The Fallow Period Plays an Important Role in Annual CH4 Emission in a Rice Paddy in Southern Brazil
Abstract
:1. Introduction
2. Materials and Methods
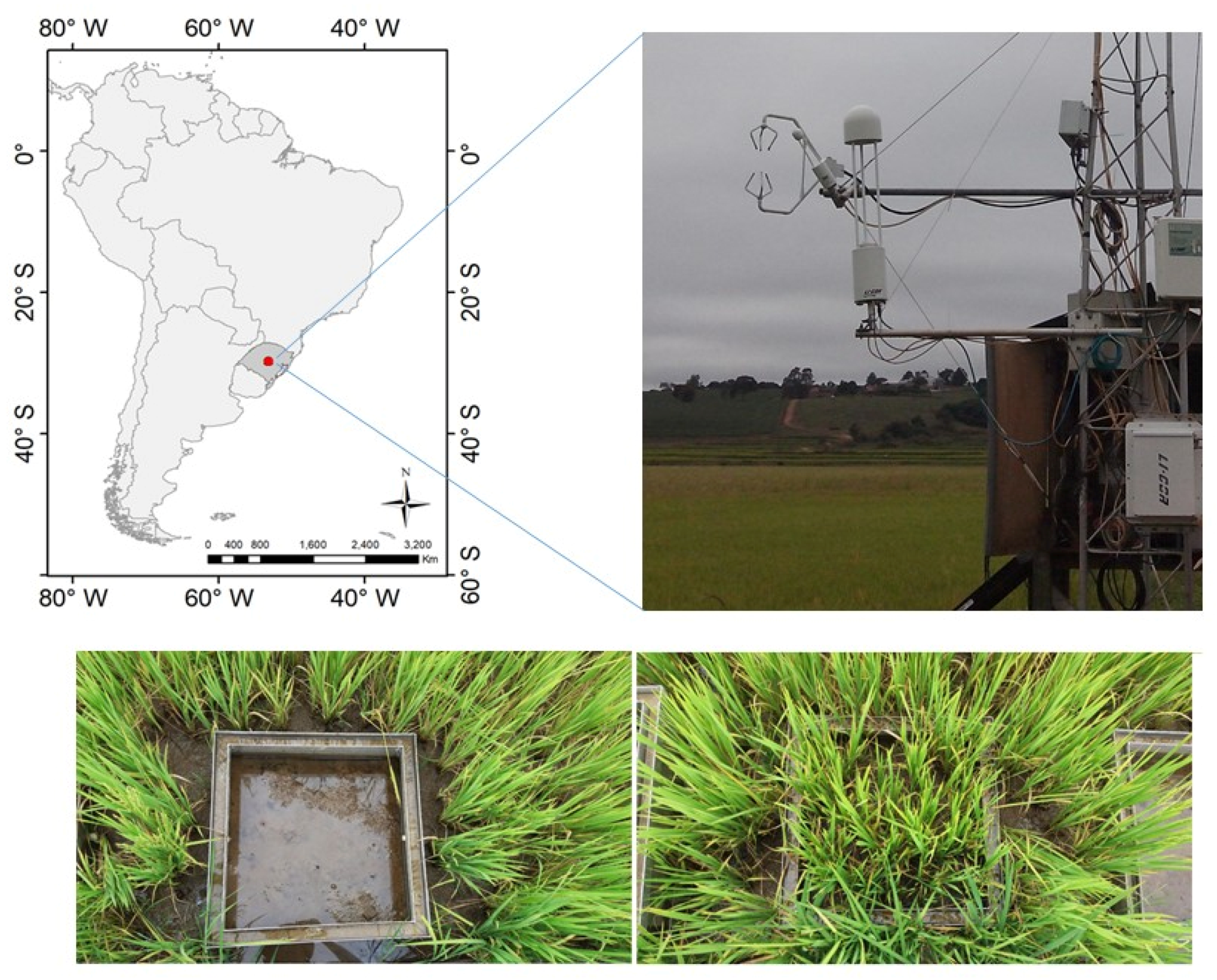
2.1. Site Description
2.2. Data Collection
2.2.1. Soil Chamber (SC)
2.2.2. Eddy Covariance and Meteorological Measurements
2.3. Eddy Covariance Flux Data Processing
2.4. Gap-Filling and Uncertainty
2.5. Data Analysis
- —
- Vegetative stage: from 21 November 2015, (16 DAP) in the vegetative stage (V4) to 9 January 2016, (62 DAP) in V13;
- —
- Reproductive stage: from 10 January 2016, (63 DAP) in the reproductive stage (R0) to 19 March 2016, (131 DAP) in R9, i.e., complete maturation stage;
- —
- Pre-harvest stage: from 20 March 2016, (132 DAP) to 19 April 2016, (162 DAP);
- —
- No rice stage: 19 April 2016, to 1 August 2016;
- —
- Land preparation stage: the period of land preparation is when the land is plowed, from 2 August 2016, to 18 August 2016.
3. Results and Discussion
3.1. Atmospheric and Soil Conditions
3.2. Correlation between the Meteorological Conditions and CH4 Fluxes Not Gap-Filled
3.3. CH4 Flux Gap-Filling
3.4. Seasonal and Diurnal CH4 Fluxes
3.4.1. EC
3.4.2. SC
3.4.3. Annual Integrated Flux
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Intergovernamental Panel on Climate Change: Working Group I Contribution to the Fifth Assessment Report of the Intergovernamental Panel on Climate Change; Stocker, T., Qin, D., Plattner, G., Tignor, M., Allen, S., Boschung, J., Nauels, A., Xia, Y., Vincent Bex, P.M., Eds.; Cambridge University Press: New York, NY, USA, 2013; ISBN 9781107661820. [Google Scholar]
- Wuebbles, D.J.; Hayhoe, K. Atmospheric methane and global change. Earth-Sci. Rev. 2002, 57, 177–210. [Google Scholar] [CrossRef]
- Lelieveld, J. Climate change: A nasty surprise in the greenhouse. Nature 2006, 443, 405–406. [Google Scholar] [CrossRef]
- Baldocchi, D.; Detto, M.; Sonnentag, O.; Verfaillie, J.; Teh, Y.A.; Silver, W.; Kelly, N.M. The challenges of measuring methane fluxes and concentrations over a peatland pasture. Agric. For. Meteorol. 2012, 153, 177–187. [Google Scholar] [CrossRef]
- Ul-Haq, Z.; Tariq, S.; Ali, M. Atmospheric variability of methane over Pakistan, Afghanistan and adjoining areas using retrievals from SCIAMACHY/ENVISAT. J. Atmos. Solar-Terr. Phys. 2015, 135, 161–173. [Google Scholar] [CrossRef]
- Savi, F.; Di Bene, C.; Canfora, L.; Mondini, C.; Fares, S. Environmental and biological controls on CH4 exchange over an evergreen Mediterranean forest. Agric. For. Meteorol. 2016, 226–227, 67–79. [Google Scholar] [CrossRef]
- Conrad, R. Microbial Ecology of Methanogens and Methanotrophs. Adv. Agron. 2007, 96, 1–63. [Google Scholar]
- Malyan, S.K.; Bhatia, A.; Kumar, A.; Gupta, D.K.; Singh, R.; Kumar, S.S.; Tomer, R.; Kumar, O.; Jain, N. Methane production, oxidation and mitigation: A mechanistic understanding and comprehensive evaluation of influencing factors. Sci. Total Environ. 2016, 572, 874–896. [Google Scholar] [CrossRef] [PubMed]
- Conab Companhia Nacional de Abastecimento. Available online: https://www.conab.gov.br/ (accessed on 10 April 2021).
- USDA (United States Department of Agriculture). Foreign Agricultural Service. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 9 September 2021).
- IRGA Instituto Rio Grandense do Arroz. Available online: https://irga.rs.gov.br/inicial (accessed on 10 April 2021).
- Alberto, M.C.R.; Wassmann, R.; Hirano, T.; Miyata, A.; Hatano, R.; Kumar, A.; Padre, A.; Amante, M. Comparisons of energy balance and evapotranspiration between flooded and aerobic rice fields in the Philippines. Agric. Water Manag. 2011, 98, 1417–1430. [Google Scholar] [CrossRef]
- Hossen, M.S.; Mano, M.; Miyata, A.; Baten, M.A.; Hiyama, T. Surface energy partitioning and evapotranspiration over a double-cropping paddy field in Bangladesh. Hydrol. Process. 2012, 26, 1311–1320. [Google Scholar] [CrossRef]
- da Silva, L.S.; Griebeler, G.; Moterle, D.F.; Bayer, C.; Zschornack, T.; Pocojeski, E. Dinâmica da emissão de metano em solos sob cultivo de arroz irrigado no sul do Brasil. Rev. Bras. Cienc. Solo 2011, 35, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Bayer, C.; Costa, F.D.S.; Pedroso, G.M.; Zschornack, T.; Camargo, E.S.; de Lima, M.A.; Frigheto, R.T.S.; Gomes, J.; Marcolin, E.; Macedo, V.R.M. Yield-scaled greenhouse gas emissions from flood irrigated rice under long-term conventional tillage and no-till systems in a Humid Subtropical climate. Field Crops Res. 2014, 162, 60–69. [Google Scholar] [CrossRef]
- Bayer, C.; Zschornack, T.; Pedroso, G.M.; da Rosa, C.M.; Camargo, E.S.; Boeni, M.; Marcolin, E.; dos Reis, C.E.S.; dos Santos, D.C. A seven-year study on the effects of fall soil tillage on yield-scaled greenhouse gas emission from flood irrigated rice in a humid subtropical climate. Soil Tillage Res. 2015, 145, 118–125. [Google Scholar] [CrossRef]
- Camargo, E.S.; Pedroso, G.M.; Minamikawa, K.; Shiratori, Y.; Bayer, C. Intercontinental comparison of greenhouse gas emissions from irrigated rice fields under feasible water management practices: Brazil and Japan. Soil Sci. Plant Nutr. 2018, 64, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Chirinda, N.; Arenas, L.; Katto, M.; Loaiza, S.; Correa, F.; Isthitani, M.; Loboguerrero, A.; Martínez-Barón, D.; Graterol, E.; Jaramillo, S.; et al. Sustainable and Low Greenhouse Gas Emitting Rice Production in Latin America and the Caribbean: A Review on the Transition from Ideality to Reality. Sustainability 2018, 10, 671. [Google Scholar] [CrossRef] [Green Version]
- Reba, M.L.; Fong, B.N.; Rijal, I. Fallow season CO2 and CH4 fluxes from US mid-south rice-waterfowl habitats. Agric. For. Meteorol. 2019, 279, 107709. [Google Scholar] [CrossRef]
- Zschornack, T.; da Rosa, C.M.; dos Reis, C.E.S.; Pedroso, G.M.; Camargo, E.S.; Dossantos, D.C.; Boeni, M.; Bayer, C. Soil CH4 and N2O emissions from rice paddy fields in southern Brazil as affected by crop management levels: A three-year field study. Rev. Bras. Cienc. Solo 2018, 42. [Google Scholar] [CrossRef] [Green Version]
- Alberto, M.C.R.; Wassmann, R.; Buresh, R.J.; Quilty, J.R.; Correa, T.Q.; Sandro, J.M.; Centeno, C.A.R. Measuring methane flux from irrigated rice fields by eddy covariance method using open-path gas analyzer. Field Crops Res. 2014, 160, 12–21. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Tomer, R.; Harit, R.C.; Jain, N.; Bhowmik, A.; Kaushik, R. Mitigation of yield-scaled greenhouse gas emissions from irrigated rice through Azolla, Blue-green algae, and plant growth–promoting bacteria. Environ. Sci. Pollut. Res. 2021, 28, 51425–51439. [Google Scholar] [CrossRef] [PubMed]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Kumar, S.S.; Fagodiya, R.K.; Pugazhendhi, A.; Duc, P.A. Mitigation of greenhouse gas intensity by supplementing with Azolla and moderating the dose of nitrogen fertilizer. Biocatal. Agric. Biotechnol. 2019, 20, 101266. [Google Scholar] [CrossRef]
- Morin, T.H. Advances in the Eddy Covariance Approach to CH 4 Monitoring Over Two and a Half Decades. J. Geophys. Res. Biogeosci. 2019, 124, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Denmead, O.T. Approaches to measuring fluxes of methane and nitrous oxide between landscapes and the atmosphere. Plant Soil 2008, 309, 5–24. [Google Scholar] [CrossRef]
- Hendriks, D.M.D.; van Huissteden, J.; Dolman, A.J. Multi-technique assessment of spatial and temporal variability of methane fluxes in a peat meadow. Agric. For. Meteorol. 2010, 150, 757–774. [Google Scholar] [CrossRef]
- McDermitt, D.; Burba, G.; Xu, L.; Anderson, T.; Komissarov, A.; Riensche, B.; Schedlbauer, J.; Starr, G.; Zona, D.; Oechel, W.; et al. A new low-power, open-path instrument for measuring methane flux by eddy covariance. Appl. Phys. B Lasers Opt. 2011, 102, 391–405. [Google Scholar] [CrossRef]
- Peltola, O.; Hensen, A.; Belelli Marchesini, L.; Helfter, C.; Bosveld, F.C.; van den Bulk, W.C.M.; Haapanala, S.; van Huissteden, J.; Laurila, T.; Lindroth, A.; et al. Studying the spatial variability of methane flux with five eddy covariance towers of varying height. Agric. For. Meteorol. 2015, 214–215, 456–472. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Hicks, B.B.; Meyers, T.P. Measuring biosphere-atmosphere exchanges of biologically related gases with micrometeorological methods. Ecology 1988, 69, 1331–1340. [Google Scholar] [CrossRef]
- Baldocchi, D.D. How eddy covariance flux measurements have contributed to our understanding of Global Change Biology. Glob. Chang. Biol. 2020, 26, 242–260. [Google Scholar] [CrossRef]
- Schrier-Uijl, A.P.; Kroon, P.S.; Hensen, A.; Leffelaar, P.A.; Berendse, F.; Veenendaal, E.M. Comparison of chamber and eddy covariance-based CO2 and CH4 emission estimates in a heterogeneous grass ecosystem on peat. Agric. For. Meteorol. 2010, 150, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Meijide, A.; Manca, G.; Goded, I.; Magliulo, V.; Di Tommasi, P.; Seufert, G.; Cescatti, A. Seasonal trends and environmental controls of methane emissions in a rice paddy field in Northern Italy. Biogeosciences 2011, 8, 3809–3821. [Google Scholar] [CrossRef] [Green Version]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.B.; Roberti, D.R.; Carneiro, J.V.; Souza, V.D.A.; de Moraes, O.L.L. Dynamics of the superficial fluxes over a flooded rice paddy in southern Brazil. Agric. For. Meteorol. 2019, 276–277, 107650. [Google Scholar] [CrossRef]
- EMBRAPA. Adubação e Calagem para o Arroz Irrigado no Rio Grande do Sul. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/745950/1/Circular62.pdf (accessed on 4 October 2021).
- Steinmetz, S.; Cuadra, S.V.; Pereira, C.B.; Santos, E.L.; Almeida, I.R. GD Arroz: Programa Baseado em Graus-Dia como Suporte ao Planejamento e à Tomada de Decisão no Manejo do Arroz Irrigado; Embrapa Clima Temperado, Circular Técnica, 162; Embrapa Clima Temperado: Pelotas, Brazil, 2015; 8p. (In Portuguese) [Google Scholar]
- Souza, V.D.A.; Roberti, D.R.; Ruhoff, A.L.; Zimmer, T.; Adamatti, D.S.; de Gonçalves, L.G.G.; Diaz, M.B.; Alves, R.D.C.M.; de Moraes, O.L.L. Evaluation of MOD16 algorithm over irrigated rice paddy using flux tower measurements in Southern Brazil. Water 2019, 11, 1911. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.D.S.; Bayer, C.; De Lima, M.A.; Frighetto, R.T.S.; Macedo, V.R.M.; Marcolin, E. Variação diária da emissão de metano em solo cultivado com arroz irrigado no Sul do Brasil. Cienc. Rural 2008, 38, 2049–2053. [Google Scholar] [CrossRef] [Green Version]
- Vickers, D.; Mahrt, L. Quality control and flux sampling problems for tower and aircraft data. J. Atmos. Ocean. Technol. 1997, 14, 512–526. [Google Scholar] [CrossRef]
- Mauder, M.; Cuntz, M.; Drüe, C.; Graf, A.; Rebmann, C.; Schmid, H.P.; Schmidt, M.; Steinbrecher, R. A strategy for quality and uncertainty assessment of long-term eddy-covariance measurements. Agric. For. Meteorol. 2013, 169, 122–135. [Google Scholar] [CrossRef]
- Wilczak, J.M.; Oncley, S.P.; Stage, S.A. Sonic anemometer tilt correction algorithms. Bound.-Layer Meteorol. 2001, 99, 127–150. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water vapour transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Gash, J.H.C.; Culf, A.D. Applying a linear detrend to eddy correlation data in realtime. Bound.-Layer Meteorol. 1996, 79, 301–306. [Google Scholar] [CrossRef]
- Moncrieff, J.; Clement, R.; Finnigan, J.; Meyers, T. Averaging, Detrending, and Filtering of Eddy Covariance Time Series. In Handbook of Micrometeorology; Kluwer Academic Publishers: Norwell, MA, USA, 2004; pp. 7–31. [Google Scholar]
- Moncrieff, J.; Valentini, R.; Greco, S.; Seufert, G.; Ciccioli, P. Trace gas exchange over terrestrial ecosystems: Methods and perspectives in micrometeorology. J. Exp. Bot. 1997, 48, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Dengel, S.; Levy, P.E.; Grace, J.; Jones, S.K.; Skiba, U.M. Methane emissions from sheep pasture, measured with an open-path eddy covariance system. Glob. Chang. Biol. 2011, 17, 3524–3533. [Google Scholar] [CrossRef]
- Ge, H.X.; Zhang, H.S.; Zhang, H.; Cai, X.H.; Song, Y.; Kang, L. The characteristics of methane flux from an irrigated rice farm in East China measured using the eddy covariance method. Agric. For. Meteorol. 2018, 249, 228–238. [Google Scholar] [CrossRef]
- Dai, S.; Ju, W.; Zhang, Y.; He, Q.; Song, L.; Li, J. Variations and drivers of methane fluxes from a rice-wheat rotation agroecosystem in eastern China at seasonal and diurnal scales. Sci. Total Environ. 2019, 690, 973–990. [Google Scholar] [CrossRef]
- Ruppert, J.; Mauder, M.; Thomas, C.; Lüers, J. Innovative gap-filling strategy for annual sums of CO2 net ecosystem exchange. Agric. For. Meteorol. 2006, 138, 5–18. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Kim, Y.; Johnson, M.S.; Knox, S.H.; Black, T.A.; Dalmagro, H.J.; Kang, M.; Kim, J.; Baldocchi, D. Gap-filling approaches for eddy covariance methane fluxes: A comparison of three machine learning algorithms and a traditional method with principal component analysis. Glob. Chang. Biol. 2019, 26, 1499–1518. [Google Scholar] [CrossRef] [PubMed]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Knox, S.H.; Matthes, J.H.; Sturtevant, C.; Oikawa, P.Y.; Verfaillie, J.; Baldocchi, D. Biophysical controls on interannual variability in ecosystem-scale CO2 and CH4 exchange in a California rice paddy. J. Geophys. Res. Biogeosci. 2016, 121, 978–1001. [Google Scholar] [CrossRef]
- Anderson, F.E.; Bergamaschi, B.; Sturtevant, C.; Knox, S.; Hastings, L.; Windham-Myers, L.; Detto, M.; Hestir, E.L.; Drexler, J.; Miller, R.L.; et al. Variation of energy and carbon fluxes from a restored temperate freshwater wetland and implications for carbon market verification protocols. J. Geophys. Res. Biogeosci. 2016, 121, 777–795. [Google Scholar] [CrossRef]
- Knox, S.H.; Jackson, R.B.; Poulter, B.; McNicol, G.; Fluet-Chouinard, E.; Zhang, Z.; Hugelius, G.; Bousquet, P.; Canadell, J.G.; Saunois, M.; et al. FluXNET-CH4 synthesis activity objectives, observations, and future directions. Bull. Am. Meteorol. Soc. 2019, 100, 2607–2632. [Google Scholar]
- Richardson, A.D.; Hollinger, D.Y. A method to estimate the additional uncertainty in gap-filled NEE resulting from long gaps in the CO2 flux record. Agric. For. Meteorol. 2007, 147, 199–208. [Google Scholar] [CrossRef]
- Wilks, D.S. Statistical Methods in the Atmospheric Sciences, 59th ed.; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Meek, D.W.; Hatfield, J.L.; Howell, T.A.; Idso, S.B.; Reginato, R.J. Generalized relationship between photosynthetically active radiation and solar radiation. Agron. J. 1984, 76, 939–945. [Google Scholar] [CrossRef]
- Carrer, D.; Pique, G.; Ferlicoq, M.; Ceamanos, X.; Ceschia, E. What is the potential of cropland albedo management in the fight against global warming? A case study based on the use of cover crops. Environ. Res. Lett. 2018, 13, 44030. [Google Scholar] [CrossRef] [Green Version]
- Timm, A.U.; Roberti, D.R.; Streck, N.A.; de Gonçalves, L.G.G.; Acevedo, O.C.; Moraes, O.L.L.; Moreira, V.S.; Degrazia, G.A.; Ferlan, M.; Toll, D.L. Energy partitioning and evapotranspiration over a rice paddy in Southern Brazil. J. Hydrometeorol. 2014, 15, 1975–1988. [Google Scholar] [CrossRef]
- Weller, S.; Kraus, D.; Butterbach-Bahl, K.; Wassmann, R.; Tirol-Padre, A.; Kiese, R. Diurnal patterns of methane emissions from paddy rice fields in the Philippines. J. Plant Nutr. Soil Sci. 2015, 178, 755–767. [Google Scholar] [CrossRef]
- Tseng, K.H.; Tsai, J.L.; Alagesan, A.; Tsuang, B.J.; Yao, M.H.; Kuo, P.H. Determination of methane and carbon dioxide fluxes during the rice maturity period in Taiwan by combining profile and eddy covariance measurements. Agric. For. Meteorol. 2010, 150, 852–859. [Google Scholar] [CrossRef]
- Wang, Z.P.; Han, X.G. Diurnal variation in methane emissions in relation to plants and environmental variables in the Inner Mongolia marshes. Atmos. Environ. 2005, 39, 6295–6305. [Google Scholar] [CrossRef]
- Dengel, S.; Zona, D.; Sachs, T.; Aurela, M.; Jammet, M.; Parmentier, F.J.W.; Oechel, W.; Vesala, T. Testing the applicability of neural networks as a gap-filling method using CH4 flux data from high latitude wetlands. Biogeosciences 2013, 10, 8185–8200. [Google Scholar] [CrossRef] [Green Version]
- Moffat, A.M.; Papale, D.; Reichstein, M.; Hollinger, D.Y.; Richardson, A.D.; Barr, A.G.; Beckstein, C.; Braswell, B.H.; Churkina, G.; Desai, A.R.; et al. Comprehensive comparison of gap-filling techniques for eddy covariance net carbon fluxes. Agric. For. Meteorol. 2007, 147, 209–232. [Google Scholar] [CrossRef]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T.; et al. Towards a standardized processing of Net Ecosystem Exchange measured with eddy covariance technique: Algorithms and uncertainty estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Conrad, R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 1996, 60, 609–640. [Google Scholar] [CrossRef]
- Whalen, S.C. Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ. Eng. Sci. 2005, 22, 73–94. [Google Scholar] [CrossRef]
- Hatala, J.A.; Detto, M.; Sonnentag, O.; Deverel, S.J.; Verfaillie, J.; Baldocchi, D.D. Greenhouse gas (CO2, CH4, H2O) fluxes from drained and flooded agricultural peatlands in the Sacramento-San Joaquin Delta. Agric. Ecosyst. Environ. 2012, 150, 1–18. [Google Scholar] [CrossRef]
- Holzapfel-Pschorn, A.; Seiler, W. Methane emission during a cultivation period from an Italian rice paddy. J. Geophys. Res. 1986, 91, 11803. [Google Scholar] [CrossRef]
- Schütz, H.; Seiler, W.; Conrad, R. Processes involved in formation and emission of methane in rice paddies. Biogeochemistry 1989, 7, 33–53. [Google Scholar] [CrossRef]
- Schütz, H.; Seiler, W.; Conrad, R. Influence of soil temperature on methane emission from rice paddy fields. Biogeochemistry 1990, 11, 77–95. [Google Scholar] [CrossRef]
- Liu, G.; Si, B.C. Multi-layer diffusion model and error analysis applied to chamber-based gas fluxes measurements. Agric. For. Meteorol. 2009, 149, 169–178. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Korhonen, J.F.J.; Juszczak, R.; Giebels, M.; Pihlatie, M. Assessing the effects of chamber placement, manual sampling and headspace mixing on CH4 fluxes in a laboratory experiment. Plant Soil 2011, 343, 171–185. [Google Scholar] [CrossRef]
- Meijide, A.; Gruening, C.; Goded, I.; Seufert, G.; Cescatti, A. Water management reduces greenhouse gas emissions in a Mediterranean rice paddy field. Agric. Ecosyst. Environ. 2017, 238, 168–178. [Google Scholar] [CrossRef]
- Chaichana, N.; Bellingrath-Kimura, S.; Komiya, S.; Fujii, Y.; Noborio, K.; Dietrich, O.; Pakoktom, T. Comparison of Closed Chamber and Eddy Covariance Methods to Improve the Understanding of Methane Fluxes from Rice Paddy Fields in Japan. Atmosphere 2018, 9, 356. [Google Scholar] [CrossRef] [Green Version]
- Riederer, M.; Serafimovich, A.; Foken, T. Net ecosystem CO2 exchange measurements by the closed chamber method and the eddy covariance technique and their dependence on atmospheric conditions. Atmos. Meas. Tech. 2014, 7, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.A.K.; Butenhoff, C.L. Spatial variability of methane emissions from rice fields and implications for experimental design. J. Geophys. Res. 2008, 113, G00A09. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Guidelines for National Greenhouse Gas Inventories (Miscellaneous)|ETDEWEB. Available online: https://www.osti.gov/etdeweb/biblio/20880391 (accessed on 21 August 2020).
- Davidson, E.A.; Swank, W.T.; Perry, T.O. Distinguishing between Nitrification and Denitrification as Sources of Gaseous Nitrogen Production in Soil. Appl. Environ. Microbiol. 1986, 52, 1280–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, S.N.; Bonatti, J.P.; Kubota, P.Y.; Grell, G.A.; Morrison, H.; Barros, S.R.M.; Fernandez, J.P.R.; Ramirez, E.; Siqueira, L.; Luzia, G.; et al. The Brazilian Global Atmospheric Model (BAM): Performance for Tropical Rainfall Forecasting and Sensitivity to Convective Scheme and Horizontal Resolution. Weather Forecast. 2016, 31, 1547–1572. [Google Scholar] [CrossRef]





| Entire Period | Vegetative | Reproductive | Pre Harv. | No Rice | Land Prep | |
|---|---|---|---|---|---|---|
| CH4(EC) data coverage (%) | 26.5 | 47.3 | 40.4 | 21.3 | 8.2 | 30.5 |
| RMSE (μmol m−2 s−1) | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 |
| PBias (%) | 0.40 | 0.30 | 0.60 | −1.90 | 3.80 | −0.20 |
| r | 0.98 | 0.99 | 0.97 | 0.97 | 0.94 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maboni, C.; Bremm, T.; Aguiar, L.J.G.; Scivittaro, W.B.; de Arruda Souza, V.; Zimermann, H.R.; Teichrieb, C.A.; de Oliveira, P.E.S.; Herdies, D.L.; Degrazia, G.A.; et al. The Fallow Period Plays an Important Role in Annual CH4 Emission in a Rice Paddy in Southern Brazil. Sustainability 2021, 13, 11336. https://doi.org/10.3390/su132011336
Maboni C, Bremm T, Aguiar LJG, Scivittaro WB, de Arruda Souza V, Zimermann HR, Teichrieb CA, de Oliveira PES, Herdies DL, Degrazia GA, et al. The Fallow Period Plays an Important Role in Annual CH4 Emission in a Rice Paddy in Southern Brazil. Sustainability. 2021; 13(20):11336. https://doi.org/10.3390/su132011336
Chicago/Turabian StyleMaboni, Cristiano, Tiago Bremm, Leonardo José Gonçalves Aguiar, Walkyria Bueno Scivittaro, Vanessa de Arruda Souza, Hans Rogério Zimermann, Claudio Alberto Teichrieb, Pablo Eli Soares de Oliveira, Dirceu Luis Herdies, Gervásio Annes Degrazia, and et al. 2021. "The Fallow Period Plays an Important Role in Annual CH4 Emission in a Rice Paddy in Southern Brazil" Sustainability 13, no. 20: 11336. https://doi.org/10.3390/su132011336







