour. Technol. 2009, 100, 1428–1434. [Google Scholar] [CrossRef]
- Dong, N.H.; Luo, K.H.; Wang, Q. Modeling of biomass pyrolysis in a bubbling fluidized bed reactor: Impact of intra-particle heat conduction. Fuel Process. Technol. 2017, 161, 199–203. [Google Scholar] [CrossRef]
- Grace, J.R.; Lim, C.J.; Brereton, C.M.H.; Chaouki, J. Circulating fluidized bed reactor design and operation. Sadhand 1987, 10, 35–48. [Google Scholar] [CrossRef]
- Chandel, M.K.; Alappat, B.J. Annular Downflow Layer in a Recirculating Fluidized Bed. Ind. Eng. Chem. Res. 2006, 45, 5748–5754. [Google Scholar] [CrossRef]
- Zhu, J.X.; Yu, Z.Q.; Jin, Y.; Grace, J.R.; Issangya, A. Cocurrent Downflow Circulating Fluidized Bed (Downer) Reactors—A State of the Art Review. Can. J. Chem. Eng. 1995, 73, 662–667. [Google Scholar] [CrossRef]
- Li, X.T.; Grace, J.R.; Lim, C.J.; Watkinson, A.P.; Chen, H.P.; Kim, J.R. Biomass gasification in a circulating fluidized bed. Biomass Bioenergy 2004, 26, 171–193. [Google Scholar] [CrossRef]
- Xianwen, D.; Chuangzhi, W.; Haibin, L.; Yong, C. The Fast Pyrolysis of Biomass in CFB Reactor. Energy Fuels 2000, 14, 552–557. [Google Scholar] [CrossRef]
- Charles, N.S. Heterogeneous Catalysis in Practice; McGraw-Hill: New York, NY, USA, 1980; p. 321. [Google Scholar] [CrossRef]
- Chawla, M.; Rafiq, S.; Jamil, F.; Usman, M.R.; Khurram, S.; Ghauri, M.; Muhammad, N.; Ala′a, H.; Aslam, M. Hydrocarbons fuel upgradation in the presence of modified bi-functional catalyst. J. Clean. Prod. 2018, 198, 683–692. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Y.; Zong, P.; Qin, S.; Wang, C.; Tian, Y. Fast pyrolysis characteristics of two typical coastal zone biomass fuels by thermal gravimetric analyzer and down tube reactor. Bioresour Technol. 2019, 283, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, M.A.F.; Uemura, Y.; Osman, N.B.; Yusup, S. Fast pyrolysis of hardwood residues using a fixed bed drop-type pyrolyzer. Energy Convers. Manag. 2015, 98, 208–214. [Google Scholar] [CrossRef]
- Ly, H.V.; Kim, S.-S.; Choi, J.H.; Woo, H.C.; Kim, J. Fast pyrolysis of Saccharina japonica alga in a fixed-bed reactor for bio-oil production. Energy Convers. Manag. 2016, 122, 526–534. [Google Scholar] [CrossRef]
- Helleur, R.; Popovic, N.; Ikura, M.; Stanciulescu, M.; Liu, D. Characterization and potential applications of pyrolytic char from ablative pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 58, 813–824. [Google Scholar] [CrossRef]
- Peacocke, G.V.C. Ablative Pyrolyser; Aston University: Birmingham, UK, 2002. [Google Scholar]
- Luo, G.; Chandler, D.S.; Anjos, L.C.A.; Eng, R.J.; Jia, P.; Resende, F.L.P. Pyrolysis of whole wood chips and rods in a novel ablative reactor. Fuel 2017, 194, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Pecocke, G.V.C.; Bridgwater, A.V. Design of a Novel Ablative Pyrolysis Reactor. In Advances in Thermochemical Biomass Conversion; Bridgwater, A.V., Ed.; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar] [CrossRef]
- Peacocke, G.V.C.; Bridgwater, A.V. Ablative Plate Pyrolysis of Biomass for Liquids. Biomass Eioenerg. 1994, 7, 147–154. [Google Scholar] [CrossRef]
- Flaxman, R.J.; Hallett, W.L. Flow and particle heating in an entrained flow reactor. Fuel 1987, 66, 607–611. [Google Scholar] [CrossRef]
- Brown, A.L.; Dayton, D.C.; Nimlos, M.R.; Daily, J.W. Design and Characterization of an Entrained Flow Reactor for the Study of Biomass Pyrolysis Chemistry at High Heating Rates. Energy Fuels 2001, 15, 1276–1285. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Guo, R.; Song, N.; Wang, J.; Cao, Y.; Pan, W.P. Mercury adsorption characteristics of HBr-modified fly ash in an entrained-flow reactor. J. Environ. Sci. 2015, 33, 156–162. [Google Scholar] [CrossRef]
- Laxminarayan, Y.; Jensen, P.A.; Wu, H.; Frandsen, F.J.; Sander, B.; Glarborg, P. Biomass fly ash deposition in an entrained flow reactor. Proc. Combust. Inst. 2019, 37, 2689–2696. [Google Scholar] [CrossRef]
- Dupont, C.; Commandré, J.-M.; Gauthier, P.; Boissonnet, G.; Salvador, S.; Schweich, D. Biomass pyrolysis experiments in an analytical entrained flow reactor between 1073K and 1273K. Fuel 2008, 87, 1155–1164. [Google Scholar] [CrossRef] [Green Version]
- Bitowft, B.; Andersson, L.A.; Bjerle, I. Fast pyrolysis of sawdust in an entrained flow reactor. Fuel 1989, 68, 561–566. [Google Scholar] [CrossRef]
- Iisa, K.; Johansson, A.-C.; Pettersson, E.; French, R.J.; Orton, K.A.; Wiinikka, H. Chemical and physical characterization of aerosols from fast pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2019. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.K.; Oh, C.H.; Park, J.W.; Kwon, E.E. Production of bio-oil from fast pyrolysis of biomass using a pilot-scale circulating fluidized bed reactor and its characterization. J. Environ. Manag. 2019, 234, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Suntivarakorn, R.; Treedet, W.; Singbua, P.; Teeramaetawat, N. Fast pyrolysis from Napier grass for pyrolysis oil production by using circulating Fluidized Bed Reactor: Improvement of pyrolysis system and production cost. Energy Rep. 2018, 4, 565–575. [Google Scholar] [CrossRef]
- Fonseca, F.G.; Funke, A.; Niebel, A.; Soares Dias, A.P.; Dahmen, N. Moisture content as a design and operational parameter for fast pyrolysis. J. Anal. Appl. Pyrolysis 2019, 139, 73–86. [Google Scholar] [CrossRef]
- Upadhyay, M.; Park, H.C.; Choi, H.S. Multiphase fluid dynamics coupled fast pyrolysis of biomass in a rectangular bubbling fluidized bed reactor: Process intensification. Chem. Eng. Process. Process Intensif. 2018, 128, 180–187. [Google Scholar] [CrossRef]
- Park, J.-W.; Heo, J.; Ly, H.V.; Kim, J.; Lim, H.; Kim, S.-S. Fast pyrolysis of acid-washed oil palm empty fruit bunch for bio-oil production in a bubbling fluidized-bed reactor. Energy 2019, 179, 517–527. [Google Scholar] [CrossRef]
- Xu, S.; Lai, D.; Zeng, X.; Zhang, L.; Han, Z.; Cheng, J.; Xu, G. Pyrolysis characteristics of waste tire particles in fixed-bed reactor with internals. Carbon Resour. Convers. 2017, 24, 1–20. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Ramachandran, S.; Subbiah, S. Modeling, experimental validation and optimization of Prosopis juliflora fuelwood pyrolysis in fixed-bed tubular reactor. Bioresour Technol. 2018, 264, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Wise, H.G.; Dichiara, A.B.; Resende, F.L.P. Ex-situ catalytic fast pyrolysis of Beetle-killed lodgepole pine in a novel ablative reactor. Fuel 2019, 241, 933–940. [Google Scholar] [CrossRef]
- Shoaib, A.M.; El-Adly, R.A.; Hassanean, M.H.M.; Youssry, A.; Bhran, A.A. Developing a free-fall reactor for rice straw fast pyrolysis to produce bio-products. Egypt. J. Pet. 2018, 27, 1305–1311. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Tan, H.; Bai, S.; Mikulčić, H.; Yang, F. Evolution of PM2.5 from biomass high-temperature pyrolysis in an entrained flow reactor. J. Energy Inst. 2018, 92, 1548–1556. [Google Scholar] [CrossRef]
- Moogi, S.; Jae, J.; Kannapu, H.P.R.; Ahmed, A.; Park, E.D.; Park, Y.K. Enhancement of aromatics from catalytic pyrolysis of yellow poplar: Role of hydrogen and methane decomposition. Bioresour. Technol. 2020, 315. [Google Scholar] [CrossRef] [PubMed]
- Pütün, E. Catalytic pyrolysis of biomass: Effects of pyrolysis temperature, sweeping gas flow rate and MgO catalyst. Energy 2010, 35, 2761–2766. [Google Scholar] [CrossRef]
- Yin, W.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Catalytic Hydrotreatment of the Pyrolytic Sugar and Pyrolytic Lignin Fractions of Fast Pyrolysis Liquids Using Nickel Based Catalysts. Energies 2020, 13, 285. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; O′connor, P. Processing biomass-derived oxygenates in the oil refinery: Catalytic cracking (FCC) reaction pathways and role of catalyst. J. Catal. 2007, 247, 307–327. [Google Scholar] [CrossRef]
- Jia, L.Y.; Raad, M.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Bettahar, M.M.; Mauviel, G.; Tarrighi, M.; Pinard, L.; Dufour, A. Catalytic fast pyrolysis of biomass: Superior selectivity of hierarchical zeolite to aromatics. R. Soc. Chem. 2017, 19, 5442–5459. [Google Scholar] [CrossRef]
- Wang, K.; Dayton, D.C.; Peters, J.E.; Mante, O.D. Reactive catalytic fast pyrolysis of biomass to produce high-quality bio-crude. Green Chem. 2017, 19, 3243–3251. [Google Scholar] [CrossRef]
- Demirbas, A.; Ahmad, W.; Alamoudi, R.; Sheikh, M. Sustainable charcoal production from biomass. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1882–1889. [Google Scholar] [CrossRef]
- Howe, D.; Westover, T.; Carpenter, D.; Santosa, D.; Emerson, R.; Deutch, S.; Starace, A.; Kutnyakov, I.; Lukins, C. Field-to-fuel performance testing of lignocellulosic feedstocks: An integrated study of the fast pyrolysis–hydrotreating pathway. Energy Fuels 2015, 29, 3188–3197. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Dalley, T.S.L.; Helleur, R.J. Preliminary laboratory production and characterization of biochars from lignocellulosic municipal waste. J. Anal. Appl. Pyrolysis 2013, 99, 71–78. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Pilon, G.; Lavoie, J.-M. Pyrolysis of switchgrass (Panicum virgatum L.) at low temperatures within N2 and CO2 environments: Product yield study. ACS Sustain. Chem. Eng. 2013, 1, 198–204. [Google Scholar] [CrossRef]
- Muradov, N.; Fidalgo, B.; Gujar, A.C.; Garceau, N.; T.-Raissi, A. Production and characterization of Lemna minor bio-char and its catalytic application for biogas reforming. Biomass Bioenergy 2012, 42, 123–131. [Google Scholar] [CrossRef]
- Kabir, G.; Mohd Din, A.T.; Hameed, B.H. Pyrolysis of oil palm mesocarp fiber and palm frond in a slow-heating fixed-bed reactor: A comparative study. Bioresour Technol. 2017, 241, 563–572. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, J.; Wang, J. Pyrolytic characteristics of pine wood in a slowly heating and gas sweeping fixed-bed reactor. J. Anal. Appl. Pyrolysis 2009, 84, 179–184. [Google Scholar] [CrossRef]
- Aramideh, S.; Xiong, Q.; Kong, S.C.; Brown, R.C. Numerical simulation of biomass fast pyrolysis in an auger reactor. Fuel 2015, 156, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Brassard, P.; Godbout, S.; Raghavan, V. Pyrolysis in auger reactors for biochar and bio-oil production: A review. Biosyst. Enineering 2017, 161, 80–92. [Google Scholar] [CrossRef]
- Prapakornrattana, P.P.U.I.P.; Charoen, K. Effect of Temperature on Product Yield from the Pyrolysis of Soybean Cake in an Auger Reactor. In Proceedings of the 3rd TIChE International Conference 2013, Khon Kaen, Thailand, 17–18 October 2013; Volume 1, pp. 1–3. [Google Scholar]
- Garcia-Perez, M.; Adams, T.T.; Goodrum, J.W.; Geller, D.P.; Das, K.C. Production and Fuel Properties of Pine Chip Bio-oil/Biodiesel Blends. Energy Fuels 2007, 21, 2363–2372. [Google Scholar] [CrossRef]
- Liaw, S.-S.; Wang, Z.; Ndegwa, P.; Frear, C.; Ha, S.; Li, C.-Z.; Garcia-Perez, M. Effect of pyrolysis temperature on the yield and properties of bio-oils obtained from the auger pyrolysis of Douglas Fir wood. J. Anal. Appl. Pyrolysis 2012, 93, 52–62. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.D.; Lappas, A.A. Catalyst deactivation, ash accumulation and bio-oil deoxygenation during ex situ catalytic fast pyrolysis of biomass in a cascade thermal-catalytic reactor system. Fuel Process. Technol. 2019, 186, 99–109. [Google Scholar] [CrossRef]
- Li, S.-Q.; Yao, Q.; Chi, Y.; Yan, J.-H.; Cen, K.-F. Pilot-scale pyrolysis of scrap tires in a continuous rotary kiln reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Montagnaro, F.; Tregambi, C.; Salatino, P.; Senneca, O.; Solimene, R. Modelling oxy-pyrolysis of sewage sludge in a rotary kiln reactor. Fuel 2018, 231, 468–478. [Google Scholar] [CrossRef]
- Li, A.M.; Li, X.D.; Li, S.Q.; Ren, Y.; Chi, Y.; Yan, J.H.; Cen, K.F. Pyrolysis of solid waste in a rotary kiln: Influence of final pyrolysis temperature on the pyrolysis products. J. Anal. Appl. Pyrolysis 1999, 50, 149–162. [Google Scholar] [CrossRef]
- Fantozzi, F.; Colantoni, S.; Bartocci, P.; Desideri, U. Rotary Kiln Slow Pyrolysis for Syngas and Char Production From Biomass and Waste—Part I: Working Envelope of the Reactor. J. Eng. Gas Turbines Power 2007, 129, 901. [Google Scholar] [CrossRef]
- Colin, B.; Dirion, J.L.; Arlabosse, P.; Salvador, S. Wood chips flow in a rotary kiln: Experiments and modeling. Chem. Eng. Res. Des. 2015, 98, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Jiang, D.; Wang, S.; Barati, B.; Gong, X.; Cao, B.; Zhang, R.; Zhang, C.; Odey, E.A. Study on catalytic pyrolysis mechanism of seaweed polysaccharide monomer. Combust. Flame 2020, 218, 1–11. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic pyrolysis of plastic waste: Moving toward pyrolysis based biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Valla, J.A.; Bollas, G.M. Characteristics and origin of char and coke from fast and slow, catalytic and thermal pyrolysis of biomass and relevant model compounds. Green Chem. 2013, 15, 3214. [Google Scholar] [CrossRef]
- Russell, S.H.; Turrion-Gomez, J.L.; Meredith, W.; Langston, P.; Snape, C.E. Increased charcoal yield and production of lighter oils from the slow pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2017, 124, 536–541. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Biswas, A.B. Pyrolysis of orange bagasse: Comparative study and parametric influence on the product yield and their characterization. J. Environ. Chem. Eng. 2019, 7, 102903. [Google Scholar] [CrossRef]
- Ghysels, S.; Ronsse, F.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar from lignin-rich digested stillage from lignocellulosic ethanol production. Biomass Bioenergy 2019, 122, 349–360. [Google Scholar] [CrossRef]
- Farrokh, N.T.; Suopajärvi, H.; Mattila, O.; Umeki, K.; Phounglamcheik, A.; Romar, H.; Sulasalmi, P.; Fabritius, T. Slow pyrolysis of by-product lignin from wood-based ethanol production—A detailed analysis of the produced chars. Energy 2018, 164, 112–123. [Google Scholar] [CrossRef]
- Babler, M.U.; Phounglamcheik, A.; Amovic, M.; Ljunggren, R.; Engvall, K. Modeling and pilot plant runs of slow biomass pyrolysis in a rotary kiln. Appl. Energy 2017, 207, 123–133. [Google Scholar] [CrossRef]
- Klose, W.; Wiest, W. Experiments and mathematical modeling of maize pyrolysis in a rotary kiln. Fuel 1999, 78, 65–72. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, N.; Zhang, L.; Li, A. Modeling and Simulation of Oil Sludge Pyrolysis in a Rotary Kiln with a Solid Heat Carrier: Considering the Particle Motion and Reaction Kinetics. Energy Fuels 2014, 28, 6029–6037. [Google Scholar] [CrossRef]
- Mao, X.; Kang, Q.; Liu, Y.; Siyal, A.A.; Ao, W.; Ran, C.; Fu, J.; Deng, Z.; Song, Y.; Dai, J. Microwave-assisted pyrolysis of furfural residue in a continuously operated auger reactor: Biochar characterization and analysis. Energy 2019, 168, 573–584. [Google Scholar] [CrossRef]
- Liaw, S.-S.; Zhou, S.; Wu, H.; Garcia-Perez, M. Effect of pretreatment temperature on the yield and properties of bio-oils obtained from the auger pyrolysis of Douglas fir wood. Fuel 2013, 103, 672–682. [Google Scholar] [CrossRef]
- Guda, V.K.; Toghiani, H. Altering bio-oil composition by catalytic treatment of pinewood pyrolysis vapors over zeolites using an auger—Packed bed integrated reactor system. Biofuel Res. J. 2016, 3, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Marmur, B.L.; Heindel, T.J. Effect of particle size, density, and concentration on granular mixing in a double screw pyrolyzer. Powder Technol. 2016, 302, 222–235. [Google Scholar] [CrossRef]
- Venderbosch, R.; Prins, W. Fast pyrolysis technology development. Biofuels 2010, 4, 178–208. [Google Scholar] [CrossRef]
- Isahak, W.N.; Hisham, M.W.; Yarmo, M.A.; Hin, T.Y.Y. A review on bio-oil production from biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923. [Google Scholar] [CrossRef]
- García-Pérez, M.; Chaala, A.; Roy, C. Vacuum pyrolysis of sugarcane bagasse. J. Anal. Appl. Pyrolysis 2002, 65, 111–136. [Google Scholar] [CrossRef]
- García-Pérez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Vacuum pyrolysis of softwood and hardwood biomass. J. Anal. Appl. Pyrolysis 2007, 78, 104–116. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, T.; Ma, L.; Zhang, Q.; Wang, L. Upgrading of liquid fuel from the vacuum pyrolysis of biomass over the Mo–Ni/γ-Al2O3 catalysts. Biomass Bioenergy 2009, 33, 1030–1036. [Google Scholar] [CrossRef]
- Foon, S.Y.; Latiff, N.S.A.; Liew, R.K.; Yek, P.N.Y.; Lam, S.S. Production of biochar for potential catalytic and energy applications via microwave vacuum pyrolysis conversion of cassava stem. Mater. Sci. Energy Technol. 2020, 3, 728–733. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Ren, S.; Wang, L.; Holladay, J.; Zhang, Q.; Tang, J.; Ruan, R. Phenol and phenolics from lignocellulosic biomass by catalytic microwave pyrolysis. Bioresour Technol. 2011, 102, 7004–7007. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, P.; Zhang, B.; Yang, C.; Liu, Y.; Lin, X.; Ruan, R. Microwave-assisted pyrolysis of biomass: Catalysts to improve product selectivity. J. Anal. Appl. Pyrolysis 2009, 86, 161–167. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Ren, S.; Wang, L.; Zhang, Q.; Tang, J.; Ruan, R. Production of phenols and biofuels by catalytic microwave pyrolysis of lignocellulosic biomass. Bioresour Technol. 2012, 108, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Yin, C. Microwave-assisted pyrolysis of biomass for liquid biofuels production. Bioresour Technol. 2012, 120, 273–284. [Google Scholar] [CrossRef]
- Robinson, J.P.; Kingman, S.W.; Barranco, R.; Snape, C.E.; Al-Sayegh, H. Microwave Pyrolysis of Wood Pellets. Ind. Eng. Chem. Res. 2010, 49, 459–463. [Google Scholar] [CrossRef]
- Mong, G.R.; Chong, C.T.; Ng, J.-H.; Chong, W.W.F.; Lam, S.S.; Ong, H.C.; Ani, F.N. Microwave pyrolysis for valorisation of horse manure biowaste. Energy Convers. Manag. 2020, 220, 113074. [Google Scholar] [CrossRef]
- Horne, P.A.; Williams, P.T. Influence of temperature on the products from the flash pyrolysis of biomass. Fuel 1996, 75, 1051–1059. [Google Scholar] [CrossRef]
- Scott, D.S.; Piskorz, J. The Flash Pyrolysis of Aspen-Poplar Wood. Can. J. Chem. Eng. 1982, 60, 666–674. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Ahmed, A.; Jeffery, D.M.; Hidayat, S.; Sukri, R.S.; Mahlia, T.M.I.; Jamil, F.; Khurrum, M.S.; Inayat, A.; Moogi, S.; et al. Pyrolysis of solid waste residues from Lemon Myrtle essential oils extraction for bio-oil production. Bioresour. Technol. 2020, 123913. [Google Scholar] [CrossRef]
- Pokorna, E.; Postelmans, N.; Jenicek, P.; Schreurs, S.; Carleer, R.; Yperman, J. Study of bio-oils and solids from flash pyrolysis of sewage sludges. Fuel 2009, 88, 1344–1350. [Google Scholar] [CrossRef] [Green Version]
- Amutio, M.; Lopez, G.; Alvarez, J.; Moreira, R.; Duarte, G.; Nunes, J.; Olazar, M.; Bilbao, J. Flash pyrolysis of forestry residues from the Portuguese Central Inland Region within the framework of the BioREFINA-Ter project. Bioresour Technol. 2013, 129, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Matamba, T.; Tahmasebi, A.; Khoshk Rish, S.; Yu, J. Promotion Effects of Pressure on Polycyclic Aromatic Hydrocarbons and H2 Formation during Flash Pyrolysis of Palm Kernel Shell. Energy Fuels 2020, 34, 3346–3356. [Google Scholar] [CrossRef]
- Tang, L.; Wang, H.; Hao, H.; Wang, Y.; Huang, H. Plasma pyrolysis of biomass for production of gaseous fuel to generate electricity. Dep. Environ. Eng. Guangdong Univ. Technol. 2010, 1, 1–5. [Google Scholar]
- An, H.; Cheng, Y.; Li, T.; Li, Y.; Cheng, Y. Numerical analysis of methane pyrolysis in thermal plasma for selective synthesis of acetylene. Fuel Process. Technol. 2018, 172, 195–199. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Bittner, D.; Baumann, H.; Klein, J. Relation between coal properties and acetylene yield in plasma pyrolysis *. Fuel 1985, 64, 1370–1374. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H. Plasma Pyrolysis of Biomass for Production of Syngas and Carbon Adsorbent. Energy Fuels 2005, 19, 1174–1178. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H.; Hao, H.; Zhao, K. Development of plasma pyrolysis/gasification systems for energy efficient and environmentally sound waste disposal. J. Electrostat. 2013, 71, 839–847. [Google Scholar] [CrossRef]
- Joardder, M.U.; Halder, P.K.; Rahim, A.; Paul, N. Solar Assisted Fast Pyrolysis: A Novel Approach of Renewable Energy Production. J. Eng. Gas Turbines Power 2014, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Huang, H.; Wu, C.; Li, H.; Chen, Y. Biomass pyrolysis in an aron/hydroen plasma reactor. Eng. Life Sci. 2001, 1, 197–199. [Google Scholar] [CrossRef]
- Morales, S.; Miranda, R.; Bustos, D.; Cazares, T.; Tran, H. Solar biomass pyrolysis for the production of bio-fuels and chemical commodities. J. Anal. Appl. Pyrolysis 2014, 109, 65–78. [Google Scholar] [CrossRef]
- Adinberg, R.; Epstein, M.; Karni, J. Solar Gasification of Biomass: A Molten Salt Pyrolysis Study. J. Sol. Energy Eng. 2004, 126, 850. [Google Scholar] [CrossRef]
- Zeng, K.; Minh, D.P.; Gauthier, D.; Weiss-Hortala, E.; Nzihou, A.; Flamant, G. The effect of temperature and heating rate on char properties obtained from solar pyrolysis of beech wood. Bioresour Technol. 2015, 182, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Cen, K.; Cao, X.; Zhang, J.; Chen, F.; Zhou, J. Upgrading of bio-oil via solar pyrolysis of the biomass pretreated with aqueous phase bio-oil washing, solar drying, and solar torrefaction. Bioresour Technol. 2020, 305, 123130. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, W.A.; Carrier, M.; Knoetze, J.H. Vacuum pyrolysis of intruder plant biomasses. J. Anal. Appl. Pyrolysis 2011, 92, 184–193. [Google Scholar] [CrossRef]
- Murwanashyaka, J.N.; Pakdel, H.; Roy, C. Step-wise and one-step vacuum pyrolysis of birch-derived biomass to monitor the evolution of phenols. J. Anal. Appl. Pyrolysis 2001, 60, 219–231. [Google Scholar] [CrossRef]
- Shepherd, B.J.; Ryan, J.; Adam, M.; Beneroso Vallejo, D.; Castaño, P.; Kostas, E.T.; Robinson, J.P. Microwave pyrolysis of biomass within a liquid medium. J. Anal. Appl. Pyrolysis 2018, 134, 381–388. [Google Scholar] [CrossRef]
- Lo, S.-L.; Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H. Microwave Pyrolysis of Lignocellulosic Biomass. Energy Procedia 2017, 105, 41–46. [Google Scholar] [CrossRef]
- Scott, D.S.; Piskorz, J. The Continuous Flash Pyrolysis of Biomass. Can. J. Chem. Eng. 1984, 62, 404–412. [Google Scholar] [CrossRef]
- Liden, A.G.; Berruti, F.; Scott, D.S. A Kinetic Model for the Production of Liquids from the Flash Pyrolysis of Biomass. Chem. Eng. Commun. 2010, 65, 207–221. [Google Scholar] [CrossRef]
- Blanquet, E.; Nahil, M.A.; Williams, P.T. Enhanced hydrogen-rich gas production from waste biomass using pyrolysis with non-thermal plasma-catalysis. Catal. Today 2019. [Google Scholar] [CrossRef]
- Van Oost, G.; Hrabovsky, M.; Kopecky, V.; Konrad, M.; Hlina, M.; Kavka, T. Pyrolysis/gasification of biomass for synthetic fuel production using a hybrid gas–water stabilized plasma torch. Vacuum 2008, 83, 209–212. [Google Scholar] [CrossRef]
- Ayala-Cortés, A.; Lobato-Peralta, D.R.; Arreola-Ramos, C.E.; Martínez-Casillas, D.C.; Pacheco-Catalán, D.E.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Villafán-Vidales, H.I. Exploring the influence of solar pyrolysis operation parameters on characteristics of carbon materials. J. Anal. Appl. Pyrolysis 2019, 140, 290–298. [Google Scholar] [CrossRef]
- Rony, A.H.; Kong, L.; Lu, W.; Dejam, M.; Adidharma, H.; Gasem, K.A.M.; Zheng, Y.; Norton, U.; Fan, M. Kinetics, thermodynamics, and physical characterization of corn stover (Zea mays) for solar biomass pyrolysis potential analysis. Bioresour Technol. 2019, 284, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Rowe, A.; Wild, P. A techno-economic analysis of using mobile distributed pyrolysis facilities to deliver a forest residue resource. Bioresour. Technol. 2013, 150, 367–376. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefining 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O. Investigating the potential for a self-sustaining slow pyrolysis system under varying operating conditions. Bioresour. Technol. 2014, 162, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, T.; Suzuki, S.; Kojima, T. Influence of biomass pyrolysis temperature, heating rate and type of biomass on produced char in a fluidized bed reactor. Energy Environ. Res. 2014, 4, 64. [Google Scholar] [CrossRef]
- Burton, A.; Wu, H. Mechanistic investigation into bed agglomeration during biomass fast pyrolysis in a fluidized-bed reactor. Energy Fuels 2012, 26, 6979–6987. [Google Scholar] [CrossRef]
- Coulson, M.; Bridgwater, A. Fast pyrolysis of annually harvested crops for bioenergy applications. In Proceedings of the 2nd World Conference on Biomass, Rome, Italy, 10–14 May 2004; pp. 1098–1101. [Google Scholar]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 2010, 44, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Shackley, S.; Sohi, S.; Brownsort, P. Prospective life cycle carbon abatement for pyrolysis biochar systems in the UK. Energy Policy 2011, 39, 2646–2655. [Google Scholar] [CrossRef]
- Ibarrola, R.; Shackley, S.; Hammond, J. Pyrolysis biochar systems for recovering biodegradable materials: A life cycle carbon assessment. Waste Manag. 2012, 32, 859–868. [Google Scholar] [CrossRef]
- Snowden-Swan, L.J.; Male, J.L. Summary of Fast Pyrolysis and Upgrading GHG Analyses; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2012. [Google Scholar]
- Hsu, D.D. Life cycle assessment of gasoline and diesel produced via fast pyrolysis and hydroprocessing. Biomass Bioenergy 2012, 45, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Dong, X.; Jiang, H.; Li, G.; Zhang, M. Ordered mesoporous carbon supported ferric sulfate: A novel catalyst for the esterification of free fatty acids in waste cooking oil. Fuel Process. Technol. 2014, 128, 10–16. [Google Scholar] [CrossRef]
- Fan, J.; Kalnes, T.N.; Alward, M.; Klinger, J.; Sadehvandi, A.; Shonnard, D.R. Life cycle assessment of electricity generation using fast pyrolysis bio-oil. Renew. Energy 2011, 36, 632–641. [Google Scholar] [CrossRef]
- Jaroenkhasemmeesuk, C.; Tippayawong, N. Technical and Economic Analysis of A Biomass Pyrolysis Plant. Energy Procedia 2015, 79, 950–955. [Google Scholar] [CrossRef] [Green Version]







| Feedstock | Ultimate Analysis | HHV (MJ/Kg) | VM | Ash | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O * | S | |||||
| Rice straw | 26 | 4 | 1 | 17 | 0.28 | 12.50 | 50 | 49.50 | [92] |
| Milkweed | 37 | 6 | 2.50 | 43 | – | 16.67 | 80 | 11.20 | [93] |
| Linseed | 65 | 9 | 2.45 | 30 | – | 28 | 83 | 6 | [89] |
| Wood chip | 51.50 | 6.18 | 0.30 | 41.67 | 0.1 | 20.70 | 86 | 0.30 | [94] |
| Sewage sludge | 25.50 | 4.46 | 4.84 | 25.87 | 2.07 | 11.10 | 54.20 | 37.20 | [95] |
| Potato skin | 71.32 | 6.13 | 2.57 | 9.38 | – | 35 | 78.55 | 10.60 | [96] |
| Forestry residue | 51.40 | 6 | 0.50 | 40 | 0.04 | 20.80 | 76.70 | 2.10 | [70] |
| Lignocellulosic Biomass | Cellulose | Hemicellulose | Lignin | Ref. |
|---|---|---|---|---|
| Cotton stalks | 41.7 | 27.3 | 18.7 | [97] |
| Chili stalks | 37.5 | 28.3 | 17.3 | |
| Pepper stalks | 35.7 | 26.2 | 18.3 | |
| Okra stalks | 36.3 | 28.7 | 17.9 | |
| Bean stalks | 31.1 | 26.0 | 16.7 | |
| Corncobs | 45.0 | 35.0 | 15.0 | [98] |
| Wheat straw | 30.0 | 50.0 | 15.0 | |
| Empty fruit bunch | 41.0 | 24.0 | 21.2 | |
| Wastepaper | 60–70 | 10–20 | 5–10 | |
| Date palm leaves | 59.11 | 16.71 | 16.43 | [99] |
| Date palm leaf base | 51.5 | 24.41 | 18.5 | |
| Date palm rachis | 32.0 | 19.0 | 11.0 | [100] |
| Feedstock | Technology | Reactor Type | Ton/Day | Yield | Cost $/Liter | Ref. |
|---|---|---|---|---|---|---|
| Corn stover | Fast pyrolysis-Aspen model | Fluidized-bed reactor | 2000 | Bio-oil | 0.68 (2010) | [102] |
| Wood | Fast pyrolysis-ChemCAD | Circulating fluidized bed | 2000 | Gasoline and diesel | 0.8 (2014) | [103] |
| Hybrid popular | Fast pyrolysis-non-linear programing | Circulating fluidized bed | 250–3600 | Gasoline and diesel | 0.60–0.90 (2013) | [104] |
| Hybrid popular | Fast pyrolysis-ChemCAD-ICARUS | Circulating fluidized bed | 2000 | Gasoline and diesel | 0.46–0.54 (2009) | [105] |
| Corn stover | Fast pyrolysis-Aspen Plus | Fluidized bed- Fischer Tropsch | 2000 | Gasoline and diesel | 1.48 (2015) | [106] |
| Biomass Feedstock: Lignocellulosic and Protein-Rich Agricultural Waste Residue, Seedcake, Distiller Grains, Sludge, MSW and SS, Food Waste, Forestry Waste | |
|---|---|
| Biomass Pretreatment: Physical Treatment: Drying, Grinding, Palatalization Composition Tuning: Harvesting Method and Timing, Storage Method, Chemical Treatment, Thermochemical Treatment, Co-Feeding | |
| Required product distribution: >> Biochar with << bio-oil & condensable gases | Required product distribution: >> Bio-oil & condensable gases with << biochar |
Type: Product (biochar)
| Type: (bio-oil)
|
| Operating parameters: (operating temperature, heating rate, residence time, pressure, particle size)
| Operating parameters: (operating temperature, heating rate, residence time, pressure, particle size)
|
Reactors
| Reactors
|
| Advanced Pyrolysis Techniques | |
| Vacuum pyrolysis of biomass | Microwave pyrolysis of biomass |
| Flash pyrolysis of biomass | Biomass pyrolysis via Solar Energy |
| Biomass pyrolysis via Plasma technology | |
| Feed Stock | Pyrolyzer Reactor | Parametric Study | Remarks | References |
|---|---|---|---|---|
| Stem wood | Fluidized-bed pyrolyzer | Aerosol concentrations and size distributions | Aerosols < 1 µm were formed and aerosols < 1 µm deposited during the cooling of pyrolysis vapors. | [141] |
| Sawdust, empty fruit bunch, and giant Miscanthus | Circulating fluidized-bed reactor | Heating value, moisture content, and ash content | Giant Miscanthus has the highest heating value amongst three biomass feedstocks. | [142] |
| Napier grass | Circulating fluidized-bed reactor | Reactor temperature, superficial velocity, and feed rate of feedstock | The new design of the pyrolysis system was developed to reduce the bio-oil production cost. | [143] |
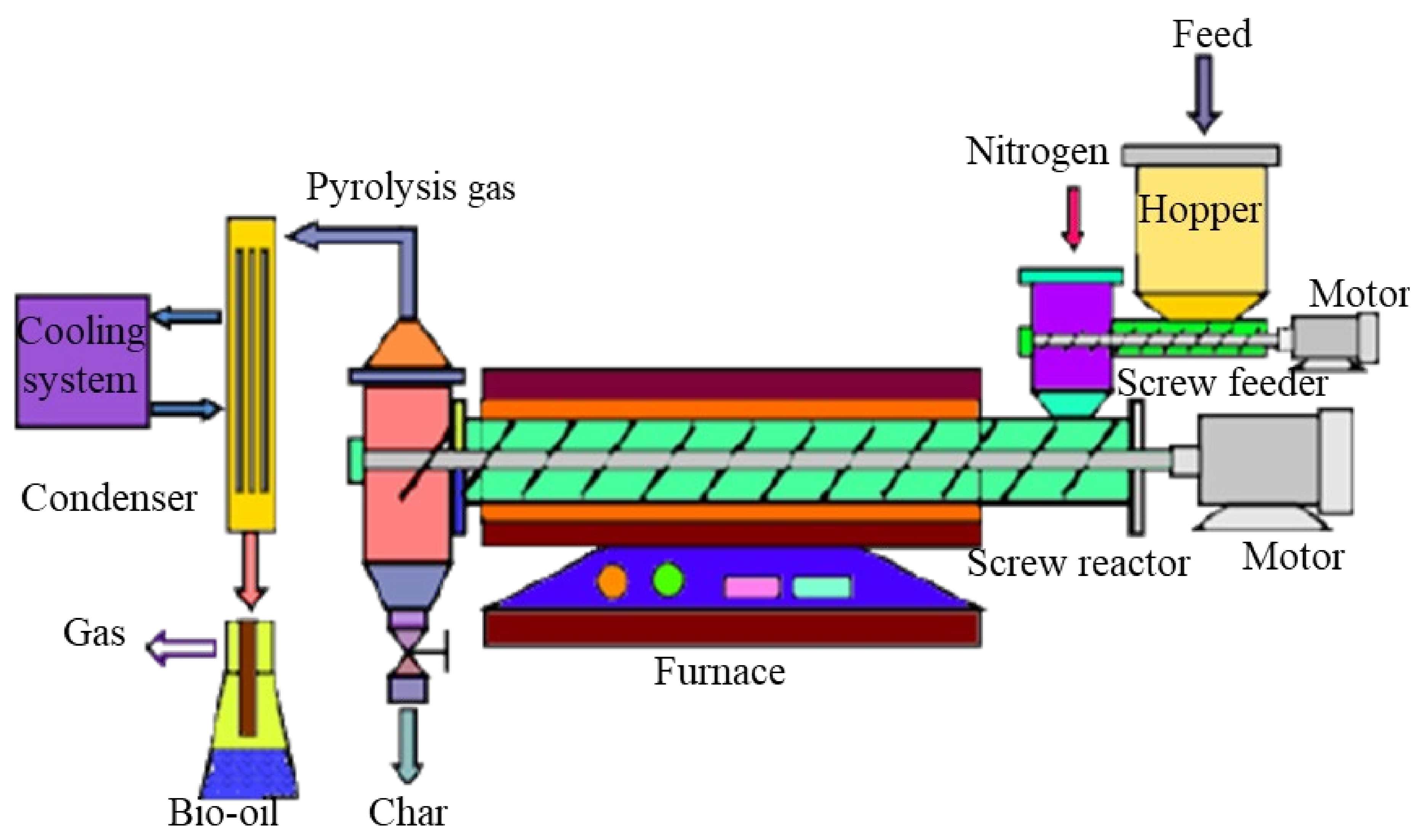
| Wheat straw | Screw reactor | Moisture content | Moisture content as design and operational parameter for the fast pyrolysis process | [144] |
| Geodae-Uksae | Bubbling fluidized-bed reactor | Reaction temperature, superficial gas velocity, and sand particle inventory | Key influencing factors were identified, and optimum conditions were proposed. | [145] |
| Oil palm empty fruit bunch | Bubbling fluidized-bed reactor | Effect of pretreatment by acid washing | The effect of pretreatment using the dilute nitric acid solution in biomass confirmed. | [146] |
| Waste tire particles | Fixed-bed reactor | Different external heating temperatures | An innovative fixed-bed reactor with internals was employed to pyrolyze waste tire particles. | [147] |
| Prosopis Juliflora | Fixed-bed tubular reactor | Particle size, operating temperature, and heating rates | The developed kinetic model was able to predict the performance of a fixed-bed tubular reactor in terms of pyrolysis product properties. | [148] |
| Beetle-killed lodgepole pine | Ablative reactor | Operating temperature and catalyst/biomass ratio | The novel ablative reactor could be converted into a portable unit without the need for biomass pretreatment. | [149] |
| Rice straw | Free-fall reactor | Particle heating rate and particle’s free-fall velocity | The designed free-fall reactor could be used for producing useful bio-products and contribute to solving problematic agriculture waste. | [150] |
| Wheat straw | Entrained flow reactor | Pyrolysis operating temperature | PM2.5 yields during biomass pyrolysis are in the range of 7–34 g/kg and proportional to a pyrolysis temperature. | [151] |
| Feed Stock | Pyrolyzer Reactor | Parametric Study | Remarks | References |
|---|---|---|---|---|
| Orange bagasse | Semi-batch reactor | Operating temperature, heating rate, and N2 gas flow rate | Biochar has an HHV of 27.76 MJ/kg because of lower O2 content than its parental biomass. | [181] |
| Lignin-rich digested stillage | Fixed-bed reactor | Operating temperature, heating rate, and holding time | It is considerably better than straw-based biochar with identical H/C and O/C ratios. | [182] |
| By-product lignin samples from wood-based bioethanol production | Laboratory-scale batch reactor (fixed bed reactor) | Pyrolysis operating temperature and heating rate | A detailed analysis of fuel characteristics, moisture uptake, and the flow properties of lignin chars derived from slow pyrolysis was presented. | [183] |
| Wood chips | Rotary kiln reactor | Bed height, bed velocity, and heat flow, flow rate nitrogen, operating temperature | A modular numerical model for the pyrolysis of biomass in a rotary kiln is presented. | [184] |
| Maize | Rotary kiln reactor | Operational temperature, solids residence time, and solid space-time | The result suggests a strong effect and pyrolysis temperature and a noticeable effect of space velocity. | [185] |
| Oil sludge pyrolysis | Rotary kiln reactor | Particle motion in rolling mode and temperature distribution | A dynamic model of oil sludge pyrolysis in a rotary kiln with a solid heat carrier was developed. | [186] |
| Furfural residue | Auger pyrolysis reactor | Effects of temperature and additives | Microwave-assisted pyrolysis of furfural residue was performed in auger reactor to optimize process parameters for maximum biochar production. | [187] |
| Douglas for wood | Auger pyrolysis reactor | Effect of thermal pretreatment temperatures | Treatment below 300 °C does not have a major effect on product yields. | [188] |
| Pinewood | Auger pyrolysis reactor | Effect of catalytic properties (acidity, pore size, and pore structure) | An integrated reactor system is reported for catalytic pyrolysis of pine wood. | [189] |
| Corn straw | Fixed-bed reactor | Effect of different moisture content, and different ash content | This work provides an overall understanding of corn combustion for a large boiler system. | [190] |
| Feed Stock | Pyrolyzer Reactor | Parametric Study | Remarks | References |
|---|---|---|---|---|
| Kraalbos, Schotzbos, and Asbos | Vacuum pyrolysis | Pyrolysis temperature, pyrolysis time, pressure, and initial moisture content | The study of vacuum pyrolysis of intruder plant species showed that it is possible to produce economically viable, high-energy charcoal and oil products. | [221] |
| Birchbark and birch sapwood | Vacuum pyrolysis | Distribution of phenols, charcoal, and water as a function of temperature | Under vacuum, stepwise thermal decomposition of biomass under low-temperature conditions is less destructive, which simplifies the analysis of pyrolysis oil. | [222] |
| Sycamore wood | Microwave pyrolysis | Effect of energy input on the pyrolysis process | A novel application of microwave pyrolysis within a liquid medium is proposed. | [223] |
| Rice straw, rice husk, corn stover, sugarcane bagasse, and bamboo leaves | Microwave pyrolysis | Empirical equations were determined to predict product yields and gaseous concentrations. | The energy return on investment of microwave pyrolysis can be approximately 3.56, so the technique should be energetically and economically feasible. | [224] |
| Hardwood waste material and wheat straw | Flash pyrolysis | Bed temperature and heating rate | A continuous atmospheric pressure flash pyrolysis process to produce liquids from biomass has been demonstrated on a scale of 2–3 kg/hr. | [225] |
| Wood | Flash pyrolysis | Operating temperature and heating rate | The proposed kinetic model can predict the organic liquid yield as a function of the operating parameters of the process. | [226] |
| Waste wood sawdust | Plasma technology pyrolysis | Comparison of catalysis, plasma, and plasma-catalysis for hydrogen-rich gas production and hydrocarbon tar reduction | Two-stage pyrolysis plasma/catalysis has been developed for enhanced H2 production. | [227] |
| Crushed wood | Plasma technology pyrolysis/gasification | The reaction temperature and heating rate | The project aims to demonstrate the economic viability, environmental performance, and safety of biofuels. | [228] |
| Agave | Solar pyrolysis | Operating temperature and heating rate | The main findings include: (i) solar pyrolysis temperature and heating rate scarcely impact char composition (ii) structure, surface area, and electrochemical performance are highly affected by both. | [229] |
| Corn stover | Solar energy-assisted pyrolysis | Kinetic, thermodynamics and physical characterization was conducted | The results indicate that the corn stover can be a great bioresource for chemical production with solar pyrolysis. | [230] |
| Feedstock | Reactor | Plant Capacity Ton/Year | Product Yield L/DT | Application | GWP | Ref. |
|---|---|---|---|---|---|---|
| Corn stover | Rotary kiln | 84,000 | - | Soil amendment | −865 | [238] |
| Barley straw | Rotary kiln | 100,000 | - | Soil amendment | −900 | [239] |
| Sewage sludge | - | 2000 | - | Energy generation | −750 | [240] |
| Poplar wood | Fluidized bed | - | 300 | Gasoline and diesel | 0.74 | [241] |
| Forest residue | Hydroprocessing | - | 350 | Gasoline | 1.21 | [242] |
| Forest residue | Fluidized bed | - | 114 | Chemicals | −0.53 | [243] |
| Wood residue | Fluidized bed | - | 320 | Bio-oil | 0.11 | [244] |
| Technology | Location | No. of Units | Max. Size Kg/h |
|---|---|---|---|
| a Fixed-bed and moving-bed | Anhui Yineng Bioenergy Ltd., China | 3 | 600 |
| a Vacuum pyrolysis | Pyrovac, Canada | 1 | 3500 |
| a Ablative reactor | PyTec, Germany | 2 | 250 |
| a Rotating cone | BTG, Netherlands | 4 | 2000 |
| a Circulating fluidized bed | Metso/UPM, Finland | 1 | 400 |
| a Fluidized-bed | RTI, Canada | 5 | 20 |
| b Transported fluidized-bed | Ensyn, Canada | 8 | 4000 |
| b Bubbling fluidized-bed | Dynamotive, Canada | 1 | 3800 |
| b Indirect heating rotary kiln | Mitsubishi Heavy Industries | 1 | 4000 |
| b Rotary cone | BTG, Malaysia | 1 | 2000 |
| b Heated kiln pyrolysis followed by gasification | Choren, Germany | 1 | 6800 |
| c Fluidized bed | Phrae, Thailand | 1 | 10–20 |
| Reactor Type | Advantages | Disadvantages | Oil Yield |
|---|---|---|---|
| Fixed-bed | Simple and reliable design Biomass size dependent | Long residence time Difficult to remove char | 35–50% |
| Bubbling fluidized-bed | Simple design and easy operation Suitable for large scale | Small particle sizes are needed | 70–75% |
| Circulating fluidized-bed | Good temp. control Large particle size could be used | Suitable for small scale Complex hydrodynamics | 70–75% |
| Rotating cone | No carrier gas required Less wear | Complex process Small particle | 65% |
| Vacuum | Produce clean oil Can process large particle (3–5 cm) No carrier gas required | Slow process Solid residence time is too high | 65% |
| Ablative | Inert gas is not required Large particle sizes can be processed | Reactor is costly Low reaction rate | 70% |
| PyRos | Compact and low cost High heat transfer Short gas residence time | Complex design High impurities in the oil High temp. required | 70–75% |
| Microwave | High heating rates Large size biomass can be processed High temperature | High electrical power consumption High operating costs | 60–70% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.-K. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability 2021, 13, 11061. https://doi.org/10.3390/su131911061
Raza M, Inayat A, Ahmed A, Jamil F, Ghenai C, Naqvi SR, Shanableh A, Ayoub M, Waris A, Park Y-K. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability. 2021; 13(19):11061. https://doi.org/10.3390/su131911061
Chicago/Turabian StyleRaza, Mohsin, Abrar Inayat, Ashfaq Ahmed, Farrukh Jamil, Chaouki Ghenai, Salman R. Naqvi, Abdallah Shanableh, Muhammad Ayoub, Ammara Waris, and Young-Kwon Park. 2021. "Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing" Sustainability 13, no. 19: 11061. https://doi.org/10.3390/su131911061








