Abstract
The high life expectancy of the world population provokes increase in demand for food and energy. As a result, the intense industrialization and the application of fossil sources is responsible for high levels of CO2 emission and waste generation. To mitigate the CO2 emission a practical solution at the very short term is urgently needed. The capture of CO2 and its application in chemical processes for the valorization of residual biomass are of great importance nowadays. The application of CO2 in the selective carboxylation of furoic acid for the production of 2,5-furandicarboxylic acid (FDCA), a bio-based monomer, has been an important step towards obtaining biopolymers to replace petroleum-based plastics such as polyethylene terephthalate (PET). In this project report, we discuss on the current challenges for obtaining the 2,5-FDCA precursor from the furfural in two main routes involving oxidation and carboxylation via heterogeneous catalysis. We present the main objectives and discuss the importance of this research for the development of more sustainable processes.
1. Introduction
Nowadays, looking for alternative renewable bio-resources is urgent and important in order to replace non-bio and non-renewable ones conventionally employed in different industrial areas. The valorization of biomass, currently experiencing a great blooming, constitutes a considerable pathway among the available and proposed solutions [1,2,3]. Moreover, the intensive exploitation of fossil sources is one of the major causes of the CO2 emissions, a hazardous greenhouse gas and a major cause of the global warming that is dangerously threatening our existence [4]. In the European Union alone, more than 75% of the greenhouse gas emissions come from the production and consumption of fossil fuels. In order to reduce the amount of CO2 released, some processes have been suggested to capture the gas and use it for several chemical reactions [4]. The carboxylation of chemical compounds with CO2 is one of these reactions that can be applied in the valorization of biomass. Part of biomass valorization consists of producing bioplastics from sugars such as xylose or glucose, which are abundantly present in agricultural productions such as wheat or beet, in agricultural residues or even in sawdust. Indeed, scientific research is increasingly concerned with the production of bioplastics, which represent about 1% of the 368 million tons of plastic produced each year in the world. Thus, the demand for the production of biopolymers for bioplastics, more resistant and even more biodegradable ones, is constantly increasing and diversifying (Figure 1). Indeed, the production of bioplastics, especially which are biodegradable, is expected to increase by 50% in the next 25 years.
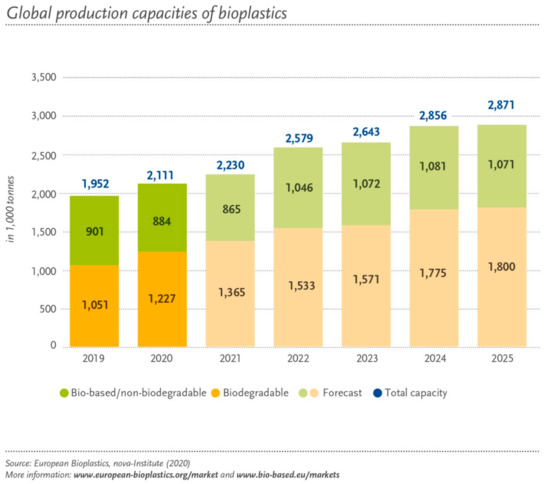
Figure 1.
Global bioplastic production capacity. Reprinted with permission from ref. [5].
It is worth noting that plastic surrounds us wherever we go, whatever we use, and it can be roughly said that currently plastic constitutes an entire portion of our daily life. However, at the same time its excessive consumption and environmental risks and impacts posed by it are very strongly linked. The progressive replacement of plastic with bioplastic allows us to fight against pollution caused by carbon dioxide.
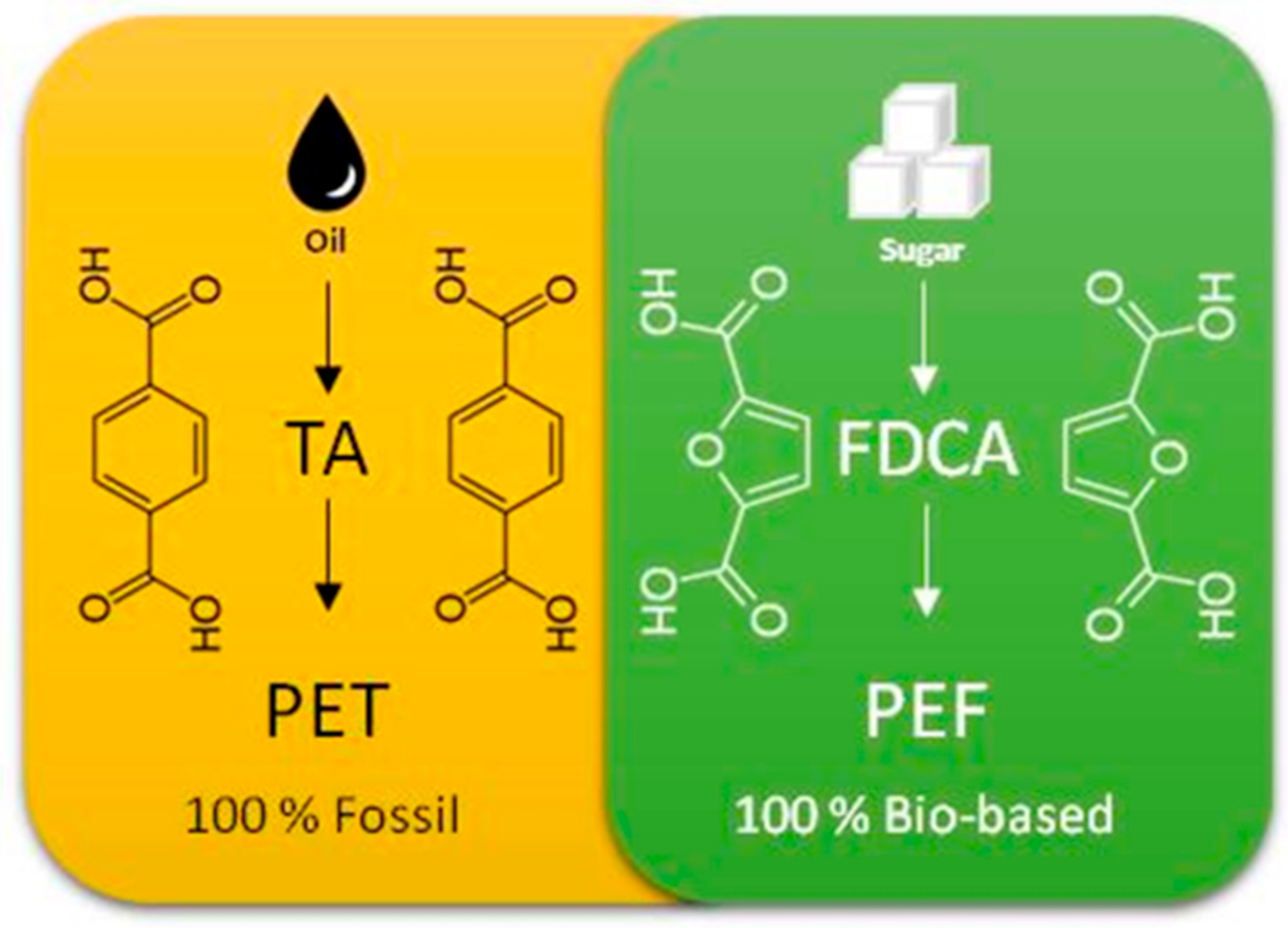
One of the most important steps in the manufacturing process of these bioplastics is achieved using CO2. This step consists of the selective production of 2,5-furandicarboxylic acid (2,5-FDCA) from a bio-sourced compound, furoic acid. 2,5-FDCA is one of the most important components for the production of biopolymers, such as polyethylene furoate (PEF), which is a natural replacement for polyethylene terephthalate (PET) derived from terephthalic acid (TA), an unsustainable molecule derived from petrochemistry (Figure 2). PEF is used for the manufacture of rigid and flexible packaging. Moreover, this biopolymer is totally recyclable and biodegradable. The properties of this biopolymer are also very interesting, offering the possibility of obtaining highly stable materials, while improving the barrier protection against oxygen and CO2 of the packaged products. In addition to packaging, other applications could be possible, including in textiles.
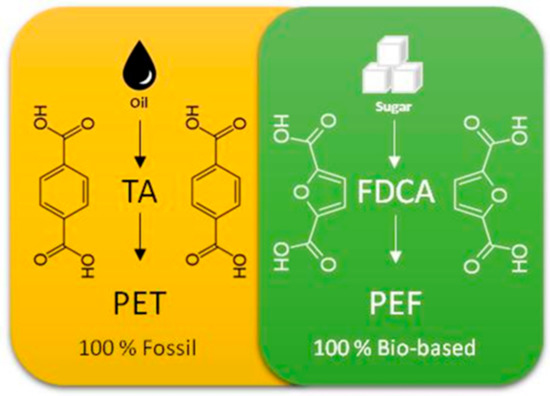
Figure 2.
Diagram of the manufacturing process of PET (100% fossil) and PEF (100% bio-based).
2. Project Objectives
Currently, two main routes are being studied by scientists for the synthesis of the biopolymer, 2,5-FDCA. The first route consists of the oxidation of 5-hydroxymethylfurfural (HMF) with oxygen using heterogeneous or chemical catalysts. This route allows attainment of the best catalytic performances, but some problems concerning the purity of 2,5-FDCA are encountered due to the formation of unstable intermediates. Moreover, the HMF generally obtained from fructose must also be of very high purity [6]. Recently, studies have shown that the dehydration and then the oxidation processes for obtaining FDCA are not always very selective, which creates more stable by-products. According to several reports, the current cost of FDCA is on average USD 2300/kg [6]. However, to obtain economically viable production, the FDCA price must be USD 1000/ton. At the present, FDCA is thus not commercialized in industrial volumes, its cost price being too high. Production only is carried out in laboratories of various sizes for sales adapted to each customer and is used to carry out scientific tests [6].
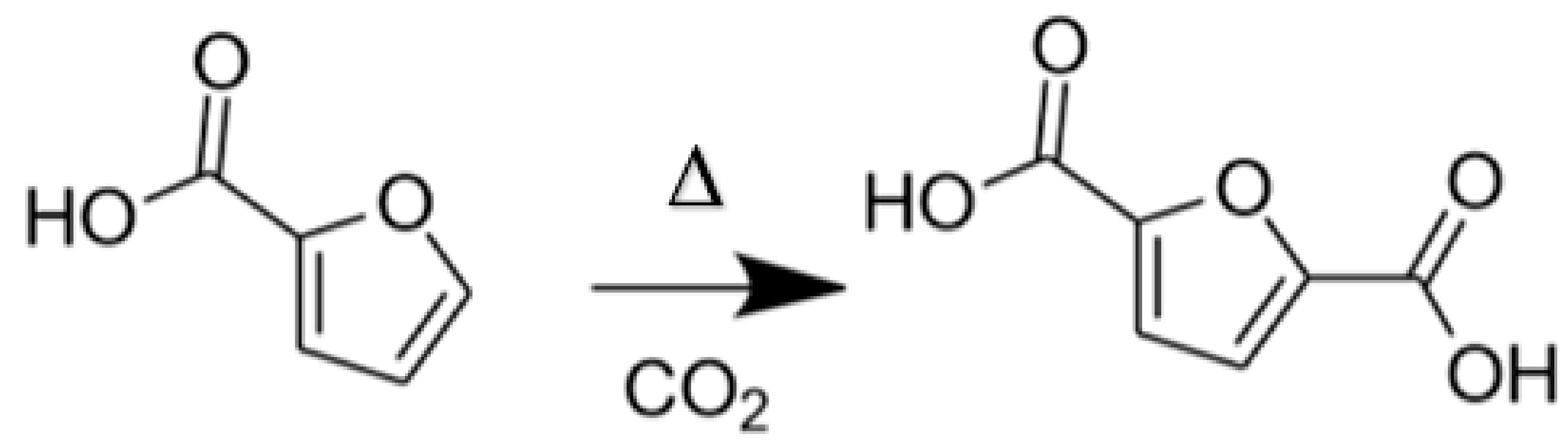
The second method consists of using furfural, which is industrially produced from non-edible bio-sourced resources (agricultural residues, wood...). Furfural undergoes an oxidation step to obtain furoic acid. Then, the carboxylation of furoic acid with CO2 allows the formation of 2,5-FDCA (Figure 3). This reaction pathway was found to be more selective than the one with HMF and therefore yields the desired product with higher purity.
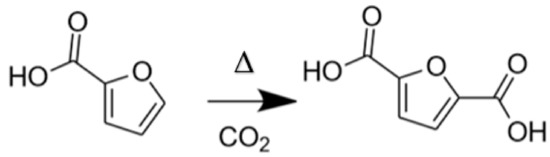
Figure 3.
Reaction scheme for the CO2 carboxylation of furoic acid to 2,5-furandicarboxylic acid (2,5-FDCA). Conditions used: substrate/M = 9, FCO2 = 45 mL min−1, 20 rpm, T = 200 °C, t = 20 h, STYFDCA = 1260 µmol kg−1 h−1).
However, the main problem in the synthesis of 2,5-FDCA from furoic acid is the insertion of carboxylate groups at the C-H bonds of the hydrocarbons [7]. As a C1 feedstock, CO2 has thermodynamic and kinetic limitations [8]. Indeed, in the esterification of aromatic hydrocarbons with CO2, low equilibrium conversion is obtained at several temperatures [8]. Therefore, several solutions have been investigated to, aiming achieve the direct carboxylation of C-H, such as the use of a base, developed by Kolbe and Schmitt [9,10,11], a Lewis acid [12], transition metal catalysts [13], and enzymes [14] as reagents. In terms of mechanism, these reagents could influence the mode of C-H cleavage, which could be an electrophilic aromatic substitution, C-H deprotonation by a base, or C- H oxidation and subsequent insertion. This reaction can take place under both basic and acidic conditions. Under basic conditions, the use of a strong base deprotonates the C-H group with the most acidic proton, which is at position 5 in the FA [15] to form a strong nucleophilic carbon atom that could react with the weakly electrophilic CO2. In an acidic environment, CO2 is activated by coordination with a Lewis acid, resulting in a reaction between the reactant and the activated CO2 [16].
Generally, this reaction is carried out using homogeneous catalysts (Henkel reaction [17,18,19,20]) which are very complicated to separate from the obtained products. This is the same for melted salt [19]. This reaction involves the thermal rearrangement or dismutation of alkali salts derived from aromatic acids into unsubstituted and symmetrical aromatic diacids known in particular for the production of TA. The selectivity in desired products cannot exceed 50%.
One of the main objectives of this project is to design heterogeneous catalysts easy to separate from the reaction medium with simple filtration. For this purpose, researchers were first interested in designing heterogeneous catalysts to activate CO2 at relatively mild conditions of temperature and pressure, known to be weakly active, in order to perform the direct carboxylation of furoic acid.
Silver nanoparticle catalysts have been shown to interact with CO2, giving the possibility of carboxylation of furoic acid to 2,5-FDCA [21]. This reaction can occur at relatively low temperatures but requires high CO2 pressure in a closed reactor. Under these conditions, the catalysts were able to obtain interesting yields of 2,5-FDCA suggesting an avenue of research for the replacement of the use of homogeneous catalysts on a laboratory scale. In addition, the use of undesirable reagents such as sodium hydroxide, a strong base, was eliminated. These results suggest the feasibility of this process at laboratory scale. However, the catalytic performances remain to be improved upon by working for example on the type of used catalyst. In order to apply a such process on an industrial scale, it will be necessary to improve the yield of 2,5-FDCA.
3. Methodology
Currently, one of the most important bio-sourced platform molecules is 2,5-furandicarboxylic acid (FDCA) due to its potential application as a building block for green polymers, which could replace petro-sourced polyethylene terephthalate (PET). Our team developed a very efficient, innovative and green process to oxidize furfural (FUR) to furoic acid (FA) with a 95% yield [22]. Recent work from our group has also shown that FA could undergo a C-H carboxylation with CO2 to form FDCA [21]. We have combined our respective processes to gain access to FDCA from FUR via FA using only O2 and CO2 as the main reactants. At this stage, we have demonstrated the feasibility of this very exciting concept. This is very encouraging and places us at the cutting-edge of innovation in the area of FDCA production. In this context, the main objectives of the project are: (i) to study the catalytic process of furoic acid transformation to FDCA via Henkel reaction (TRL 3); (ii) to design new heterogeneous catalysts to obtain a minimum yield of 90% in Henkel reaction and (iii) to assess the validity and acceptance of the technical solutions retained from the environmental, economic and societal points of view. FDCA synthesis was performed in a Glass Oven B-585 Kugelrohr (Büchi) at 200 °C, under a continuous flow of CO2 (45 mL min−1), over 20 h.
At the end of this project, we should be able to conclude on the possibility of transferring our academic research results to the socio-economic world by patenting and/or licensing our whole process to an industrial partner and, hence, on the necessity to invest in a more ambitious maturation program.
4. Conclusions
The valorization of lignocellulosic biomass and CO2 capture have been very important processes for the reduction of the greenhouse effect and the implementation of more sustainable processes. 2,5-FDCA is a promising bio-based building block used to produce bioplastics and other biopolymers in order to replace petroleum-based molecules. Recent work from our group has also shown that FA could undergo a direct C-H carboxylation with CO2 to form FDCA using only O2 (first step oxidation of furfural) and CO2 as the main reactants, where high yield in FDCA can obtained from a raw FA (un-published data) but using molten salts instead of heterogeneous catalyst. These data, as well as the several publications of our group are very encouraging and place us at the cutting-edge of innovation in the area of FDCA production.
Author Contributions
Conceptualization, D.F. and Y.S.; methodology, D.F. and R.W.; formal analysis, D.F. and Y.S.; investigation, D.F. and Y.S.; writing—original draft preparation, D.F., Y.S., I.I.J. and R.W.; writing—review and editing, I.I.J. and R.W.; supervision, R.W.; project administration, R.W.; funding acquisition, R.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by REGION HAUTS-de-FRANCE, grant number 3859523 FDCA STARTAIRR project and I-SITE ULNE grant V-Start’AIRR-18-001-Wojcieszak. This study was supported by the French government through the Programme Investissement d’Avenir (I-SITE ULNE/ANR-16- IDEX-0004 ULNE) managed by the Agence Nationale de la Recherche.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supporting data discussed in this Project Report can be found on https://www.mdpi.com/2073-4344/11/3/326 (accessed on 10 September 2021). Raw data could be obtained on demand.
Acknowledgments
The Métropole Européen de Lille (MEL) is also acknowledged for the “CatBioInnov”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itabaiana, I., Jr.; Avelar do Nascimento, M.; de Souza, R.O.M.A.; Dufour, A.; Wojcieszak, R. Levoglucosan: A Promising Platform Molecule? Green Chem. 2020, 22, 5859–5880. [Google Scholar] [CrossRef]
- Dumeignil, F.; Capron, M.; Katryniok, B.; Wojcieszak, R.; Löfberg, A.; Girardon, J.-S.; Desset, S.; Araque-Marin, M.; Jalowiecki-Duhamel, L.; Paul, S. Biomass-Derived Platform Molecules Upgrading through Catalytic Processes: Yielding Chemicals and Fuels. J. Jpn. Petrol. Inst. 2015, 58, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Jaén, S.; Virginie, M.; Bonin, J.; Robert, M.; Wojcieszak, R.; Khodakov, A.Y. Highlights and Challenges in the Selective Reduction of Carbon Dioxide to Methanol. Nat. Rev. Chem. 2021, 5, 564–579. [Google Scholar] [CrossRef]
- European-Bioplastics. Available online: https://www.european-bioplastics.org/market/ (accessed on 9 September 2021).
- Wojcieszak, R.; Itabaiana, I., Jr. Engineering the future: Perspectives in the 2,5-furandicarboxylic acid synthesis. Catal. Today 2020, 354, 211–217. [Google Scholar] [CrossRef]
- Luo, J.; Larrosa, I. C−H Carboxylation of Aromatic Compounds through CO2 Fixation. ChemSusChem 2017, 10, 3317–3332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabestani, R.; Britt, P.F.; Buchanan, A.C. Pyrolysis of Aromatic Carboxylic Acid Salts: Does Decarboxylation Play a Role in Cross-Linking Reactions? Energy Fuels 2005, 19, 365–373. [Google Scholar] [CrossRef]
- Lindsey, A.S.; Jeskey, H. The Kolbe-Schmitt Reaction. Chem. Rev. 1957, 57, 583–620. [Google Scholar] [CrossRef]
- Kolbe, H.; Lautemann, E. Constitution of Salicylic Acid and Its Bascity. Liebigs Ann. Chem. 1860, 157–206. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, R. Beitrag Zur Kenntniss Der Kolbe’schen Salicylsäure Synthese. J. Prakt. Chem. 1885, 1, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Olah, G.A.; Török, B.; Joschek, J.P.; Bucsi, I.; Esteves, P.M.; Rasul, G.; Surya Prakash, G.K. Efficient Chemoselective Carboxylation of Aromatics to Arylcarboxylic Acids with a Superelectrophilically Activated Carbon Dioxide−Al2 Cl6/Al System. J. Am. Chem. Soc. 2002, 124, 11379–11391. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.M.; Rovis, T. C–H Carboxylation Takes Gold. Nat. Chem. 2010, 2, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Wuensch, C.; Glueck, S.M.; Gross, J.; Koszelewski, D.; Schober, M.; Faber, K. Regioselective Enzymatic Carboxylation of Phenols and Hydroxystyrene Derivatives. Org. Lett. 2012, 14, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Dick, G.R.; Yoshino, T.; Kanan, M.W. Carbon Dioxide Utilization via Carbonate-Promoted C–H Carboxylation. Nature 2016, 531, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Watanabe, K.; Tanaka, Y.; Hattori, T. Cl2/2,6-Disubstituted Pyridine-Mediated Carboxylation of Alkenes with Carbon Dioxide. Org. Lett. 2016, 18, 2576–2579. [Google Scholar] [CrossRef] [PubMed]
- Drault, F.; Snoussi, Y.; Paul, S.; Itabaiana, I.; Wojcieszak, R. Recent Advances in Carboxylation of Furoic Acid into 2,5-Furandicarboxylic Acid: Pathways towards Bio-Based Polymers. ChemSusChem. 2020, 13, 5164–5172. [Google Scholar] [CrossRef] [PubMed]
- McNelis, E. Reactions of Aromatic Carboxylates. II. 1 The Henkel Reaction. J. Org. Chem. 1965, 30, 1209–1213. [Google Scholar] [CrossRef]
- Xiao, D.J.; Chant, E.D.; Frankhouser, A.D.; Chen, Y.; Yau, A.; Washton, N.M.; Kanan, M.W. A Closed Cycle for Esterifying Aromatic Hydrocarbons with CO2 and Alcohol. Nat. Chem. 2019, 11, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.R.; Frankhouser, A.D.; Banerjee, A.; Kanan, M.W. A Scalable Carboxylation Route to Furan-2,5-Dicarboxylic Acid. Green Chem. 2017, 19, 2966–2972. [Google Scholar] [CrossRef]
- Drault, F.; Snoussi, Y.; Thuriot-Roukos, J.; Itabaiana, I.; Paul, S.; Wojcieszak, R. Study of the Direct CO2 Carboxylation Reaction on Supported Metal Nanoparticles. Catalysts 2021, 11, 326. [Google Scholar] [CrossRef]
- Santarelli, F.; Wojcieszak, R.; Paul, S.; Dumeignil, F.; Cavani, F. Furoic Acid Preparation Method. World Patent WO2017158106A1, 21 September 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).