Abstract
Fat rich microorganisms, such as microalgae Schizochytrium spp., are potential biotechnological tools in the modulation of rumen microbiome towards ecofriendly and high nutritional value end-products. However, limited in vivo trials have been reported on the topic. The aim of this study was to contribute to the knowledge on the effect of fat rich microalgae on the methanogenic and feed degrading particle-associated microbes in goats’ rumen content. For the trial, twenty-four goats were divided into four homogenous clusters (six goats/treatment) according to their fat corrected (4%) milk yield, body weight and age and individually were fed with alfalfa hay and concentrate feeds (F/C = 50/50). The concentrate of the control group (CON) contained no microalgae, while those of the treated groups were supplemented daily with 20 (ALG20), 40 (ALG40), and 60 (ALG60) g of Schizochytrium spp./goat. The relative abundances of total Archaea, methanogens, Methanomassiliicoccales, Methanobrevibacter spp., Methanosphaera stadmanae and Methanobacterium formicicum were significantly (p < 0.05) decreased in microalgae-fed goats compared to the CON ones. Moreover, a significant decline in the relative abundances of Firmicutes, Ruminococcus flavefaciens, Butyrivibrio fibrosolvents, and Neocallimastigales in the rumen particle-associated microbiota of microalgae supplemented goats were observed. In conclusion, goats’ diets supplementation with Schizochytrium spp., could be considered a sustainable nutritional strategy for methanogens inhibition in their rumen particle-associated microbiota.
1. Introduction
There are approximately 7.7 billion humans on earth of which 10% do not have sufficient access to food [1]. According to population growth rate predictions, global population is anticipated to reach 9.15 billion by 2050 [2]. Consequently, both dairy products and meat consumption are expected to increase in 2050 by 58 and 73 percent, respectively, compared to their 2010 levels [2,3,4], due to the aforementioned demands. Simultaneously, methane (CH4) emissions are expected to rise by 30% until 2050, as a result of meat and milk increasing demands if mitigation strategies are not implemented [5]. Within Greece and other Mediterranean areas, small ruminants’ husbandry has a substantial socio-economic and environmental impact, while goats farming systems portraying a continuous upward trend [6]. Despite the fact that the dominant proportion of methane emission is derived from cattle and buffalo, goats produce around 4.9% of total CH4 emissions from livestock globally [7]. Hence, world concern on the unbalanced nature of this growth and its attendant environmental and socio-economic consequences are rapidly increasing. These current challenges require the design of multidisciplinary approach strategies, particularly to mitigate the greenhouse gases emissions and improve animals’ productivity and products quality. To achieve these objectives, the dietary supplementation with feed supplements such as microalgae rich in polyunsaturated fatty acids (PUFA) represents a promising tool, since it can modify the rumen fermentation towards advantageous directions [8].
Up to now, microalgae such as Schizochytrium spp. rich in docosapentaenoic (DPA) and docosahexaenoic (DHA) fatty acids (FA) have been used as a nutritional approach to enhance ruminants’ milk with PUFA [9,10,11,12] and/or improve animals’ and milk oxidative status [13]. Interestingly, high fat microalgae such as the Schizochytrium spp. could also be studied for their potential in reducing methane production in ruminants, as reported in vitro by Fievez [14]. This property could be connected to their lipid content, and especially long-chain PUFA such as DHA that are toxic in specific bacteria and potentially also on methanogens, since they can disrupt their bilayer membrane and induce malfunction in their metabolic functions [15,16]. Nonetheless, immense PUFA inclusion on ruminants’ diet might also have toxic effects on some crucial microorganisms such as the fibrolytic bacteria and consequently on animal performance [17]. Apart from its negative impact on the environment, methane production has in fact been estimated to be responsible for a loss of 2–15% of the gross energy of feeds in ruminants [18]. Among the bacteria involved in this process, microbial populations in rumen attached to feed particles have a pivotal role in feed fermentation, since they account up to 70% of the amylase activity, 91% of both endoglucanase and xylanase activity, and 75% of the protease action [19]. Furthermore, recent studies report that rumen microbiome is able to re-adapt to its initial composition after an enduring dietary supplementation with such bioactive feed additives, due to the host governance [20,21]. This evidence appears to be central challenge for the commercial application of such dietary interventions οn farm scale. Thus, the experimental duration of these dietary treatments should also be addressed.
Taking into consideration the aforementioned, it was assumed that not only the different dietary supplementation levels of Schizochytrium spp. but also the interval of administration might affect the composition of goats’ rumen particle-associated microbiota. Thus, the aim of this study was to examine the long-standing dietary inclusion of Schizochytrium spp. on three different levels (20, 40 and 60 g/animal/day) on (i) the abundance of microorganisms involved in methane production and (ii) microbes that adhere to feed particles on goats’ rumen.
2. Materials and Methods
2.1. Experimental Design and Animals’ Diets
Twenty-four dairy goats (Local (Greek) × Alpine breeds), at 150 ± 10 days in milk (DIM), of a mean body weight (BW) of 47.6 ± 5.9 kg, were separated into four (n = 6 goats) comparable groups balanced according to their fat corrected (4%) milk yield, BW, and age. During the trial, each goat was fed individually, while the dry matter intake (DMI) was recorded on a daily basis. Each goat was offered 1 kg alfalfa hay and 1 kg concentrate daily, with a fixed forage-to-concentrate ratio (F:C) of 50/50. The concentrate of the control (CON) group consisted of 36.7% maize grain; 20% barley grain; 21% wheat middlings; 8% sunflower meal; 10% soya bean meal; 1.5% calcium phosphate; 0.5% calcium carbonate; 0.3% salt; and 2% vitamin and minerals mix, while the three microalgae treated groups (ALG20, ALG40, and ALG60) were received the same basal concentrate mix supplemented with 20 g, 40 g, and 60 g Schizochytrium spp./goat, respectively. Aiming to achieve comparable energy density amongst the treatments (less than 5%); 20, 40, and 60 g of the above concentrate mixture of the microalgae groups were swapped respectively, by the same quantities of microalgae. The rations were also isonitrogenous since the microalgae protein content was such that did not significantly affect the protein content amongst the diets (Table 1). The Schizochytrium spp. is traded as commercial product termed DHAgold (DSM Human Nutrition & Health, Heerlen, The Netherlands). The trial lasted 74 days of which, the first 14 days were used as adaptation period. Further information concerning the experimental design and diets is available by Mavrommatis and Tsiplakou [12].

Table 1.
Alfalfa hay, DHAgold™ and concentrate mix chemical composition (g/kg) and fatty acids profile (g/100 g total FA).
2.2. Milk Samples Collection and Analysis
Milk samples were gathered at 20, 40, and 60 days of the experimental period and analyzed for their chemical composition as previously described by Mavrommatis and Tsiplakou [12].
2.3. Feed Samples Analysis
Feedstuff chemical and FA composition are presented in Table 1. The analytical procedures have been previously described by Mavrommatis et al. [13].
2.4. Rumen Samples Collection
Rumen samples were collected as previously described by Tsiplakou [22] using an alternative to rumen cannulation, non-surgical and non-operating procedure. Briefly, on day 20th, 40th, and 60th of the trial, rumen content was collected using a stomach tube (flexible PVC tube of 1.5 mm thickness and 10 mm I.D.) and an electric vacuum pump (MZ2CNT, Vacuubrand Gmbh & Co Kg, Wertheim, Germany) as have been described for sheep and goats by Ramos Morales et al. [23]. The stomach tube was placed into a depth up to 150 cm, while the first quantity of fluid was discarded to reduce the effect of the saliva contamination [23,24]. Immediately after gathering, the rumen digesta was filtered with cheesecloth layers (Figure 1) to separate the solid phase and then the samples were frozen at −80 °C until the DNA extraction.

Figure 1.
Rumen samples collection, (A,B) rumen digesta filtration through cheesecloth layers, (C) solid phase collection and (D) and immediate freezing in liquid nitrogen [25].
2.5. Rumen Microbial Analysis
A total of 72 samples from 24 goats (3 sampling points) were used for microorganisms’ analysis as previously described by Tsiplakou et al. [22] with some modifications. Specifically, DNA extraction accomplished using a modified cetyltrimethylammonium bromide (CTAB) protocol. One g of rumen digesta was grinded using pestle and mortar and liquid nitrogen. The grinded powder was incubated in a lysis solution consisted of 2% CTAB, 0.02 M EDTA, 0.1 M tris- HCl pH 8, 1.4 M NaCl, 2-mercaptoethanol 99% and proteinase K from Tritirachium album (Sigma-Aldrich, Missouri, USA) for 2 h at 57 °C. DNA was extracted three times with chloroform:isoamyl alcohol (24:1) and precipitated by adding 2-propanol at −20 °C. After a centrifugation at 7500 g for 15 min at 4 °C, the precipitate was washes two times with 70% and 100% ethanol, respectively. The DNA pellet obtained was re-suspended in ultra-pure water and 10 μL RNase A were added and incubated for 1 h at 37 °C to remove any RNA contamination. Afterwards, samples are purified through spin column NucleoSpin® Tissue (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany) based on the manufacturer guidelines. The quality of extracted DNA was estimated by ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA) and by using a 0.70% agarose gel electrophoresis.
The primer set used for the real-time PCR, the genomic region of PCR amplification, the PCR efficiency (%) and the slope of the standard curves are presented in Table 2. The primers for amplifying the total bacterial 16S rRNA sequences have been modified to adjust the hybridization temperature to 60 °C [26]. The general anaerobic fungi primers have been designed from multiple alignments of fungal 18S ribosomal and ITS1 gene sequences, which encompassed all available anaerobic fungal sequences [26]. A set of ciliate protozoal PCR primers were designed based on all ruminal protozoan 18S rDNA sequences according to Sylvester et al. [27]. The archaea 16S rRNA gene was amplified using an already published primer pair [28], while for methanogens detection, the methyl coenzyme-M reductase subunit A (MCRA) gene was targeted for amplification. This enzyme complex is considered to be unique to, and ubiquitous in, methanogens making it a suitable tool for their exclusive detection [29]. The primer sets for the detection and enumeration of the other bacteria and methanogens populations amplify the 16S rRNA gene sequences, described by Denman and McSweeney [26], Kim et al. [28], Yang et al. [30], Vargas-Bello-Pérez et al. [31], Duval et al. [32], and Elolimy et al. [33] (Table 2). Primers were then associated with sequences accessible at the NCBI via a BLAST search to find out primer specificity.

Table 2.
Sequences of primers used for RT-PCRs, genomic regions of PCR amplification, primer efficiency, standard curve slope, amplicon size and hybridization temperature.
Conventional PCR for the authentication of the specificity of the chosen primers against target genes were performed in 25 μL reactions with the addition of 2.5 mM MgCl2 and employing Taq polymerase following below conditions: initial step at 95 °C for 4 min, and 28 cycles of 95 °C for 30 s, 60 °C for 20 s (or relevant based on primers Tm), and 68 °C for 1 min for elongation. PCR products were analyzed for a unique band and the absence of primer dimer by running on 2% agarose gels.
Due to variation of PCR inhibitors in rumen samples it was vital to validate the PCR efficiency in order to confirm an accurate quantitation. Consecutive dilutions of pool microbial DNA were amplified (duplicate) by real-time PCR based on the conditions emerged by primers Tm and product length. Real time PCR reaction efficiencies (e) were calculated for the primers presented in Table 2 from a linear regression of the threshold cycle (Ct) for each dilution against the log dilution using the formula: e = 10−1/slope [34]. Primers efficiencies varied from 93% to 100%. Relative abundance of microbial populations was expressed as a proportion of total bacterial 16S ribosomal DNA as described by Carberry et al. [35] according to the equation: relative abundance = e (target)− (Ct target microorganism-Ct of bacterial 16s rDNA), (latest cycle detected = 32). Changes in relative abundance of specific microbes due to an effect of microalgae or sampling time were expressed as % of total bacterial 16s rDNA as described by Chen et al. [36] and Carberry et al. [35]. The relative abundance expression of the results is suitable and accurate method provided that no different taxa are compared, whilst the comparisons are limited to between treatments and time [26]. Quantitative real-time PCRs were performed using a Step-One Plus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) in a reaction volume of 10 μL: 5 μL SYBRTM Select Master Mix (Thermo Fisher Scientific, Massachusetts, USA), 4 μL primers (0.2 μmol each), and 1 μL of DNA (15 ng/uL) as template according to master mix manufacturer protocol. Primer specificity and formation of primer dimers were explored by dissociation curve analysis (melt curve).
2.6. Statistical Analysis
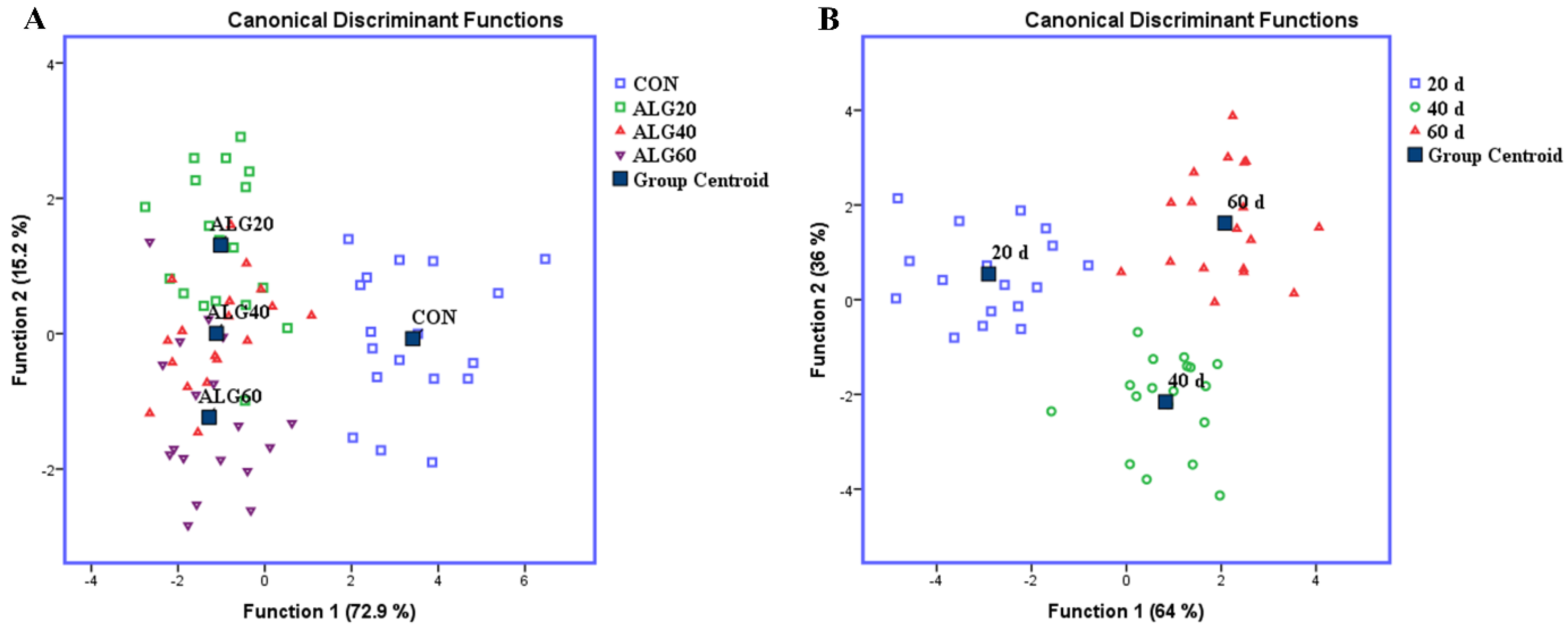
Statistical analyses were conducted using the SPSS, IBM (v. 20.0). Discriminant analyses were performed to pooled data of microbiota relative abundances to examine those variables capable of discriminating and ordering samples among (i) the four dietary groups (CON, ALG20, ALG40, and ALG60) and (ii) the three-sampling time (20th, 40th, and 60th experimental day) (Figure 2). It is worth mentioned that Control measurements were omitted by the sampling time discriminant analysis in order to evaluate the microorganism adaptation occurred by dietary effect. Wilk’s lambda (λ) criterion was used for assessing discriminant functions [3]. Twenty-four variables (microorganisms’ relative abundances) were entered to create a model to distinguish the seventy-two samples of the first case (4 groups × 6 goats/group × 3 sampling time) and fifty-four for the second (3 dietary treatments × 6 goats/group × 3 sampling time).
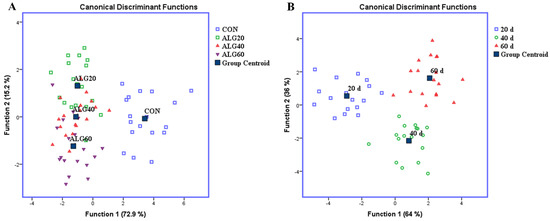
Figure 2.
Discriminant plots separating (A) the four dietary treatments (CON; blue □, ALG20; green □, ALG40; red △, and ALG60; purple ▽) according to pooled data of three sampling time (20th, 40th, and 60th experimental day) on the relative abundances of specific microbes and (B) the three-sampling time (20th; blue □, 40th; green ○, and 60th; red △) according to pooled data of three dietary treatments (ALG20, ALG40, and ALG60).
Dietary treatment effects on microorganism relative abundances were investigated using a general linear model (GLM) for repeated measures (ANOVA) since samples were taken at three sampling time (20th, 40th, and 60th experimental day) from the same subjects (n = 72; 24 goats × 3 sampling time). Specifically, dietary groups (D = CON, ALG20, ALG40, and ALG60) were inserted as fixed factor, and the sampling time (S) as the repeated variable, while their interactions (D × S) were also included to evaluate the differences over time according to the model:
where Yijk is the dependent variable, µ the overall mean, Di the effect of dietary treatment (i = 4; CON, ALG20, ALG40, and ALG60), Sj the effect of sampling time (j = 3; 20th, 40th, and 60th experimental day), Ak the animal’s random effect, (DxS)ij the interaction between dietary treatments and sampling time, and eijk the residual error.
Yijkl = µ + Di + Sj + Ak + (D × S)ij + eijkl
Post hoc analysis was performed when appropriate using a Tukey’s multiple range test [3]. GraphPad Prism 6.0 (2012) was used for interleaved bars, while errors bars represent the standard error of means (SEM), and the corresponding superscripts letters have been emerged by SPSS repeated measure analysis (Figure 3). In addition, aiming to further simplify the time effect between dietary treatments and in order to unveil any microbes’ adaptation trend, connected superimposed symbols were also used (Figure 4). However, in supplementary data (Table S1) more statistical information are available about the effect of diet, sampling time and their interaction under microalgae treatment.
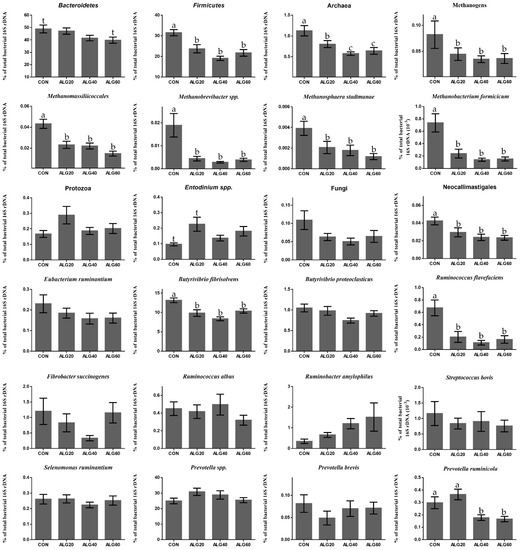
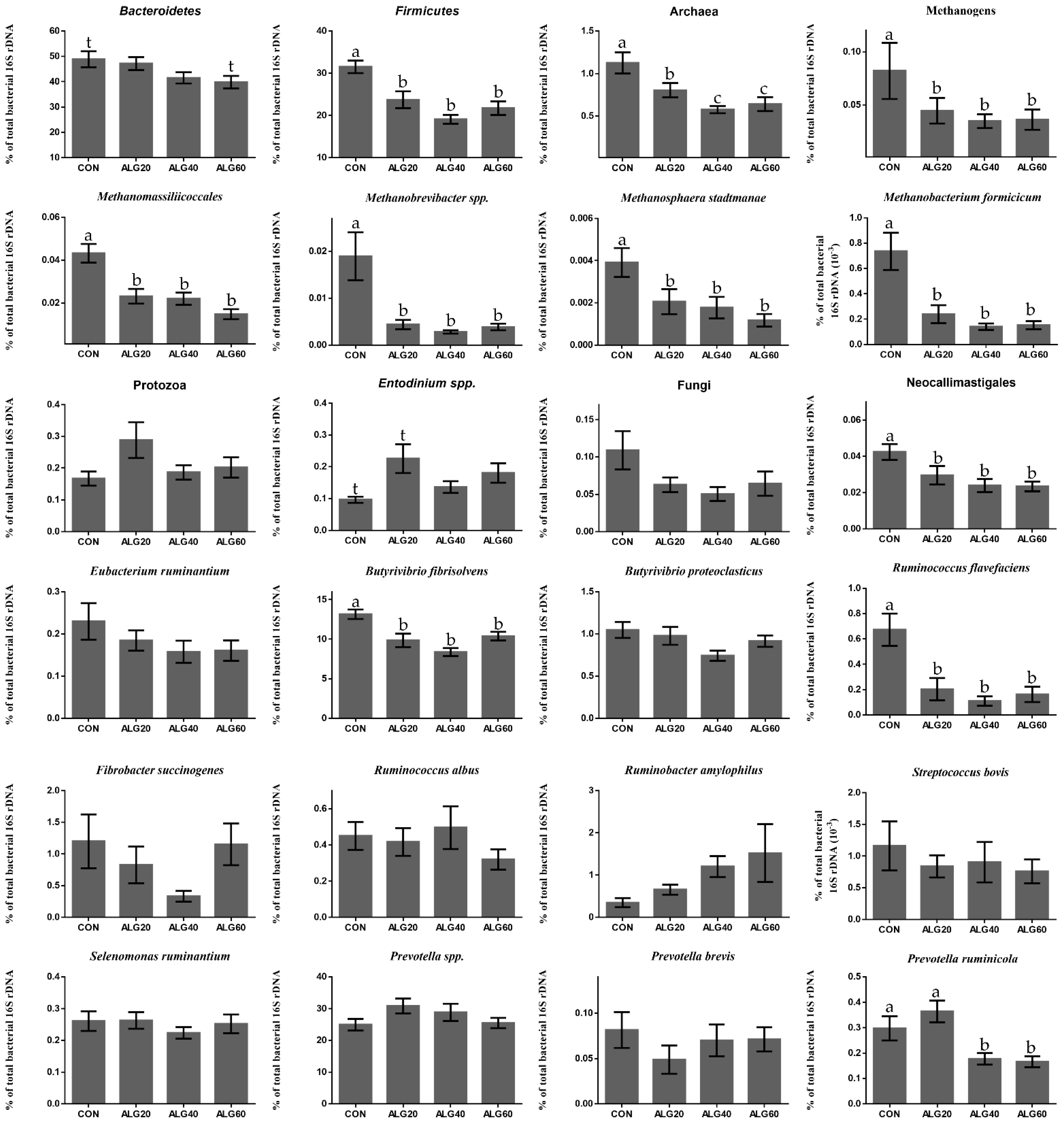
Figure 3.
Average changes of target microorganisms adhering to the feed particles in the rumen of goats of CON, ALG20, ALG40, and ALG60 groups within the three sampling times (20th, 40th, and 60th) illustrated in column bars (± Standard Error of Means) as a proportion of total rumen bacterial 16S rDNA. Bars with different superscript (a, b, c) between dietary treatments differ significantly (p ≤ 0.05) while t is referred to p-value < 0.10, according to the Analysis of Variance (ANOVA) using a general linear model for repeated measures and Post hoc analysis was performed when appropriate using Tukey’s multiple range test.
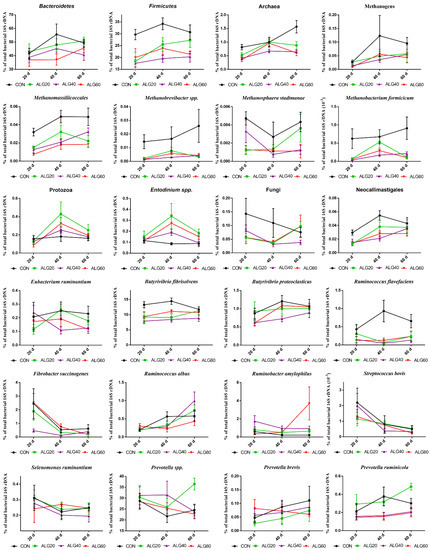
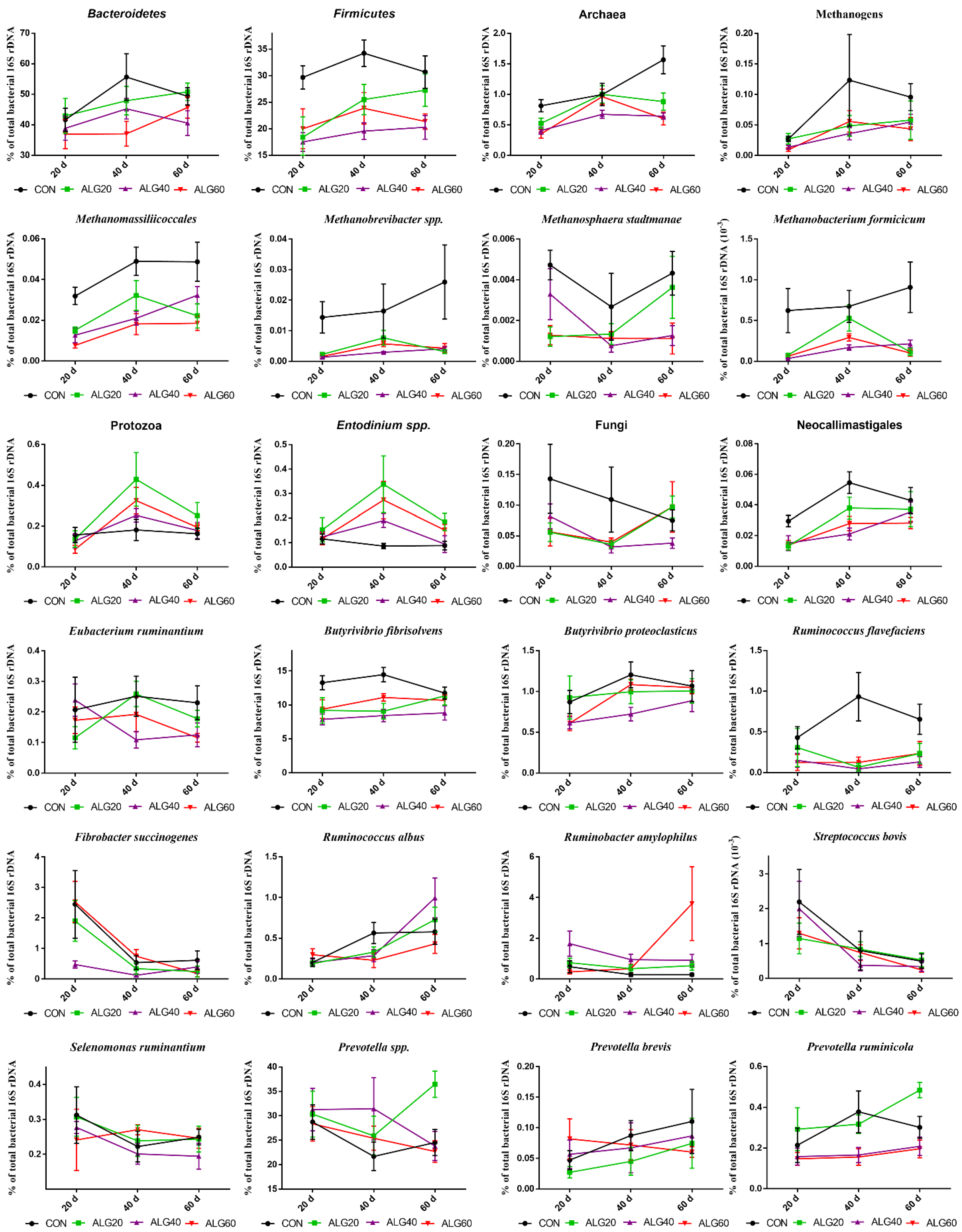
Figure 4.
Average changes of target microorganisms adhering to the feed particles in the rumen of goats of CON, ALG20, ALG40, and ALG60 groups in each of three sampling time (20th, 40th, and 60th) illustrated in connected superimposed symbols (± Standard Error of Means) as a proportion of total rumen bacterial 16S rDNA.
3. Results
3.1. Milk Performance
Briefly, the average daily concentrate intake of ALG60 goats was decreased compared to the other treatments (670 g vs. 1000 g), leading to a lower intake of the microalgae (40 g) than the scheduled one (60 g), since microalgae had been supplemented into the concentrate mixtures. Despite the decreased DMI of ALG60 group, the milk yield and BW (data not shown) were not significantly affected (Table 3). The fat content was reduced in both ALG40 and ALG60 groups, compared with the CON and ALG20 (Table 3). Further information concerning milk performance and its chemical composition have been extensively reported by Mavrommatis and Tsiplakou [12].

Table 3.
Mean milk performance of goats fed diets with different levels of Schizochytrium spp. (CON, ALG20, ALG40, and ALG60) throughout the experimental period.
3.2. Holistic Statistics
Figure 2A depicts a discriminant plot of the four-dietary treatments (CON; blue □, ALG20; green □, ALG40; red △, and ALG60; purple ▽) throughout the experimental period. The proportions of the samples that were correctly classified were 88.1%. Wilks’ lambda was observed at 0.063 for Function 1 (p < 0.001) and 0.321 for Function 2 (p = 0.036), while the relative abundance of Firmicutes, Ruminococcus flavefaciens, Methanomassiliicoccales, Methanobacterium formicicum, Methanobrevibacter spp., Archaea, Neocallimastigales, Fungi and Methanosphaera stadtmanae were the variables that contributed the most. CON variables were significantly (Function 1) classified apart of the microalgae fed groups, while ALG20, 40, and 60 showed a minor overlapping (Function 2), indicating that there was not significant dose-depended effect and especially between ALG40 and ALG60 groups, which consumed comparable microalgae amount.
In addition, a second discriminant test was performed to pooled data of three microalgae treatments (ALG20, ALG40, and ALG60) on rumen microbial community (Figure 2B) in order to examine if the samples can be discriminated according to the three-sampling times (20th; blue □, 40th; green ○, and 60th; red △). The proportions of the samples that were correctly classified, according to the sampling time, were 100%, while Wilks’ lambda was observed at 0.047 for Function 1 (p < 0.001) and 0.272 for Function 2 (p = 0.001) and the relative population of Fibrobacter succinogenes, Streptococcus bovis, Neocallimastigales, Fungi, Prevotella sp., Butyrivibrio proteoclasticus, Methanomassiliicoccales, and methanogens were the variables that contributed the most.
Overall, the supplementation of microalgae Schizochytrium spp. in goats’ diet, significantly affected the investigated variables. The variables of microbial community in 20th days interval were clearly separated by the others (40th and 60th day) in Function 1 representing a 64% of eigenvalue variance.
3.3. Relative Abundance of Specific Microbes
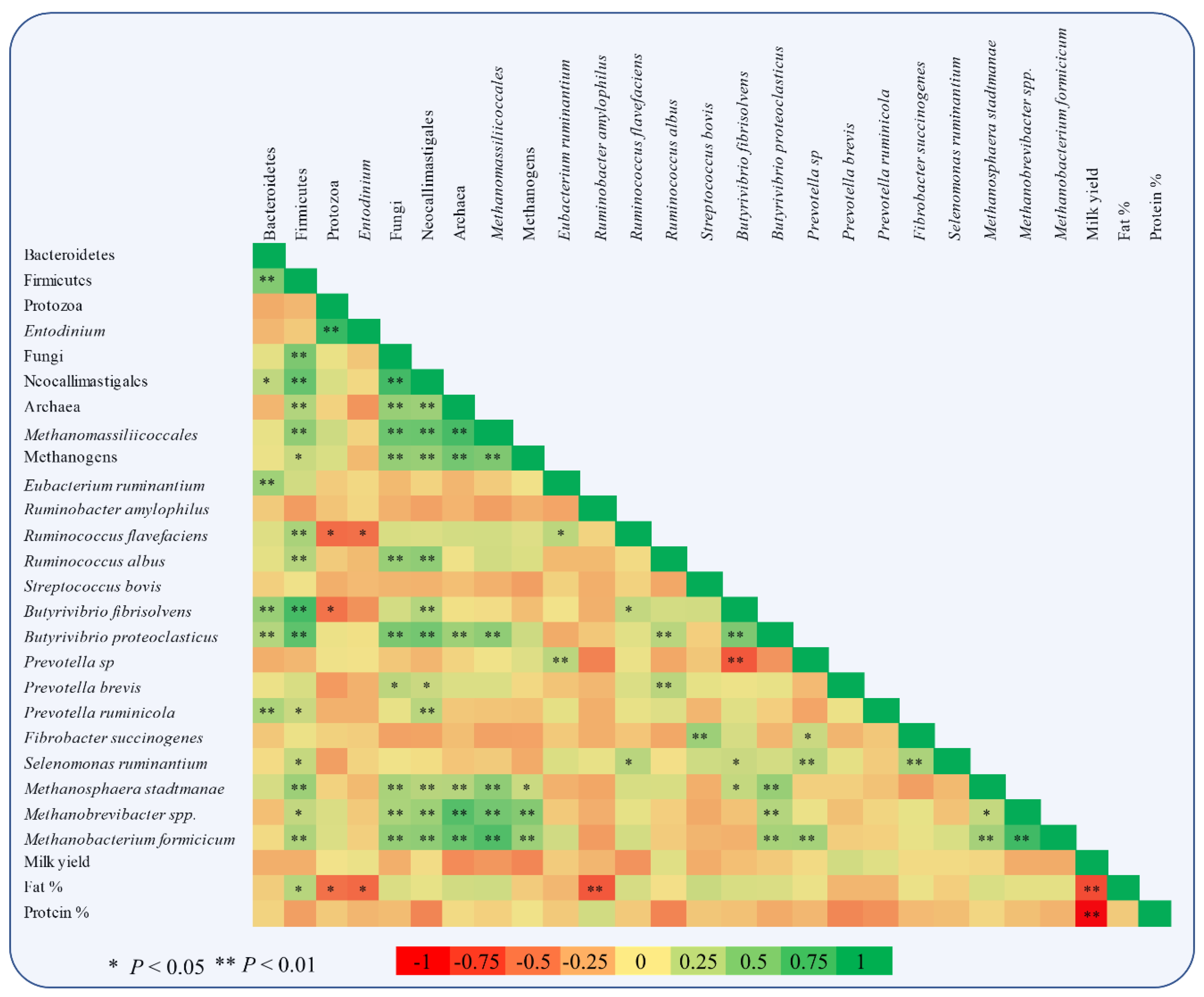
Figure 3 depicts the mean relative abundance of studied microorganisms adhering to feed particles in the rumen of goats of CON, ALG20, ALG40, and ALG60 groups on three alternate sampling times (20th, 40th, and 60th) illustrated in column bars (± Standard Error of Means) as a proportion of total rumen bacterial 16S ribosomal DNA. The dominant rumen phylum, Bacteroidetes, showed a tendency to decrease (p = 0.065) in ALG60 compared to the CON group. In the same way, the phylum Firmicutes, were significantly decreased (p < 0.001) in microalgae fed goats by 25, 40, and 32% in ALG20, ALG40, and ALG60 groups respectively, compared to the CON group. Rumen protozoa were numerically increased (p = 0.102) in ALG20 goats compared to the CON group, while the predominant ciliate rumen protozoa, Entodinium, showed a 1.3-fold increase (p = 0.076) in the aforementioned dietary group. Milk fat content was positively correlated with Firmicutes relative abundance, while total protozoa and Entodinium relative abundance were negatively correlated with fat content (Figure 5). The anaerobic fungi Neocallimastigales were decreased significantly (p = 0.016) in microalgae fed goats by 25, 50, and 50% in ALG20, ALG40, and ALG60 groups respectively, compared to the CON group. Furthermore, rumen particle-associated Neocallimastigales were increased significantly (p < 0.001) after the 20th day of the trial (Figure 4; Table S1). Total Archaea relative proportion were mitigated (p < 0.001) by 28, 46, and 46% in ALG20, ALG40, and ALG60 respectively, compared to the CON group.
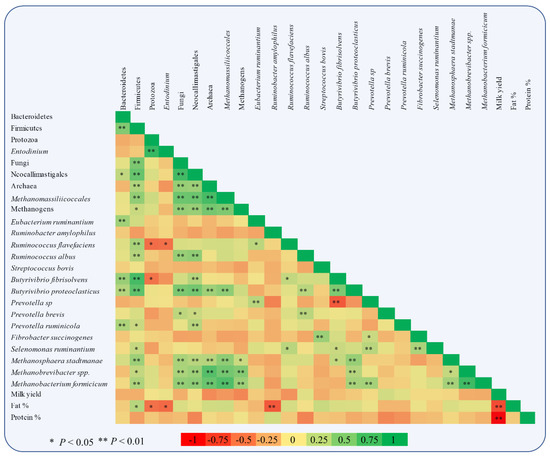
Figure 5.
Pearson correlation heatmap of relative abundances of specific microbes adhering to rumen feed particles and milk performance of goats (milk yield, fat, and protein content).
The same trends were observed in relative abundance of Methanomassiliicoccales (p < 0.001) and total methanogens (p = 0.041). Particularly, in Methanomassiliicoccales were observed a decrease of 47, 47, and 66% in ALG20, ALG40, and ALG60 respectively, compared to the CON group. Similarly, methanogens were mitigated 46, 58, and 58% in microalgae fed goats compared to the CON group. In addition, methanogens and Methanomassiliicoccales in CON group showed a significant increase after the 20th day of the experiment (Figure 4). These alterations were also found at species level since Methanosphaera stadmanae, Methanobrevibacter spp. and Methanobacterium formicicum were significantly decreased by the inclusion of Schizochytrium spp. in goats diet compared to the CON group. In detail, Methanosphaera stadmanae were decreased (p = 0.026) by 50, 50, and 75%; Methanobrevibacter spp. were decreased (p = 0.048) about 80, 85, and 80% and Methanobacterium formicicum were decreased (p = 0.001) by 72, 85, and 72% in ALG20, ALG40, and ALG60 groups respectively, compared to the CON group. Collectively, the studied methanogenic microbes were positively correlated with each other (Figure 5). Regarding the fibrolytic bacteria, Eubacterium ruminantium, Fibrobacter succinogenes, and Ruminococcus albus did not show significant alterations. However, Ruminococcus flavefaciens were decreased (p = 0.004) in rumen bacteria adhering to feed particles of goats fed with microalgae up to 85% compared to the CON group. Amylolytic bacteria, Streptococcus bovis and Ruminobacter amylophilous were not significantly affected between dietary treatments, despite their remarkable fluctuations. However, Ruminobacter amylophilous in ALG60 group showed a significant upward trend after 40 days of interval that was accompanied by a high SEM (Figure 4). Biohydrogenating bacterium, of Butyrivibrio proteoclasticus did not show significant alterations between dietary treatments, howbeit, in B. fibrisolvens a significant (p < 0.000) decrease by 25, 37, and 22% were observed in ALG20, ALG40, and ALG60 respectively, compared to the CON group.
Likewise, the dominant protein utilization bacteria genus of Prevotella were also not significantly affected by microalgae dietary inclusion, while a significant negative correlation was observed within Prevotella and B. fibrisolvens (Figure 5). Interestingly, Prevotella ruminicola were decreased by approximately 50% (p = 0.009) in ALG40 and ALG60 groups compared to CON and ALG20 groups.
4. Discussion
In the present study, significant milk fat depression (MFD) occurred simultaneously with a mitigation of Firmicutes abundance in the rumen particle-associated digesta in high microalgae supplementation levels (ALG40 and ALG60) as also supported by their positive correlation. Ruminants are fully dependent on their rumen microbiota for feed fermentation and consequently the composition of their products is deeply affected by the symbiotic microorganisms and their metabolites. This creates a linkage between the bacterial metabolites of the rumen microbiome and the productive characteristics of the host animal during lactation. Interestingly, Jami [38] observed that the ratio of rumen Firmicutes to Bacteroidetes is positively correlated with milk fat percentage. Indeed, the same trend was observed for the relative abundance of Bacteroidetes that remained unaffected, while the Firmicutes tended to decrease (p = 0.100) by the dietary supplementation with 20.8 g Schizochytrium spp./day in MFD ewes [9,39].
Dietary supplementation levels with Schizochytrium spp. mitigated the relative abundance of Archaea and methanogens in rumen solid adherent microbes of treated goats compared to the CON group. Members of domain Archaea constitute about 0.3 to 4% of rumen microbial DNA (16s and 18s rRNAs) [40]. Approximately all of these Archaea, are methanogens that produce methane (CH4) via two main pathways: (a) the hydrogenotrophic pathway (Methanobrevibacter spp., Methanobacterium formicicum) [1], converts H2 and CO2 formed by the bacteria, protozoa, and fungi to CH4, and (b) the methylotrophic pathway (Methanosphaera stadtmanae) in which methanol resulting by the hydrolysis of methanolic side-groups of plant polysaccharides metabolized into CH4 [41].
Considering that Methanobrevibacter spp. population is the dominant methanogen and represents at least 65% of the rumen methanogens [42] and that CH4 is produced primarily via the hydrogenotrophic pathway, the results of our study suggest that the dietary supplementation of goats with Schizochytrium spp., might be a promising nutritional strategy to reduce the methanogens population with beneficial effects on the environment. Moreover, in relation to its involvement on environmental issues, CH4 production in rumen, negatively affects ruminants feed efficiency representing a loss of 2–15% of the gross energy [18]. Performing a whole rumen metagenome sequencing, Delgado [43] observed that individuals with lower relative abundances of Firmicutes and methanogenic Archaea were more efficient in feed utilization. Indeed, in ALG60 goats, where Firmicutes and methanogens relative abundances were mitigated, the milk yield according to FCM4% and body weight were not significantly affected, despite the reduction in concentrate intake compared to the other dietary groups (33% reduced concentrate DMI) suggesting a more efficient feed utilization. The latter may be attributed to a potential enhancement of reductive acetogenesis activity due to methanogenesis inhibition [44]. Specifically, the acetogenic bacteria may benefit from a higher availability of H2 in rumen dependent on the methanogenesis suppression; therefore, the host may exploit the produced acetic acid for productive purposes. A significant inhibition in the methanogens has been previously observed by the addition of fish oil [45] and red algae; Asparagopsis taxiformis [46] in the rumen liquid of cows in vitro, and by the supplementation with DHA-rich microalgae in sheep rumen digesta using RUSITEC technique [47], while to the best of our knowledge no in vivo studies exist concerning the impact of DHA-rich microalgae such as the Schizochytrium spp. on methanogens population in goats’ rumen.
Although CH4 is produced mainly by the above pathways, methanogens interact with other protozoa, bacteria, and fungi within the rumen, via interspecies H2 transfer. Such interactions are pivotal for rumen fermentation as they prevent H2 accumulation and the consequent feedback fermentation inhibition. The majority of methanogenic microbes float in rumen liquid or live as a consortia within the biofilm adhering to feed particles [42]. Protozoa indirectly contribute to the CH4 formation through their high production of H2. In detail, protozoa in rumen form two volatile fatty acids (VFA); butyrate and acetate, whose synthesis produces 2 and 4 M of hydrogen respectively, per M of fermented glucose [48]. Approximately 50% of this H2 is converted to CH4 by methanogenic Archaea which are either ectosymbionts or endosymbionts in protozoa cells [49,50]. A meta-analysis by Guyader [49] reported that the protozoa abundances were decreased in the majority of the experiments testing lipids supplementation on ruminants’ diet, due to changes in membrane permeability, resulting in cell lysis [51,52]. However, the study of Jordan [53] clarified that protozoa membranes are more susceptible to the action of medium chain fatty acids (lauric acid rather than the PUFA action). Indeed, the fact that Schizochytrium spp., which were supplemented in goats’ diets were rich in PUFAs (DPA and DHA), further support the aforementioned assumption. A previous study [54] also failed to show any significant change in protozoa population when DHA-rich oil was infused in goats’ rumen at 2.5, 5, and 10 g/day, while in the study by Vargas [47] ciliate protozoa were increased about 1.3-fold by the supplementation with DHA-rich microalgae in sheep rumen digesta using the RUSITEC technique. Despite the mutualistic relationship of protozoa and methanogenic Archaea in regard to CH4 production, the selective mitigation of methanogens in response to dietary supplementation with Schizochytrium spp. levels, may underlie a potential beneficial impact of a large-scale microalgae application in ruminants’ diet. Specifically, a meta-analysis by Newbold [50] summarizing the effect of protozoa defaunation, indicated that rumen organic matter digestibility and specifically NDF and ADF digestibility were significantly decreased (−7%, p = 0.008; −20%, p = 0.040 and −16%, p = 0.100 respectively) as a result of the loss of protozoal fibrolytic activity with consequent feed efficiency depression. This observation may further supports the negative correlation between protozoa abundance and milk fat content that was observed in the present study.
Furthermore, a reciprocal relationship has also been reported between Archaea-methanogens and fungi in in vitro co-cultures since the first one is physically attached to anaerobic fugal biomass and enhances their fibrolytic activity, while simultaneously convert fungal degradation end-products to CH4 [55]. Indeed, the observed positive correlation between total anerobic fungi and their single order Neocallimastigales with methanogens, indicating the aforementioned complementary link and highlights in vivo and in vitro similarities. In partially agreement with our study the supplementation of DHA-rich microalgae oil in sheep rumen digesta [47] decreased anaerobic fungi by 50%; however, the results in this case were not significant.
The nutritional interventions with high dietary PUFA should also be evaluated by taking into account their effect in vivo on the whole rumen microbiota, since PUFA might have toxic effects on various microorganisms such as the cellulolytic bacteria [17]. Rumen microbiota adhering to feed particles account up to 91% of both endoglucanase and xylanase activity, 70% of the amylase action, and 75% of the protease activity in the rumen [19]. Hence, the particle-associated microbiota constitutes the most representative rumen fraction in evaluating the abundance of feed degrading microbes with emphasis on fibrolytics [56,57].
In our study, the cellulolytic bacteria E. ruminantium, F. succinogenes, and R. albus did not show significant alterations, while R. flavefaciens were decreased in microalgae fed goats. Indeed, in the study of Maia [17], cellulolytic bacteria were the most sensitive microbial group to PUFA toxicity. However, the most intense sensitivity was observed on R. flavefaciens that were unable to grow even with the minimum experimental linoleic acid level in vitro (20 lg LA mL−1). In the study of Vargas [47], the supplementation of DHA-rich microalgae oil in sheep diet did not affect the relative abundance of F. succinogenes, R. albus, and total Butyrivibrio spp. in the rumen digesta, while S. bovis were decreased significantly in a similar manner with the present study, although our reduction was only numerical. Interestingly, it has been reported that either DHA supplementation on fistulated goats [54] or fish oil inclusion in cows’ diet [58] affect the majority of cellulolytic bacteria in rumen, while in our study, Schizochytrium spp. dietary supplementation decreased only R. flavefaciens. Taking into account these sets of evidence, apart from potential species variations between ruminants, the source of these PUFA (microalgae) may also play a central role in microbiome modulation, since a proportion of PUFA may be protected by microalgae’ cell wall.
Except of the fibrolytic species per se, also non-fibrolytic bacteria are pivotal for fiber utilization in the rumen due to the ability of the second category to enhance fibrolytic activity through an interaction termed "cross-feeding" [59]. Non-fibrolytic P. ruminicola and S. ruminantium have been reported to synergize with fibrolytic bacteria in improving fiber digestion. Indeed, S. ruminantium enhances fiber digestion when co-cultured with R. flavefaciens by the conversion of succinate into propionate [59]. In our study, non-fibrolytic bacteria were not significantly affected by the microalgae supplementation, while P. ruminicola showed a peak in ALG20 goats. In agreement with our results, the dietary supplementation with 20.8 g Schizochytrium spp./day in MFD ewes [9,39] or the inclusion of pure DHA in batch cultures of cattle and sheep rumen microorganisms [60] did not significantly affect Selenomonas genus.
Regarding Prevotella genus, the existing knowledge appears to be quite controversial; in both in vivo [39] and in vitro [47] trials, the supplementation of DHA-rich microalgae did not affect Prevotella abundance in rumen digesta, while the inclusion of pure DHA in batch cultures of rumen microorganisms significantly decreased their abundance [60]. Such mixed outcomes point out the importance of the in vivo trials in which, the holistic rumen habitat is assessed.
B. fibrisolvens are involved in the initial step of rumen biohydrogenation in which PUFA are hydrogenated to trans-11 C18:1 (Vaccenic acid, VA), while B. proteoclasticus are further hydrogenate the VA to C18:0 (Stearic acid, SA) [61,62]. In our previous study [12], Schizochytrium spp. dietary supplementation decreased SA proportion up to 60% and 80%, while increased VA up to 21- and 6-fold in blood plasma and milk respectively, indicating a severe biohydrogenation inhibition. Nevertheless, B. proteoclasticus relative abundance were not significantly decreased in the particle-associated rumen digesta despite the formation of the above intermediates, while B. fibrisolvens were decreased. Likewise, in some other in vivo studies the Schizochytrium spp. did not reduce the population of B. proteoclasticus when added in goats’ diets at 18.6 g/kg DM [63] or 6.1 and 18.3 g, respectively [64], or when lactating ewes were supplemented with 16 or 24 g/kg DM with DHA Gold [65] despite the significant accumulation of VA in rumen. These evidences support the hypothesis by Maia [66], which suggested that specific PUFAs toxicity is probably occurred through a metabolic effect in Butyrivibrio group rather than a disruption of their bilayer membrane.
5. Conclusions
The dietary supplementation with 20 g of Schizochytrium spp. inhibited the methanogens populations and Ruminococcus flavefaciens in rumen particle-associated microbiota. In this level, Schizochytrium spp. may be a promising, sustainable, and alternative to fish oil dietary strategy to mitigate livestock carbon footprint. The higher levels of microalgae inclusion showed a trend to further negatively affect the microbes that involved to feed utilization within the rumen. However, further research is needed in order to bridge the knowledge of the rumen microbiome network with its biochemistry activity and clarify if the Ruminococcus flavefaciens suppression is a substantial factor to influence the feed efficiency on farm scale. Targeted microbial reprogramming in rumen, through innovative dietary supplementations, could own a pivotal role in future’s animal husbandry.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/13/2/607/s1, Table S1. Relative abundance (e−ΔCt) of several microorganisms to the total bacterial 16s rDNA in the rumen solid adherent of goats were fed with the four diets (CON, ALG20, ALG40, and ALG60) at three sampling time (20, 40, 60 experimental days).
Author Contributions
Data curation, A.M.; formal analysis, A.M.; funding acquisition, A.M.; investigation, E.T.; methodology, A.M., D.S., and E.F.; project administration, E.T.; supervision, E.T.; validation, M.S., F.R., and E.T.; writing—original draft, A.M. and E.T.; writing—review and editing, A.M., M.S., F.R., E.F., and E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers—2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (ΙΚΥ).

Institutional Review Board Statement
The study was conducted according to the guidelines of the European Union Directive on the protection of animals used for scientific purposes (EU 63/ 2010; Council of the European Union 2010), while taking into account an extended experimental design report, the Bioethical Committee of Faculty of Animal Science (currently known as: Agricultural University of Athens Ethical Committee in Research; FEK 38/A/2-3-2018, eide.aua) approved the experimental protocol under the No. 000012/10-5-2015.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
We sincerely thank the editor and the anonymous reviewers for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huws, S.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Muñoz-Tamayo, R.; Forano, E.; Waters, S.M.; Hess, M.; et al. Addressing Global Ruminant Agricultural Challenges through Understanding the Rumen Microbiome: Past, Present, and Future. Front. Microbiol. 2018, 9, 2161. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper; FAO: Rome, Italy, 2012. [Google Scholar]
- Mavrommatis, A.; Mitsiopoulou, C.; Christodoulou, C.; Karabinas, D.; Nenov, V.; Zervas, G.; Tsiplakou, E. Dietary Supplementation of a Live Yeast Product on Dairy Sheep Milk Performance, Oxidative and Immune Status in Peripartum Period. J. Fungi 2020, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; FAO: Rome, Italy, 2013; ISBN 978-92-5-107920-1. [Google Scholar]
- Höglund-Isaksson, L.; Gómez-Sanabria, A.; Klimont, Z.; Rafaj, P.; Schöpp, W. Technical potentials and costs for reducing global anthropogenic methane emissions in the 2050 timeframe –results from the GAINS model. Environ. Res. Commun. 2020, 2. [Google Scholar] [CrossRef]
- Ruiz-Morales, F.; Castel Genís, J.; Guerrero, Y. Current status, challenges and the way forward for dairy goat production in Europe. Anim. Biosci. 2019, 32, 1256–1265. [Google Scholar] [CrossRef]
- Fernández, C. Dynamic model development of enteric methane emission from goats based on energy balance measured in indirect open circuit respiration calorimeter. Glob. Ecol. Conser. 2018, e00439. [Google Scholar] [CrossRef]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Review: Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12, s295–s309. [Google Scholar] [CrossRef]
- Bichi, E.; Hervas, G.; Toral, P.G.; Loor, J.J.; Frutos, P. Milk fat depression induced by dietary marine algae in dairy ewes: Persistency of milk fatty acid composition and animal performance responses. J. Dairy Sci. 2013, 96, 524–532. [Google Scholar] [CrossRef]
- Franklin, S.; Martin, K.; Baer, R.; Schingoethe, D.; Hippen, A. Dietary Marine Algae (Schizochytrium sp.) Increases Concentrations of Conjugated Linoleic, Docosahexaenoic and Transvaccenic Acids in Milk of Dairy Cows. J. Nutr. 2019, 12, 2048–2054. [Google Scholar] [CrossRef]
- Fougère, H.; Delavaud, C.; Bernard, L. Diets supplemented with starch and corn oil, marine algae, or hydrogenated palm oil differentially modulate milk fat secretion and composition in cows and goats: A comparative study. J. Dairy Sci. 2018, 101, 8429–8445. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Tsiplakou, E. The impact of the dietary supplementation level with Schizochytrium sp., on milk chemical composition and fatty acid profile of both blood plasma and milk of goats. Small Rum. Res. 2020, 193, 106252. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Chronopoulou, G.; Sotirakoglou, K.; Labrou, N.; Zervas, G.; Tsiplakou, E. The impact of the dietary supplementation level with Schizochytrium sp, on the oxidative capacity of both goats’ organism and milk. Livest. Sci. 2018, 218, 37–43. [Google Scholar] [CrossRef]
- Fievez, V.; Boeckaert, C.; Vlaeminck, B.; Mestdagh, J.; Demeyer, D. In vitro examination of DHA-edible micro-algae. Anim. Feed Sci. Tech. 2007, 136, 80–95. [Google Scholar] [CrossRef]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.J.; Mosley, E.E. Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J. Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2009, 4, 351–365. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Fonseca, A.J.M.; Oliveira, H.M.; Mendonça, C.; Cabrita, A.R.J. The Potential Role of Seaweeds in the Natural Manipulation of Rumen Fermentation and Methane Production. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane Emissions from Cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Miron, J.; Ben-Ghedalia, D.; Morrison, M. Invited Review: Adhesion Mechanisms of Rumen Cellulolytic Bacteria. J. Dairy Sci. 2001, 84, 1294–1309. [Google Scholar] [CrossRef]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.R.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Chen, Y.; Liu, J.; Zhang, C.; Irving, B.; Fitzsimmons, C.; Plastow, G.; Guan, L.L. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome 2019, 7, 92. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Abdullah, M.A.M.; Skliros, D.; Chatzikonstantinou, M.; Flemetakis, E.; Labrou, N.; Zervas, G. The effect of dietary Chlorella vulgaris supplementation on micro-organism community, enzyme activities and fatty acid profile in the rumen liquid of goats. J. Anim. Physiol. Anim. Nutr. 2016, 101, 275–283. [Google Scholar] [CrossRef]
- Ramos-Morales, E.; Arco-Pérez, A.; Martín-García, A.I.; Yáñez-Ruiz, D.R.; Frutos, P.; Hervás, G. Use of stomach tubing as an alternative to rumen cannulation to study ruminal fermentation and microbiota in sheep and goats. Anim. Feed Sci. Tech. 2014, 198, 57–66. [Google Scholar] [CrossRef]
- Shen, J.S.; Chai, Z.; Song, L.J.; Liu, J.X.; Wu, Y.M. Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows. J. Dairy Sci. 2012, 95, 5978–5984. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A. The effect of supplementation with microalgae Schizochytrium sp. in goats’ diet, in milk and blood biochemical parameters. Doctoral Dissertation, Agricultural University Athens, Greece, Athens, 2019. National Archive of PhD Theses. Available online: http://hdl.handle.net/10442/hedi/46071 (accessed on 10 November 2020).
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, J.T.; Karnati, S.K.; Yu, Z.; Morrison, M.; Firkins, J.L. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 2004, 134, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, T.; Yu, Z. Invited Review — Metagenomic investigation of gastrointestinal microbiome in cattle. Asian-Australasian J. Anim. Sci. 2017, 30, 1515–1528. [Google Scholar] [CrossRef]
- Luton, P.E.; Wayne, J.M.; Sharp, R.J.; Riley, P.W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill b. Microbiology 2002, 148, 3521–3530. [Google Scholar] [CrossRef]
- Yang, S.L.; Bu, D.P.; Wang, J.Q.; Hu, Z.Y.; Li, D.; Wei, H.Y.; Loor, J.J. Soybean oil and linseed oil supplementation affect profiles of ruminal microorganisms in dairy cows. Animal 2009, 3, 1562–1569. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Cancino-Padilla, N.; Romero, J.; Garnsworthy, P.C. Quantitative analysis of ruminal bacterial populations involved in lipid metabolism in dairy cows fed different vegetable oils. Animal 2016, 10, 1821–1828. [Google Scholar] [CrossRef]
- Duval, S.M.; McEwan, N.R.; Graham, R.C.; Wallace, R.J.; Newbold, C.J. Effect of a blend of essential oil compounds on the colonization of starch-rich substrates by bacteria in the rumen. J. Appl. Microbiol. 2007, 103, 2132–2141. [Google Scholar] [CrossRef]
- Elolimy, A.A.; Arroyo, J.M.; Batistel, F.; Iakiviak, M.A.; Loor, J.J. Association of residual feed intake with abundance of ruminal bacteria and biopolymer hydrolyzing enzyme activities during the peripartal period and early lactation in Holstein dairy cows. J. Anim. Sci. Biotechnol. 2018, 9. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Carberry, C.A.; Kenny, D.A.; Han, S.; McCabe, M.S.; Waters, S.M. Effect of Phenotypic Residual Feed Intake and Dietary Forage Content on the Rumen Microbial Community of Beef Cattle. Appl. Environ. Microbiol. 2012, 78, 4949–4958. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Wang, J.K.; Wu, Y.M.; Liu, J.X. Effects of chemical treatments of rice straw on rumen fermentation characteristics, fibrolytic enzyme activities and populations of liquid- and solid-associated ruminal microbes in vitro. Anim. Feed Sci. Tech. 2008, 141, 1–14. [Google Scholar] [CrossRef]
- Bahl, M.I.; Bergström, A.; Licht, T.R. Freezing fecal samples prior to DNA extraction affects the Firmicutes to Bacteroidetes ratio determined by downstream quantitative PCR analysis. FEMS Microbiol. Lett. 2012, 329, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; White, B.A.; Mizrahi, I. Potential Role of the Bovine Rumen Microbiome in Modulating Milk Composition and Feed Efficiency. PLoS One 2014, 9, e85423. [Google Scholar] [CrossRef]
- Castro-Carrera, T.; Toral, P.G.; Frutos, P.; McEwan, N.R.; Hervás, G.; Abecia, L.; Belenguer, A. Rumen bacterial community evaluated by 454 pyrosequencing and terminal restriction fragment length polymorphism analyses in dairy sheep fed marine algae. J. Dairy Sci. 2014, 97, 1661–1669. [Google Scholar] [CrossRef]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2014, 8. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Forano, E.; Martin, C.; Newbold, C.J. Microbial ecosystem and methanogenesis in ruminants. Animal 2010, 4, 1024–1036. [Google Scholar] [CrossRef]
- Patra, A.K.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8. [Google Scholar] [CrossRef]
- Delgado, B.; Bach, A.; Guasch, I.; González, C.; Elcoso, G.; Pryce, J.E.; Gonzalez-Recio, O. Whole rumen metagenome sequencing allows classifying and predicting feed efficiency and intake levels in cattle. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Wright, A.D.G.; Klieve, A.V. Does the complexity of the rumen microbial ecology preclude methane mitigation? Anim. Feed Sci. Technol. 2011, 166–167, 248–253. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of coconut and fish oils on ruminal methanogenesis, fermentation, and abundance and diversity of microbial populations in vitro. J. Dairy Sci. 2013, 96, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Roque, B.M.; Brooke, C.G.; Ladau, J.; Polley, T.M.; Marsh, L.J.; Najafi, N.; Pandey, P.; Singh, L.; Salwen, J.K.; Eloe-Fadrosh, E.; et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim. Microb. 2019, 1, 1–14. [Google Scholar] [CrossRef]
- Vargas, J.E.; Andrés, S.; Snelling, T.J.; López-Ferreras, L.; Yáñez-Ruíz, D.R.; García-Estrada, C.; López, S. Effect of Sunflower and Marine Oils on Ruminal Microbiota, In vitro Fermentation and Digesta Fatty Acid Profile. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Belanche, A.; de la Fuente, G.; Newbold, C.J. Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol. Ecol. 2014, 90, 663–677. [Google Scholar] [CrossRef]
- Guyader, J.; Eugène, M.; Nozière, P.; Morgavi, D.P.; Doreau, M.; Martin, C. Influence of rumen protozoa on methane emission in ruminants: A meta-analysis approach. Animal 2014, 8, 1816–1825. [Google Scholar] [CrossRef]
- Newbold, C.J.; de la Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The Role of Ciliate Protozoa in the Rumen. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Doreau, M.; Ferlay, A. Effect of dietary lipids on nitrogen metabolism in the rumen: A review. Livest. Prod. Sci. 1995, 43, 97–110. [Google Scholar] [CrossRef]
- Goel, G.; Puniya, A.; Aguilar, C.; Singh, K. Interaction of gut microflora with tannins in feeds. Die Naturwissenschaften. 2005, 92, 497–503. [Google Scholar] [CrossRef]
- Jordan, E.; Lovett, D.K.; Monahan, F.J.; Callan, J.; Flynn, B.; O’Mara, F.P. Effect of refined coconut oil or copra meal on methane output and on intake and performance of beef heifers. J. Anim. Sci. 2006, 84, 162–170. [Google Scholar] [CrossRef]
- Lv, X.; Mao, S.; Zhu, W. A 22:6 n-3 Rich Supplement Affects the Ruminal Microbial Community and Fermentation and Alters Plasma Metabolites. Ann. Anim. Sci. 2016, 16, 533–550. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Edwards, J.E.; Allison, G.G.; Zhu, W.Y.; Theodorou, M.K. Diversity and activity of enriched ruminal cultures of anaerobic fungi and methanogens grown together on lignocellulose in consecutive batch culture. Bioresour. Technol. 2009, 100, 4821–4828. [Google Scholar] [CrossRef] [PubMed]
- De Mulder, T.; Goossens, K.; Peiren, N.; Vandaele, L.; Haegeman, A.; De Tender, C.; Ruttink, T.; Van de Wiele, T.; De Campeneere, S. Exploring the methanogen and bacterial communities of rumen environments: Solid adherent, fluid and epimural. FEMS Microbiol. Ecol. 2016, 93, fiw251. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mu, C.; Xu, Y.; Shen, J.; Zhu, W. Changes in the Solid-, Liquid-, and Epithelium-Associated Bacterial Communities in the Rumen of Hu Lambs in Response to Dietary Urea Supplementation. Front. Microbiol. 2020, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Huws, S.A.; Lee, M.R.F.; Muetzel, S.M.; Scott, M.B.; Wallace, R.J.; Scollan, N.D. Forage type and fish oil cause shifts in rumen bacterial diversity. FEMS Microbiol. Ecol. 2010, 73, 396–702. [Google Scholar] [CrossRef] [PubMed]
- Sawanon, S.; Koike, S.; Kobayashi, Y. Evidence for the possible involvement of Selenomonas ruminantium in rumen fiber digestion. FEMS Microbiol. Lett. 2011, 325, 170–179. [Google Scholar] [CrossRef]
- Carreño, D.; Toral, P.G.; Pinloche, E.; Belenguer, A.; Yáñez-Ruiz, D.R.; Hervás, G.; McEwan, N.R.; Newbold, C.J.; Frutos, P. Rumen bacterial community responses to DPA, EPA and DHA in cattle and sheep: A comparative in vitro study. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Wallace, R.J.; Chaudhary, L.C.; McKain, N.; McEwan, N.R.; Richardson, A.J.; Vercoe, P.E.; Walker, N.D.; Paillard, D. Clostridium proteoclasticum: A ruminal bacterium that forms stearic acid from linoleic acid. FEMS Microbiol. Lett. 2006, 265, 195–201. [Google Scholar] [CrossRef]
- McKain, N.; Shingfield, K.J.; Wallace, R.J. Metabolism of conjugated linoleic acids and 18:1 fatty acids by ruminal bacteria: Products and mechanisms. Microbiology. 2010, 156, 579–588. [Google Scholar] [CrossRef]
- Dewanckele, L.; Vlaeminck, B.; Hernandez-Sanabria, E.; Ruiz-González, A.; Debruyne, S.; Jeyanathan, J.; Fievez, V. Rumen Biohydrogenation and Microbial Community Changes Upon Early Life Supplementation of 22:6n-3 Enriched Microalgae to Goats. Front. Microbiol. 2018, 27, 573. [Google Scholar] [CrossRef]
- Zhu, H.; Fievez, V.; Mao, S.; He, W.; Zhu, W. Dose and time response of ruminally infused algae on rumen fermentation characteristics, biohydrogenation and Butyrivibrio group bacteria in goats. J. Anim. Sci. Biotechnol. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Toral, P.G.; Belenguer, A.; Shingfield, K.J.; Hervás, G.; Toivonen, V.; Frutos, P. Fatty acid composition and bacterial community changes in the rumen fluid of lactating sheep fed sunflower oil plus incremental levels of marine algae. J. Dairy Sci. 2012, 95, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.R.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R.; Wallace, R.J. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).