A Comprehensive Review on Oil Extraction and Biodiesel Production Technologies
Abstract
1. Introduction
2. Feedstocks for Biodiesel Production
Type and Availability
3. Oil Extraction Processes
3.1. Mechanical Extraction
3.2. Steam Distillation
3.3. Solvent Extraction
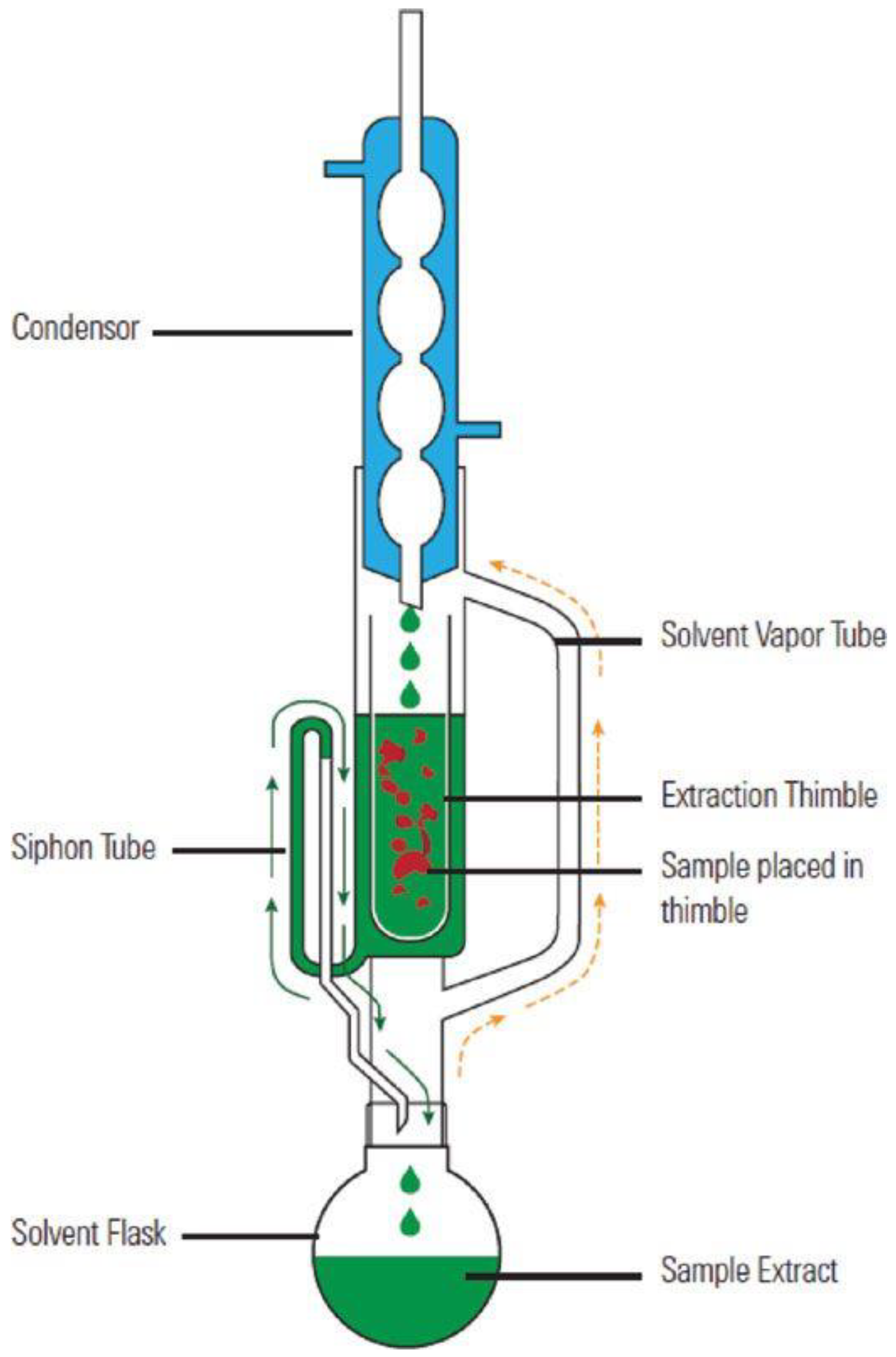
3.3.1. Soxhlet Extraction
3.3.2. Chemical Leaching
3.3.3. Enzymatic Oil Extraction
3.3.4. Supercritical Fluid Extraction
3.3.5. Microwave-Assisted Extraction
3.3.6. Ultrasound-Assisted Extraction
3.4. Oil Refining
4. Biodiesel Synthesis Processes
4.1. Direct Blending
4.2. Microemulsions
4.3. Catalytic Cracking
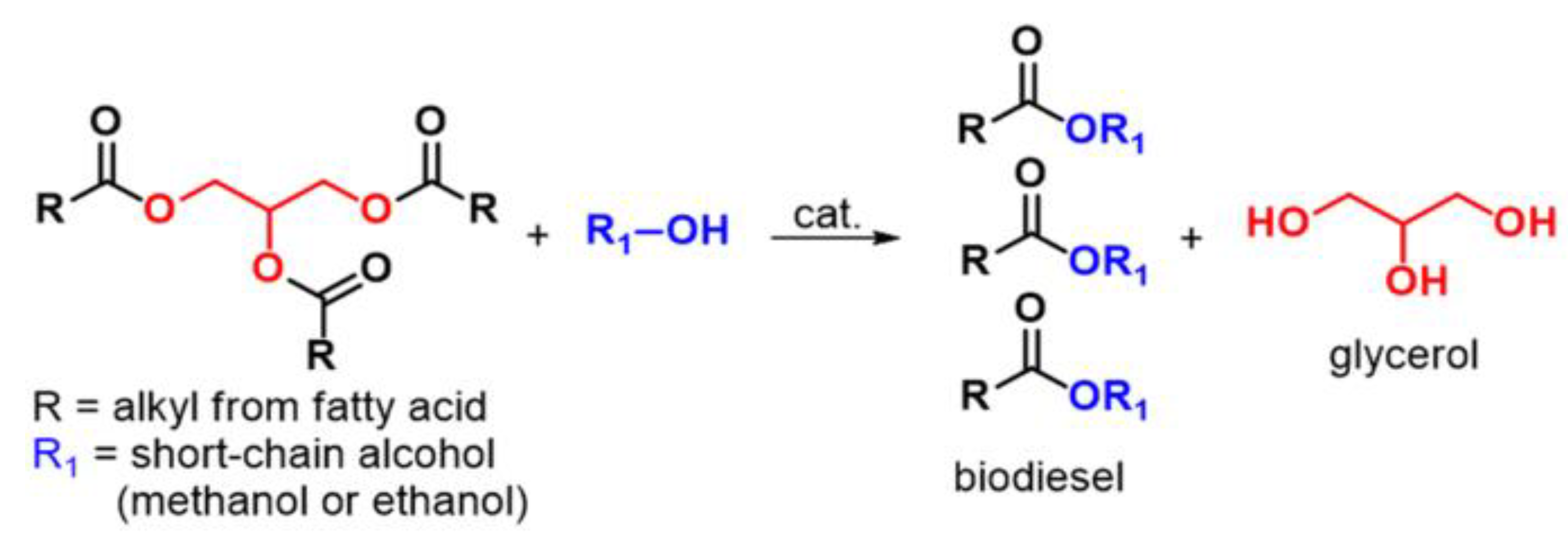
4.4. Transesterification
4.4.1. Edible Oil Use for Biodiesel Production
4.4.2. Biodiesel Synthesis from Non-Edible Oil
4.4.3. Biodiesel Synthesis from Waste/Crude Oils
5. Critical Reaction Parameters Influencing the Biodiesel Synthesis
5.1. Reaction Temperature
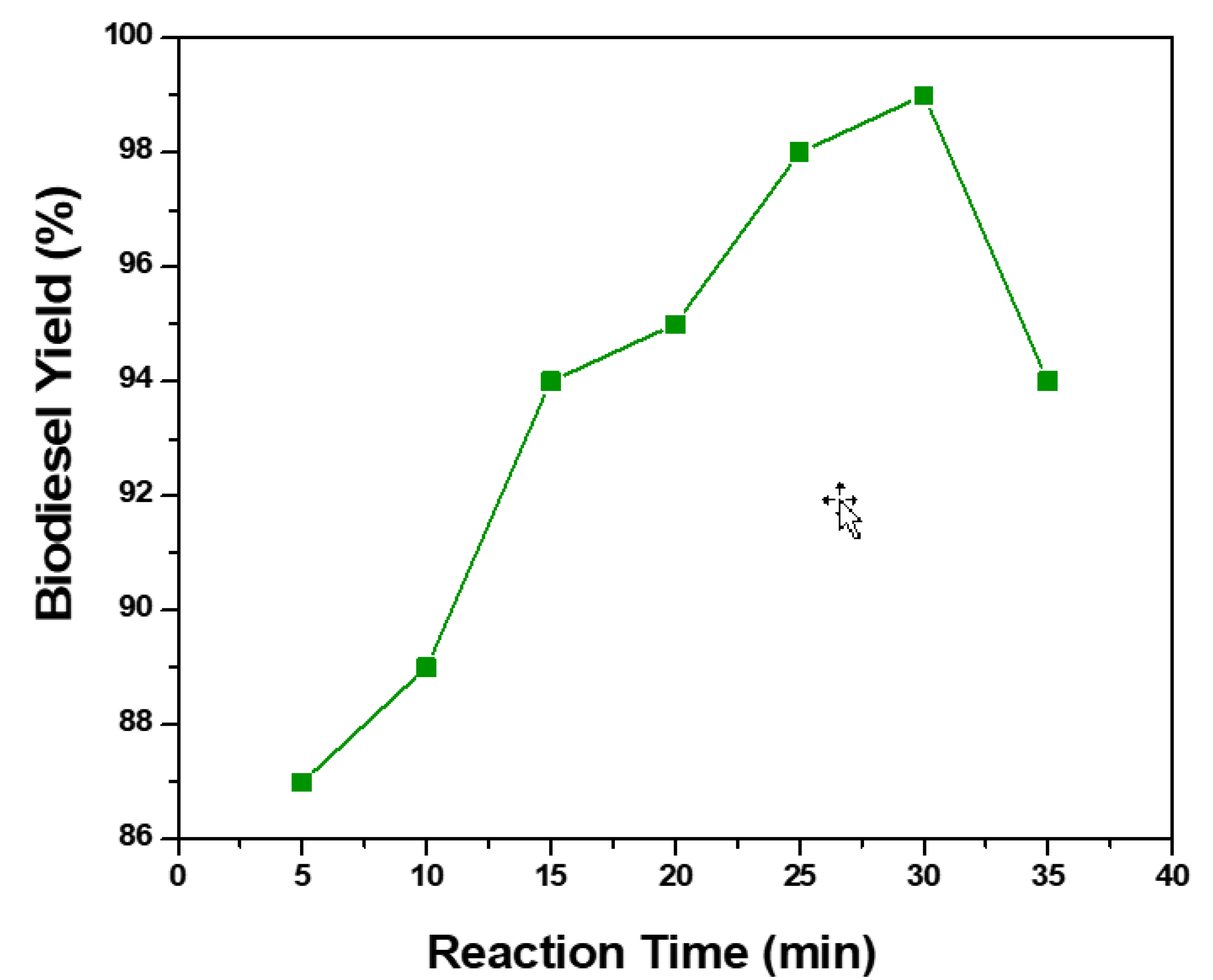
5.2. Reaction Time
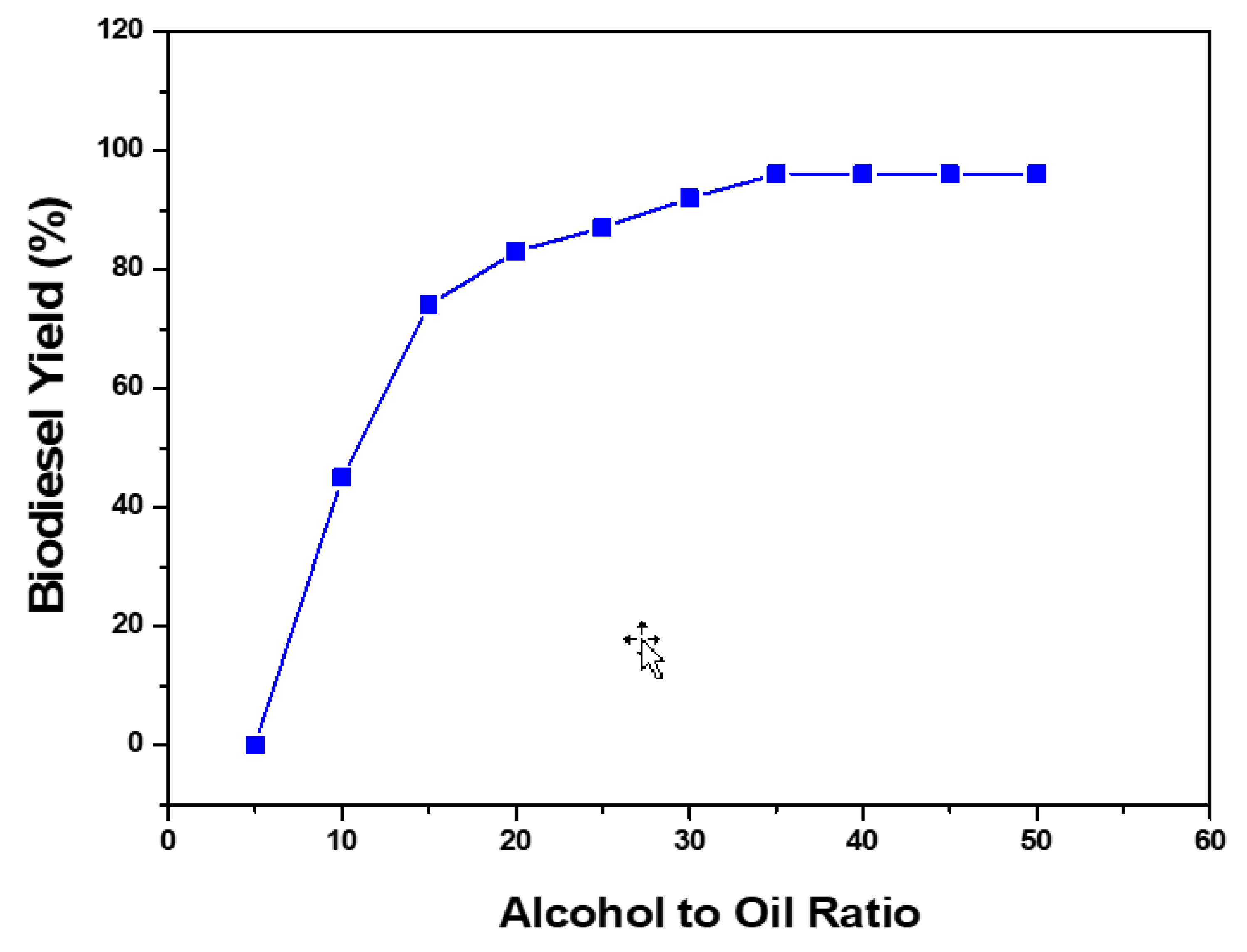
5.3. Alcohol to Oil Ratio
5.4. Catalyst Amount and Type
5.5. Effect of Water and FFA Content
5.6. Mixing Intensity
5.7. Kinetics of Biodiesel Synthesis
6. Conclusions and Future Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, H.B.; Sarmah, A.K.; Dubey, B.K. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Zulqarnain; Yusoff, M.H.M.; Ayoub, M.; Jusoh, N.; Abdullah, A.Z. The Challenges of a Biodiesel Implementation Program in Malaysia. Processes 2020, 8, 1244. [Google Scholar] [CrossRef]
- Luque, R.; Clark, J. Handbook of Biofuels Production: Processes and Technologies; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Sharma, Y.; Singh, B.; Upadhyay, S. Advancements in development and characterization of biodiesel: A review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
- Yusuf, N.; Kamarudin, S.; Yaakub, Z. Overview on the current trends in biodiesel production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Subbarayan, M.; Kumaar, J.S.; Padmanaban, M.A. Experimental investigation of evaporation rate and exhaust emissions of diesel engine fuelled with cotton seed methyl ester and its blend with petro-diesel. Transp. Res. Part D Transp. Environ. 2016, 48, 369–377. [Google Scholar] [CrossRef]
- Mazanov, S.V.; Gabitova, A.R.; Usmanov, R.A.; Gumerov, F.M.; Labidi, S.; Ben Amar, M.; Passarello, J.-P.; Kanaev, A.; Volle, F.; Le Neindre, B. Continuous production of biodiesel from rapeseed oil by ultrasonic assist transesterification in supercritical ethanol. J. Supercrit. Fluids 2016, 118, 107–118. [Google Scholar] [CrossRef]
- Lin, J.-J.; Chen, Y.-W. Production of biodiesel by transesterification of Jatropha oil with microwave heating. J. Taiwan Inst. Chem. Eng. 2017, 75, 43–50. [Google Scholar] [CrossRef]
- Ayodele, O.O.; Dawodu, F.A. Production of biodiesel from Calophyllum inophyllum oil using a cellulose-derived catalyst. Biomass Bioenergy 2014, 70, 239–248. [Google Scholar] [CrossRef]
- Kılıç, M.; Uzun, B.B.; Pütün, E.; Pütün, A.E. Optimization of biodiesel production from castor oil using factorial design. Fuel Process. Technol. 2013, 111, 105–110. [Google Scholar] [CrossRef]
- Cremonez, P.A.; Feroldi, M.; Nadaleti, W.C.; De Rossi, E.; Feiden, A.; De Camargo, M.P.; Cremonez, F.E.; Klajn, F.F. Biodiesel production in Brazil: Current scenario and perspectives. Renew. Sustain. Energy Rev. 2015, 42, 415–428. [Google Scholar] [CrossRef]
- Peer, M.S.; Kasimani, R.; Rajamohan, S.; Ramakrishnan, P. Experimental evaluation on oxidation stability of biodiesel/diesel blends with alcohol addition by rancimat instrument and FTIR spectroscopy. J. Mech. Sci. Technol. 2017, 31, 455–463. [Google Scholar] [CrossRef]
- Kafuku, G.; Mbarawa, M. Biodiesel production from Croton megalocarpus oil and its process optimization. Fuel 2010, 89, 2556–2560. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Atabani, A.; Mahlia, T.; Masjuki, H.; Badruddin, I.A.; Mekhilef, S. A review on prospect of Jatropha curcas for biodiesel in Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 3733–3756. [Google Scholar] [CrossRef]
- Atabani, A.; Silitonga, A.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Janaun, J.; Ellis, N. Perspectives on biodiesel as a sustainable fuel. Renew. Sustain. Energy Rev. 2010, 14, 1312–1320. [Google Scholar] [CrossRef]
- Cao, P.; Tremblay, A.Y.; Dubé, M.A. Kinetics of canola oil transesterification in a membrane reactor. Ind. Eng. Chem. Res. 2009, 48, 2533–2541. [Google Scholar] [CrossRef]
- Sumathi, S.; Chai, S.; Mohamed, A. Utilization of oil palm as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2008, 12, 2404–2421. [Google Scholar] [CrossRef]
- Razzaq, L.; Farooq, M.; Mujtaba, M.; Sher, F.; Farhan, M.; Hassan, M.T.; Soudagar, M.E.M.; Atabani, A.; Kalam, M.; Imran, M. Modeling viscosity and density of ethanol-diesel-biodiesel ternary blends for sustainable environment. Sustainability 2020, 12, 5186. [Google Scholar] [CrossRef]
- Kansedo, J.; Lee, K.T.; Bhatia, S. Biodiesel production from palm oil via heterogeneous transesterification. Biomass Bioenergy 2009, 33, 271–276. [Google Scholar] [CrossRef]
- Gugule, S.; Fatimah, F.; Maanari, C.P.; Tallei, T.E. Data on the use of virgin coconut oil and bioethanol produced from sugar palm sap as raw materials for biodiesel synthesis. Data Brief 2020, 29, 105199. [Google Scholar] [CrossRef]
- Çelikten, I.; Koca, A.; Arslan, M.A. Comparison of performance and emissions of diesel fuel, rapeseed and soybean oil methyl esters injected at different pressures. Renew. Energy 2010, 35, 814–820. [Google Scholar] [CrossRef]
- Hamza, M.; Ayoub, M.; Bin Shamsuddin, R.; Mukhtar, A.; Saqib, S.; Zahid, I.; Ameen, M.; Ullah, S.; Al-Sehemi, A.G.; Ibrahim, M. A review on the waste biomass derived catalysts for biodiesel production. Environ. Technol. Innov. 2020, in press. [Google Scholar] [CrossRef]
- Zulqarnain, M.H.M.Y.; Ayoub, M.; Qadeer, M.U. Comparison of kinetic and thermodynamic modeling of biodiesel production using supercritical transesterification for batch and continuous reactor. Solid State Technol. 2020, 4055–4067. Available online: https://www.researchgate.net/publication/344946868_Comparison_of_kinetic_and_thermodynamic_modeling_of_biodiesel_production_using_supercritical_transesterification_for_batch_and_continuous_reactor (accessed on 15 December 2020).
- Nazir, M.H.; Ayoub, M.; Shamsuddin, R.B.; Malik, Z. Production of Biodiesel from Waste Cooking Oil using Sugarcane Bagasse Derived Acid Activated Catalyst and Microwave as Heating Source. Solid State Technol. 2020, 3839–3846. Available online: https://www.researchgate.net/publication/344946807_Production_of_Biodiesel_from_Waste_Cooking_Oil_using_Sugarcane_Bagasse_Derived_Acid_Activated_Catalyst_and_Microwave_as_Heating_Source (accessed on 15 December 2020).
- Lang, X. Preparation and characterization of bio-diesels from various bio-oils. Bioresour. Technol. 2001, 80, 53–62. [Google Scholar] [CrossRef]
- Rattanaphra, D.; Srinophakun, P. Biodiesel production from crude sunflower oil and crude jatropha oil using immobilized lipase. J. Chem. Eng. Jpn. 2010, 43, 104–108. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Yew, G.Y.; Koyande, A.K.; Chew, K.W.; Vo, D.-V.N.; Show, P.L. Green technology for the industrial production of biofuels and bioproducts from microalgae: A review. Environ. Chem. Lett. 2020, 18, 1967–1985. [Google Scholar] [CrossRef]
- Apraku, A.; Liu, L.; Ayisi, C.L. Trends and status of dietary coconut oil in aquaculture feeds. Rev. Fish. Sci. Aquac. 2017, 25, 126–132. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef]
- Li, Y.; Khanal, S.K. Bioenergy: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Kumari, D. Chemical compositions, properties, and standards for different generation biodiesels: A review. Fuel 2019, 253, 60–71. [Google Scholar] [CrossRef]
- Ghazali, W.N.M.W.; Mamat, R.; Masjuki, H.; Najafi, G. Effects of biodiesel from different feedstocks on engine performance and emissions: A review. Renew. Sustain. Energy Rev. 2015, 51, 585–602. [Google Scholar] [CrossRef]
- Istadi, I.; Prasetyo, S.A.; Nugroho, T.S. Characterization of K2O/CaO-ZnO catalyst for transesterification of soybean oil to biodiesel. Procedia Environ. Sci. 2015, 23, 394–399. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Zhang, Z.; Doherty, W.O.; O’Hara, I.M. The prospect of microbial oil production and applications from oil palm biomass. Biochem. Eng. J. 2019, 143, 9–23. [Google Scholar] [CrossRef]
- Oltean-Dumbrava, C.; Watts, G.R.; Miah, A. Transport infrastructure: Making more sustainable decisions for noise reduction. J. Clean. Prod. 2013, 42, 58–68. [Google Scholar] [CrossRef]
- Lee, S.L.; Wong, Y.C.; Tan, Y.P.; Yew, S.Y. Transesterification of palm oil to biodiesel by using waste obtuse horn shell-derived CaO catalyst. Energy Convers. Manag. 2015, 93, 282–288. [Google Scholar] [CrossRef]
- Rashtizadeh, E.; Farzaneh, F. Transesterification of soybean oil catalyzed by Sr–Ti mixed oxides nanocomposite. J. Taiwan Inst. Chem. Eng. 2013, 44, 917–923. [Google Scholar] [CrossRef]
- Pilorgé, E. Sunflower in the global vegetable oil system: Situation, specificities and perspectives. OCL 2020, 27, 34. [Google Scholar] [CrossRef]
- Fadel, H.; Marx, F.; El-Sawy, A.; El-Ghorab, A. Effect of extraction techniques on the chemical composition and antioxidant activity of Eucalyptus camaldulensis var. brevirostris leaf oils. Z. Lebensm. und-Forsch. A 1999, 208, 212–216. [Google Scholar] [CrossRef]
- Antolın, G.; Tinaut, F.V.; Briceño, Y.; Castaño, V.; Pérez, C.; Ramírez, A.I. Optimisation of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 2002, 83, 111–114. [Google Scholar] [CrossRef]
- Brachet, A.; Christen, P.; Veuthey, J.-L. Focused microwave-assisted extraction of cocaine and benzoylecgonine from coca leaves. Phytochem. Anal. 2002, 13, 162–169. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Ferhat, M.A.; Meklati, B.Y.; Smadja, J.; Chemat, F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J. Chromatogr. A 2006, 1112, 121–126. [Google Scholar] [CrossRef]
- Farhat, A.; Ginies, C.; Romdhane, M.; Chemat, F. Eco-friendly and cleaner process for isolation of essential oil using microwave energy: Experimental and theoretical study. J. Chromatogr. A 2009, 1216, 5077–5085. [Google Scholar] [CrossRef]
- Assami, K.; Pingret, D.; Chemat, S.; Meklati, B.Y.; Chemat, F. Ultrasound induced intensification and selective extraction of essential oil from Carum carvi L. seeds. Chem. Eng. Process. Process. Intensif. 2012, 62, 99–105. [Google Scholar] [CrossRef]
- Gayas, B.; Kaur, G.; Singh, A. Ultrasound assisted extraction of apricot kernel oil: Effect on physicochemical, morphological characteristics, and fatty acid composition. Acta Aliment. 2020, 49, 23–31. [Google Scholar] [CrossRef]
- Mittelbach, M.; Remschmidt, C. Biodiesel: The Comprehensive Handbook; Boersedruck Ges. MBH: Vienna, Austria, 2004. [Google Scholar]
- Knothe, G. Biodiesel and renewable diesel: A comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373. [Google Scholar] [CrossRef]
- Zahid, I.; Ayoub, M.; Abdullah, B.B.; Nazir, M.H.; Ameen, M.; Zulqarnain; Yusoff, M.H.M.; Inayat, A.; Danish, M. Production of Fuel Additive Solketal via Catalytic Conversion of Biodiesel-Derived Glycerol. Ind. Eng. Chem. Res. 2020, 59, 20961–20978. [Google Scholar] [CrossRef]
- Demirbas, A. Potential resources of non-edible oils for biodiesel. Energy Sources Part B 2009, 4, 310–314. [Google Scholar] [CrossRef]
- Nguyen, T.; Do, L.; Sabatini, D.A. Biodiesel production via peanut oil extraction using diesel-based reverse-micellar microemulsions. Fuel 2010, 89, 2285–2291. [Google Scholar] [CrossRef]
- Baskar, G.; Soumiya, S. Production of biodiesel from castor oil using iron (II) doped zinc oxide nanocatalyst. Renew. Energy 2016, 98, 101–107. [Google Scholar] [CrossRef]
- Nan, Y.; Liu, J.; Lin, R.; Tavlarides, L.L. Production of biodiesel from microalgae oil (Chlorella protothecoides) by non-catalytic transesterification in supercritical methanol and ethanol: Process optimization. J. Supercrit. Fluids 2015, 97, 174–182. [Google Scholar] [CrossRef]
- Meher, L.; Sagar, D.V.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Biodiesel from vegetable oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Gargalo, C.L.; Udugama, I.A.; Ramin, P.; Sales-Cruz, M.; Sin, G.; Gernaey, K.V. Economic risk analysis and critical comparison of biodiesel production systems. In Biodiesel; Springer: Berlin, Germany, 2019; pp. 127–148. [Google Scholar]
- Vujicic, D.; Comic, D.; Zarubica, A.; Micic, R.; Boskovic, G.C. Kinetics of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel 2010, 89, 2054–2061. [Google Scholar] [CrossRef]
- Noureddini, H.; Zhu, D. Kinetics of transesterification of soybean oil. J. Am. Oil Chem. Soc. 1997, 74, 1457–1463. [Google Scholar] [CrossRef]
- Reyero, I.; Arzamendi, G.; Zabala, S.; Gandía, L.M. Kinetics of the NaOH-catalyzed transesterification of sunflower oil with ethanol to produce biodiesel. Fuel Process. Technol. 2015, 129, 147–155. [Google Scholar] [CrossRef]
- Nasreen, S.; Liu, H.; Skala, D.; Waseem, A.; Wan, L. Preparation of biodiesel from soybean oil using La/Mn oxide catalyst. Fuel Process. Technol. 2015, 131, 290–296. [Google Scholar] [CrossRef]
- Mohan Kuppusamy, T.K.K. Optimization and production of biodiesel from cottonseed oil and neem oil. Int. J. Mod. Sci. Technol. 2016, 1, 23–28. [Google Scholar]
- Gui, X.; Chen, S.; Yun, Z. Continuous production of biodiesel from cottonseed oil and methanol using a column reactor packed with calcined sodium silicate base catalyst. Chin. J. Chem. Eng. 2016, 24, 499–505. [Google Scholar] [CrossRef]
- Onukwuli, D.O.; Emembolu, L.N.; Ude, C.N.; Aliozo, S.O.; Menkiti, M.C. Optimization of biodiesel production from refined cotton seed oil and its characterization. Egypt. J. Pet. 2017, 26, 103–110. [Google Scholar] [CrossRef]
- Qiu, T.; Guo, X.; Yang, J.; Zhou, L.; Li, L.; Wang, H.; Niu, Y. The synthesis of biodiesel from coconut oil using novel Brønsted acidic ionic liquid as green catalyst. Chem. Eng. J. 2016, 296, 71–78. [Google Scholar] [CrossRef]
- Saydut, A.; Erdoğan, S.; Kafadar, A.B.; Kaya, C.; Aydin, F.; Hamamci, C. Process optimization for production of biodiesel from hazelnut oil, sunflower oil and their hybrid feedstock. Fuel 2016, 183, 512–517. [Google Scholar] [CrossRef]
- Akia, M.; Yazdani, F.; Motaee, E.; Han, D.; Arandiyan, H. A review on conversion of biomass to biofuel by nanocatalysts. Biofuel Res. J. 2014, 1, 16–25. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H. Synthesis of Ti (SO4) O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. Appl. Catal. A Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Feyzi, M.; Nourozi, L.; Zakarianezhad, M. Preparation and characterization of magnetic CsH2PW12O40/Fe–SiO2 nanocatalysts for biodiesel production. Mater. Res. Bull. 2014, 60, 412–420. [Google Scholar] [CrossRef]
- Rashtizadeh, E.; Farzaneh, F.; Talebpour, Z. Synthesis and characterization of Sr3Al2O6 nanocomposite as catalyst for biodiesel production. Bioresour. Technol. 2014, 154, 32–37. [Google Scholar] [CrossRef]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Heterogeneous solid base nanocatalyst: Preparation, characterization and application in biodiesel production. Bioresour. Technol. 2011, 102, 4150–4156. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef]
- Feyzi, M.; Shahbazi, E. Catalytic performance and characterization of Cs–Ca/SiO2–TiO2 nanocatalysts for biodiesel production. J. Mol. Catal. A Chem. 2015, 404, 131–138. [Google Scholar] [CrossRef]
- Sanchez, F.; Vasudevan, P.T. Enzyme catalyzed production of biodiesel from olive oil. Appl. Biochem. Biotechnol. 2006, 135, 1–14. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Tominaga, Y. Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J. Mol. Catal. B Enzym. 2002, 17, 133–142. [Google Scholar] [CrossRef]
- Rathore, V.; Tyagi, S.; Newalkar, B.; Badoni, R. Jatropha and Karanja oil derived DMC–biodiesel synthesis: A kinetics study. Fuel 2015, 140, 597–608. [Google Scholar] [CrossRef]
- Olutoye, M.; Adeniyi, O.; Yusuff, A. Synthesis of biodiesel from palm kernel oil using mixed clay-eggshell heterogeneous catalysts. Iran. J. Energy Environ. 2016, 7, 308–314. [Google Scholar]
- Abbah, E.; Nwandikom, G.I.; Egwuonwu, C.C.; Nwakuba, N.R. Effect of reaction temperature on the yield of biodiesel from neem seed oil. Am. J. Energy Sci. 2016, 3, 16–20. [Google Scholar]
- Samsudeen, N.; Dammalapati, S.; Mondal, S.; Unnithan, L. Production of Biodiesel from Neem Oil Feedstock Using Bifunctional Catalyst. In Materials, Energy and Environment Engineering; Springer: Berlin, Germany, 2017; pp. 187–195. [Google Scholar]
- Dhawane, S.H.; Bora, A.P.; Kumar, T.; Halder, G. Parametric optimization of biodiesel synthesis from rubber seed oil using iron doped carbon catalyst by Taguchi approach. Renew. Energy 2017, 105, 616–624. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Sudyoadsuk, T.; Promarak, V. Rubber seed oil as potential non-edible feedstock for biodiesel production using heterogeneous catalyst in Thailand. Renew. Energy 2017, 101, 937–944. [Google Scholar] [CrossRef]
- Bokhari, A.; Chuah, L.F.; Yusup, S.; Klemeš, J.J.; Akbar, M.M.; Kamil, R.N.M. Cleaner production of rubber seed oil methyl ester using a hydrodynamic cavitation: Optimisation and parametric study. J. Clean. Prod. 2016, 136, 31–41. [Google Scholar] [CrossRef]
- Patel, R.L.; Sankhavara, C. Biodiesel production from Karanja oil and its use in diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 71, 464–474. [Google Scholar] [CrossRef]
- Sahani, S.; Roy, T.; Sharma, Y.C. Clean and efficient production of biodiesel using barium cerate as a heterogeneous catalyst for the biodiesel production; kinetics and thermodynamic study. J. Clean. Prod. 2019, 237, 117699. [Google Scholar] [CrossRef]
- Singh, V.; Hameed, B.H.; Sharma, Y.C. Economically viable production of biodiesel from a rural feedstock from eastern India, P. pinnata oil using a recyclable laboratory synthesized heterogeneous catalyst. Energy Convers. Manag. 2016, 122, 52–62. [Google Scholar] [CrossRef]
- Gargari, M.H.; Sadrameli, S. Investigating continuous biodiesel production from linseed oil in the presence of a Co-solvent and a heterogeneous based catalyst in a packed bed reactor. Energy 2018, 148, 888–895. [Google Scholar] [CrossRef]
- Baskar, G.; Selvakumari, I.A.E.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A.; Mukti, N.I.F.; Handoko, B.; Sutrisno, B. Biodiesel production from rice bran oil over modified natural zeolite catalyst. Int. J. Technol. 2018, 9, 400–411. [Google Scholar] [CrossRef]
- Deep, A.; Sandhu, S.; Chander, S. Optimization of reaction parameters of Transesterification for Castor oil. J. Sci. Ind. Res. 2017, 76, 115–118. [Google Scholar]
- Fadhil, A.B.; Saleh, L.A.; Altamer, D.H. Production of biodiesel from non-edible oil, wild mustard (Brassica juncea L.) seed oil through cleaner routes. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 1831–1843. [Google Scholar] [CrossRef]
- Sani, Y.M.; Raji-Yahya, A.O.; Alaba, P.A.; Aziz, A.R.A.; Daud, W.M.A.W. Palm frond and spikelet as environmentally benign alternative solid acid catalysts for biodiesel production. BioResources 2015, 10, 3393–3408. [Google Scholar] [CrossRef]
- Ghadge, S.V.; Raheman, H. Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass Bioenergy 2005, 28, 601–605. [Google Scholar] [CrossRef]
- Srivastava, P.; Verma, M. Methyl ester of karanja oil as an alternative renewable source energy. Fuel 2008, 87, 1673–1677. [Google Scholar] [CrossRef]
- Patil, P.D.; Deng, S. Optimization of biodiesel production from edible and non-edible vegetable oils. Fuel 2009, 88, 1302–1306. [Google Scholar] [CrossRef]
- Ramadhas, A.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Venkanna, B.; Reddy, C.V. Biodiesel production and optimization from Calophyllum inophyllum linn oil (honne oil)—A three stage method. Bioresour. Technol. 2009, 100, 5122–5125. [Google Scholar] [CrossRef] [PubMed]
- Sahar; Sadaf, S.; Iqbal, M.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Rehman, H.U.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Tangy, A.; Pulidindi, I.N.; Perkas, N.; Gedanken, A. Continuous flow through a microwave oven for the large-scale production of biodiesel from waste cooking oil. Bioresour. Technol. 2017, 224, 333–341. [Google Scholar] [CrossRef]
- Tshizanga, N.; Aransiola, E.F.; Oyekola, O. Optimisation of biodiesel production from waste vegetable oil and eggshell ash. S. Afr. J. Chem. Eng. 2017, 23, 145–156. [Google Scholar] [CrossRef]
- Suwanno, S.; Rakkan, T.; Yunu, T.; Paichid, N.; Kimtun, P.; Prasertsan, P.; Sangkharak, K. The production of biodiesel using residual oil from palm oil mill effluent and crude lipase from oil palm fruit as an alternative substrate and catalyst. Fuel 2017, 195, 82–87. [Google Scholar] [CrossRef]
- dos Santos, L.K.; Hatanaka, R.R.; de Oliveira, J.E.; Flumignan, D.L. Production of biodiesel from crude palm oil by a sequential hydrolysis/esterification process using subcritical water. Renew. Energy 2019, 130, 633–640. [Google Scholar] [CrossRef]
- Román-Figueroa, C.; Olivares-Carrillo, P.; Paneque, M.; Palacios-Nereo, F.J.; Quesada-Medina, J. High-yield production of biodiesel by non-catalytic supercritical methanol transesterification of crude castor oil (Ricinus communis). Energy 2016, 107, 165–171. [Google Scholar] [CrossRef]
- Rabu, R.A.; Janajreh, I.; Honnery, D. Transesterification of waste cooking oil: Process optimization and conversion rate evaluation. Energy Convers. Manag. 2013, 65, 764–769. [Google Scholar] [CrossRef]
- Dorado, M.P.; Ballesteros, E.; Mittelbach, A.M.; López, F.J. Kinetic parameters affecting the alkali-catalyzed transesterification process of used olive oil. Energy Fuels 2004, 18, 1457–1462. [Google Scholar] [CrossRef]
- Baskar, G.; Aiswarya, R. Trends in catalytic production of biodiesel from various feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504. [Google Scholar] [CrossRef]
- Adewale, P.; Dumont, M.-J.; Ngadi, M. Enzyme-catalyzed synthesis and kinetics of ultrasonic assisted methanolysis of waste lard for biodiesel production. Chem. Eng. J. 2016, 284, 158–165. [Google Scholar] [CrossRef]
- Šánek, L.; Pecha, J.; Kolomaznik, K.; Bařinová, M. Pilot-scale production of biodiesel from waste fats and oils using tetramethylammonium hydroxide. Waste Manag. 2016, 48, 630–637. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Betiku, E.; Ikhuomoregbe, D.I.O.; Ojumu, T.V. Production of biodiesel from crude neem oil feedstock and its emissions from internal combustion engines. Afr. J. Biotechnol. 2012, 11, 6178–6186. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S. Sustainable utilization of waste palm oil and sulfonated carbon catalyst derived from coconut meal residue for biodiesel production. Bioresour. Technol. 2018, 248, 199–203. [Google Scholar] [CrossRef]
- Mathiyazhagan, M.; Ganapathi, A. Factors affecting biodiesel production. Res. Plant Biol. 2011. [Google Scholar]
- Ogbu, I.; Ajiwe, V. Biodiesel production via esterification of free fatty acids from Cucurbita pepo L. seed oil: Kinetic studies. Int. J. Sci. Technol. 2013, 2, 616–621. [Google Scholar]
- Darnoko, D.; Cheryan, M. Kinetics of palm oil transesterification in a batch reactor. J. Am. Oil Chem. Soc. 2000, 77, 1263–1267. [Google Scholar] [CrossRef]
- Clark, W.M.; Medeiros, N.J.; Boyd, D.J.; Snell, J.R. Biodiesel transesterification kinetics monitored by pH measurement. Bioresour. Technol. 2013, 136, 771–774. [Google Scholar] [CrossRef]
- Yunus, R.; Fakhru’L-Razi, A.; Ooi, T.L.; Biak, D.R.A.; Iyuke, S.E. Kinetics of transesterification of palm-based methyl esters with trimethylolpropane. J. Am. Oil Chem. Soc. 2004, 81, 497–503. [Google Scholar] [CrossRef]
- Karmee, S.K.; Mahesh, P.; Ravi, R.; Chadha, A. Kinetic study of the base-catalyzed transesterification of monoglycerides from pongamia oil. J. Am. Oil Chem. Soc. 2004, 81, 425–430. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, P.; Garg, S. Alkali transesterification of linseed oil for biodiesel production. Fuel 2013, 104, 553–560. [Google Scholar] [CrossRef]
- Ishak, M.A.M.; Nawawi, W.I.; Jawad, A.H.; Ani, A.; Zakaria, Z.; Ismail, K. In-Situ Transesterification of Jatropha Curcas L. Seeds for Biodiesel Production Using Supercritical Methanol. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2017. [Google Scholar]
- Eevera, T.; Rajendran, K.; Saradha, S. Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew. Energy 2009, 34, 762–765. [Google Scholar] [CrossRef]
- Leung, D.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Wen, D.; Jiang, H.; Zhang, K. Supercritical fluids technology for clean biofuel production. Prog. Nat. Sci. 2009, 19, 273–284. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil 1. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Farobie, O.; Leow, Z.Y.M.; Samanmulya, T.; Matsumura, Y. New insights in biodiesel production using supercritical 1-propanol. Energy Convers. Manag. 2016, 124, 212–218. [Google Scholar] [CrossRef]
- Alamu, O.; Waheed, M.; Jekayinfa, S. Biodiesel production from Nigerian palm kernel oil: Effect of KOH concentration on yield. Energy Sustain. Dev. 2007, 11, 77–82. [Google Scholar] [CrossRef]
- Hossain, A.; Boyce, A. Biodiesel production from waste sunflower cooking oil as an environmental recycling process and renewable energy. Bulg. J. Agric. Sci. 2009, 15, 312–317. [Google Scholar]
- Zhang, Y.; Dubé, M.; McLean, D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.; Dastidar, M. Kinetic and thermodynamic studies on biodiesel production from Spirulina platensis algae biomass using single stage extraction–transesterification process. Fuel 2014, 135, 228–234. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M. Kinetics of acid base catalyzed transesterification of Jatropha curcas oil. Bioresour. Technol. 2010, 101, 7701–7706. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Rasheed, T.; Sher, F. An Experimental Investigation on Tribological Behaviour of Tire-Derived Pyrolysis Oil Blended with Biodiesel Fuel. Sustainability 2020, 12, 9975. [Google Scholar] [CrossRef]
- Guo, Y. Alkaline-Catalyzed Production of Biodiesel Fuel from Virgin Canola Oiland Recycled Waste Oils. Ph.D. Thesis, Hong Kong University, Hong Kong, China, 2005. [Google Scholar]
- Rashid, U.; Anwar, F. Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 2008, 87, 265–273. [Google Scholar] [CrossRef]
- Akhihiero, E.; Oghenejoboh, K.; Umukoro, P. Effects of process variables on transesterification reaction of jatropha curcas seed oil for the production of biodiesel. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 388–393. [Google Scholar]
- Pullen, J.; Saeed, K. Investigation of the factors affecting the progress of base-catalyzed transesterification of rapeseed oil to biodiesel FAME. Fuel Process. Technol. 2015, 130, 127–135. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel; Springer: Berlin, Germany, 2008. [Google Scholar]
- Jagadale, S.; Jugulkar, L. Review of various reaction parameters and other factors affecting on production of chicken fat based biodiesel. Int. J. Mod. Eng. Res. 2012, 2, 407–411. [Google Scholar]
- Kusdiana, D.; Saka, S. Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol. 2004, 91, 289–295. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Panahi, H.K.S.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci. 2019, 74, 239–303. [Google Scholar] [CrossRef]
- Shahbazi, M.R.; Khoshandam, B.; Nasiri, M.; Ghazvini, M. Biodiesel production via alkali-catalyzed transesterification of Malaysian RBD palm oil–Characterization, kinetics model. J. Taiwan Inst. Chem. Eng. 2012, 43, 504–510. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, B.; Xin, Z.; Liu, Q.; Sun, S. Kinetics of transesterification of palm oil and dimethyl carbonate for biodiesel production at the catalysis of heterogeneous base catalyst. Bioresour. Technol. 2010, 101, 8144–8150. [Google Scholar] [CrossRef] [PubMed]
- Birla, A.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresour. Technol. 2012, 106, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Z.; Xu, Y.; Liu, Q.; Qian, G. CaFeAl mixed oxide derived heterogeneous catalysts for transesterification of soybean oil to biodiesel. Bioresour. Technol. 2015, 190, 438–441. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.; Rajvanshi, S. Acid base catalyzed transesterification kinetics of waste cooking oil. Fuel Process. Technol. 2011, 92, 32–38. [Google Scholar] [CrossRef]
- Likozar, B.; Levec, J. Effect of process conditions on equilibrium, reaction kinetics and mass transfer for triglyceride transesterification to biodiesel: Experimental and modeling based on fatty acid composition. Fuel Process. Technol. 2014, 122, 30–41. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Comparative kinetics of transesterification for biodiesel production from palm oil and mustard oil. Can. J. Chem. Eng. 2012, 90, 342–350. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; He, S.; Shen, W.; Song, Y.; Liu, Y. Reaction Kinetics of Transesterification between Vegetable Oil and Methanol under Supercritical Conditions. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 30, 681–688. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Aziz, A.M.; Al-Tamer, M.H. Biodiesel production from Silybum marianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification. Energy Convers. Manag. 2016, 108, 255–265. [Google Scholar] [CrossRef]
- Berrios, M.; Martín, M.; Chica, A.F.; Martin, A. Study of esterification and transesterification in biodiesel production from used frying oils in a closed system. Chem. Eng. J. 2010, 160, 473–479. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Sánchez, N. An improvement to the transesterification process by the use of co-solvents to produce biodiesel. Fuel 2016, 166, 51–58. [Google Scholar] [CrossRef]
- Gurunathan, B.; Ravi, A. Process optimization and kinetics of biodiesel production from neem oil using copper doped zinc oxide heterogeneous nanocatalyst. Bioresour. Technol. 2015, 190, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Asri, N.P.; Machmudah, S.; Wahyudiono, W.; Suprapto, S.; Budikarjono, K.; Roesyadi, A.; Goto, M. Non catalytic transesterification of vegetables oil to biodiesel in sub-and supercritical methanol: A kinetic’s study. Bull. Chem. React. Eng. Catal. 2013, 7, 215–223. [Google Scholar] [CrossRef]
- Valle, P.; Velez, A.R.; Hegel, P.E.; Mabe, G.; Brignole, E. Biodiesel production using supercritical alcohols with a non-edible vegetable oil in a batch reactor. J. Supercrit. Fluids 2010, 54, 61–70. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Han, L.; Fan, T.; Wang, X. Study on Kinetics of Transesterification of Biodiesel in Zanthoxylum bungeanum seed Oil Ethyl Ester. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020. [Google Scholar]
- Kurhade, A.; Dalai, A.K. Kinetic modeling, mechanistic, and thermodynamic studies of HPW-MAS-9 catalysed transesterification reaction for biodiesel synthesis. Fuel Process. Technol. 2019, 196, 106164. [Google Scholar] [CrossRef]
- Booramurthy, V.K.; Ramesh, K.; Subramanian, D.; Pandian, S. Production of biodiesel from tannery waste using a stable and recyclable nano-catalyst: An optimization and kinetic study. Fuel 2020, 260, 116373. [Google Scholar] [CrossRef]
- Jamil, F.; Kumar, P.S.M.; Al-Haj, L.; Myint, M.T.Z.; Al-Muhtaseb, A.H. Heterogeneous carbon-based catalyst modified by alkaline earth metal oxides for biodiesel production: Parametric and kinetic study. Energy Convers. Manag. X 2020, 100047, in press. Available online: https://pubs.acs.org/doi/full/10.1021/ef700250g (accessed on 15 December 2020).
- Qadeer, M.U.; Ayoub, M.; Bilad, Z.M.R. Biodiesel production from waste cooking oil by using methyl acetate with sodium methylate as a catalyst. Solid State Technol. 2020, 63, 3847–3854. [Google Scholar]



| Classification | Type of Feedstock | C16H32O2 | C18H36O2 | C18H34O2 | C18H32O2 | C18H30O2 |
|---|---|---|---|---|---|---|
| 16:0 Saturated | 18:0 Saturated | 18:1 Mono Saturated | 18:2 Di Unsaturated | 18:3 Poly-Unsaturated | ||
| First-generation feedstocks | Soybean | 10.4–24.8 | 2.6–4.7 | 16.5–24.8 | 51.8–53.0 | 6.5–7.0 |
| Palm | 37.80–43.79 | 2.7–4.76 | 39.90–42.6 | 9.59–12.20 | 0.17–0.53 | |
| Olive | 9.7 | 1.74 | 82.3 | - | - | |
| Rapeseed | 3.49–4.0 | 0.55–2.3 | 62–77.8 | 1.8–8.23 | 1.8–8.23 | |
| sunflower | 10.58 | 4.76 | 22.52 | 8.19 | 8.19 | |
| Second-generation feedstocks | Tallow | 29.0 | 24.5 | 44.5 | - | - |
| Jatropha. C oil | 14.2 | 7.0 | 44.7 | 32.8 | - | |
| P. pinnata | 10.2 | 7.0 | 51.8 | 17.7 | 0.2 | |
| M. indica | 24.5 | 22.7 | 37.0 | 14.3 | 3.6 | |
| Neem oil | 13.8 | 18.2 | 52.6 | 13.6 | - | |
| Rubber seed oil | 9.1 | 5.6 | 24.0 | 46.2 | 14.2 | |
| Linseed oil | 5.61 | 4.04 | 19.34 | 17.15 | 48.79 | |
| Castor oil | 0.92 | 0.16 | 3.53 | 4.21 | 0.91 | |
| Mustard oil | 2.80 | 1.09 | 24.98 | 11.64 | 8.61 | |
| Third-generation feedstocks | Crude castor oil | 1.06 | 1.15 | 3.71 | 5.41 | 0.58 |
| WCO | 4.1–26.5 | 1.4–10.9 | 38.6–44.7 | 32.8–36.0 | 0.2 | |
| Chicken fat | 19.82 | - | 37.62 | - | 1.45 | |
| Yellow grease | 23.24 | - | 44.32 | 2.43 | 0.80 | |
| Waste frying oil | 6.90 | 2.35 | 61.58 | 20.01 | 4.74 | |
| Waste animal fat | 22.31 | 17.02 | 43.26 | 9.76 | 1.71 | |
| Crude neem oil | 18.1 | 18.1 | 44.5 | 18.3 | 0.2 |
| Feedstock | Oil Yield (kg Oil/ha) | Cultivation Cost (USD/ton) |
|---|---|---|
| Edible oil | ||
| Palm | 5000 | 950 |
| Soybean | 375 | 615 |
| Rapeseed | 1000 | 336 |
| Non-edible oil | ||
| Castor | 1188 | 160 |
| Rubber seed | 120 | N/A |
| Jatropha | 1590 | 620 |
| Technology | Advantage | Disadvantage |
|---|---|---|
| Mechanical extraction |
|
|
| Steam distillation |
|
|
| Solvent extraction |
|
|
| Enzymatic extraction |
|
|
| Supercritical fluids extraction |
|
|
| Microwave-assisted extraction |
|
|
| Ultrasound-assisted extraction |
|
|
| Transesterification | Advantages | Disadvantages |
|---|---|---|
| Homogeneous | ||
| Acid-catalyzed (HCl, H2SO4) | There is no soap formation, and it can catalyze the esterification and transesterification simultaneously. | Acid presence causes corrosion, high temperature and slower reaction rate. The acid catalysts have low catalytic activity. |
| Basic-catalyzed (KOH, NaOH) | Higher activity of catalysts and reaction rate, low cost, and easier availability. | Soap formation and difficult separation of product. |
| Heterogeneous | ||
| Acid-catalyzed | No formation of soap and catalyst recyclable. Esterification and transesterification can take place simultaneously. | High cost, low activity, and diffusional problems. |
| Basic-catalyzed (CaO, CaCO3, Al2O3) | Non-corrosive, recyclable catalysts for a long time and higher selectivity. | Higher cost, high energy requirement, sensitive to the presence of water, low diffusion, and hence lower yield of biodiesel. |
| Enzymatic-catalyzed | No side reactions taking place, easier separation, and environmentally feasible process. | Slow reaction rate, degradation and higher cost of enzymes used. |
| Supercritical | Very high reaction rate, no catalyst requirement, easier separation of products. | Higher operation costs due to reaction taking place at high temperature and pressure. |
| Feedstock | Catalyst | Temperature (°C) | Time (min) | Oil to Alcohol Ratio | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| Sunflower oil | Cs/Al/Fe3O4 | 58 | 120 | 14:1 | 94.8 | [69] |
| Sunflower oil | MgO/MgAl2O4 | 110 | 180 | 12:1 | 95.7 | [70] |
| Sunflower oil | CsH2PW12O40 /FeSiO2 | 60 | 240 | 12:1 | 81 | [71] |
| Soybean oil | Sr3Al2O6 | - | 61 | 25:1 | 96.2 | [72] |
| Soybean oil | ZrO2/C4H4O6HK | 60 | 120 | 16:1 | 98.03 | [73] |
| Palm oil | ZnO | 60 | 300 | 6:1 | 83.2 | [74] |
| Palm oil | TiO2-ZnO | 60 | 300 | 6:1 | 92.2 | [74] |
| Vegetable oil | Cs-Ca/SiO2-TiO2 | 60 | 120 | 12:1 | 98 | [75] |
| Olive oil | C. Antarctica | 60 | 1920 | 88:1 | 93 | [76] |
| Soybean oil | Rhizopus oryzae | 45 | 180 | 15:1 | 99 | [77] |
| Feedstock | Catalyst | Temperature (°C) | Time (min) | Oil to Alcohol Ratio | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| Mahua oil | KOH | - | - | 6:1 | 98 | [93] |
| Mahua oil | KOH | 60 | 30 | 4:1 | - | [94] |
| Karanja oil | NaOH | 50 | 70 | - | 84 | [95] |
| Jatropha oil | KOH | 55 | 60 | 9:1 | 90–95 | [96] |
| Jatropha oil | H2SO4 | 60 | 120 | 9:1 | 80 | [96] |
| Canola oil | KOH | 50 | 60 | 9:1 | 90–95 | [96] |
| Rubberseed oil | NaOH | - | - | 9:1 | 80 | [97] |
| Honne oil | KOH | 45–65 | 30–150 | 4:1 | 89 | [98] |
| Feedstock | Temperature (°C) | Main Outcomes | Reference |
|---|---|---|---|
| Palm oil | 50–65 | Temperature effect on biodiesel yield was negligible. Biodiesel yield varied between 70 to 90%. | [114] |
| Canola oil | 25–45 | A second-order reaction kinetics was observed in the range of 25–45 °C with an activation energy of 66 kJ/mol. | [115] |
| Palm oil | 70–110 | Initially, the reaction was slower. The increase of temperature increased the reaction rate and reached the equilibrium at 80 °C. The biodiesel yield was maximum at 80 °C. | [116] |
| Pongamia oil | 30–60 | Initially, a slower reaction rate was observed. The increase in temperature increased the triglycerides conversion at 45 °C and 55 °C. The highest biodiesel yield of 98.8% was obtained at 55 °C. | [117] |
| Sunflower oil | 60–120 | The biodiesel yield was maximum at 80 °C. | |
| Linseed oil | 40–60 | The biodiesel yield remained between 88–96%, which raised due to increase in temperature. | [118] |
| Feedstock | Molar Ratio | Time (min) | Catalyst Loading | Temperature (°C) | Agitation Speed (rpm) | Type of Transesterification | Yield (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Palm oil | 6:1 (methanol) | 60 | 1% KOH | 60 | 600 | Homogeneous base | 88 | [139] |
| 6:1 | 60 | 1% NaOH | 60 | 600 | 93 | [139] | ||
| 9:1 | 480 | 8.5% KOH | 65–75 | - | 96.2 | [140] | ||
| 10:1 | - | 0.4% KOH | 70–110 | - | 98 | [116] | ||
| Jatropha oil Waste frying oil | 10:1 | 480 | 9% KOH | 60–80 | - | Homogeneous base | 96.8 | [78] |
| 4.83:1 to 9.65:1 | 300–480 | 1–4% | 50–65 | - | 87.3 | [141] | ||
| Soybean oil | 12:1 | 60 | 6% CaFeAl | 60 | 270 | Heterogeneous transesterification | 90 | [142] |
| Jatropha oil | 3:7 | 180 | 1% H2SO4 | 65 | 400 | Homogeneous acid and base | 21.2 | [129] |
| 3:7 | 1% NaOH | 50 | 400 | 90.2 | ||||
| Waste cooking oil | 3:7 | 180 | 1% H2SO4 | 65 | 400 | Homogeneous acid and base | 21.2 | [143] |
| 3:7 | 180 | 1% NaOH | 50 | 400 | 90.6 | |||
| Canola oil | 3:1 to 8:1 | 25–75 | 0.2–1.2% KOH | 30–70 | 100–600 | Homogeneous base | - | [144] |
| Mustard oil | - | 30 | KOH | 40–60 | 450 | Homogeneous base | - | [145] |
| Sunflower oil | 6:1 | 90–330 | 1% CaO | 23–60 | - | Heterogeneous | 91 | [60] |
| 6:1 | ||||||||
| 12:1 | - | 0.06–0.34 | 23–60 | 400 | Homogeneous base | 99 | [62] | |
| 24:1 | ||||||||
| Peanut oil | 30:1 | 30–360 | - | 250–310 | 500 | Supercritical transesterification | >90 | [146] |
| Waste lard | 6:1 | 20 | 4–6 wt.% enzyme | 50 | - | Ultrasound-assisted transesterification | 96.8 | [108] |
| Silybum Marianum seed oil | 6:1 | 75 | 4–6% sulfonated solid acid catalyst | 60 | 600 | Carbon acid esterification and homogeneous base transesterification | 96.9 | [147] |
| Canola oil | 6:1 | - | 0.5% KOH | 45 | - | Homogeneous base | 95 | [115] |
| Used frying oil | 6.03:1 | 120 | 0.55% KOH | 60–100 | - | Homogeneous base | - | [148] |
| Rapeseed oil | 3.5:1 to 42:1 | 120 | - | 200–500 | - | Supercritical transesterification | 95 | [149] |
| Neem oil | 10:1 | 60 | 10% CZO | 55 | - | Heterogeneous transesterification | 97.1 | [150] |
| Parameter | Effect on Transesterification |
|---|---|
| Temperature | The temperature increase also enhances the rate of reaction and shortens the time for the reaction completion by decreasing the viscosity. The temperature of 50 °C is recommended for conventional transesterification. |
| Time | The increase in reaction time increases the fatty acid alkyl esters concentration in the product up to optimum value. The reaction time during conventional transesterification is kept between 30 to 60 min. |
| Alcohol/oil molar ratio | The increase of the molar ratio of alcohol favors the forward reaction by avoiding the reverse reaction. |
| Catalyst amount and type | The most frequently used catalysts in biodiesel synthesis are sodium hydroxide (NaOH) and potassium hydroxide (KOH). The amount of fatty acid methyl esters increases by increasing the amount of catalyst. |
| Agitation speed | Mixing intensity is an important parameter that helps to complete the reaction resulting in the formation of product (methyl esters or biodiesel yield). The moderate stirring speed of 400 rpm is recommended to catalyze the biodiesel production reaction. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulqarnain; Ayoub, M.; Yusoff, M.H.M.; Nazir, M.H.; Zahid, I.; Ameen, M.; Sher, F.; Floresyona, D.; Budi Nursanto, E. A Comprehensive Review on Oil Extraction and Biodiesel Production Technologies. Sustainability 2021, 13, 788. https://doi.org/10.3390/su13020788
Zulqarnain, Ayoub M, Yusoff MHM, Nazir MH, Zahid I, Ameen M, Sher F, Floresyona D, Budi Nursanto E. A Comprehensive Review on Oil Extraction and Biodiesel Production Technologies. Sustainability. 2021; 13(2):788. https://doi.org/10.3390/su13020788
Chicago/Turabian StyleZulqarnain, Muhammad Ayoub, Mohd Hizami Mohd Yusoff, Muhammad Hamza Nazir, Imtisal Zahid, Mariam Ameen, Farooq Sher, Dita Floresyona, and Eduardus Budi Nursanto. 2021. "A Comprehensive Review on Oil Extraction and Biodiesel Production Technologies" Sustainability 13, no. 2: 788. https://doi.org/10.3390/su13020788
APA StyleZulqarnain, Ayoub, M., Yusoff, M. H. M., Nazir, M. H., Zahid, I., Ameen, M., Sher, F., Floresyona, D., & Budi Nursanto, E. (2021). A Comprehensive Review on Oil Extraction and Biodiesel Production Technologies. Sustainability, 13(2), 788. https://doi.org/10.3390/su13020788










