Estimation of Current and Future Suitable Areas for Tapirus pinchaque in Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
2.2.1. Predictive Model
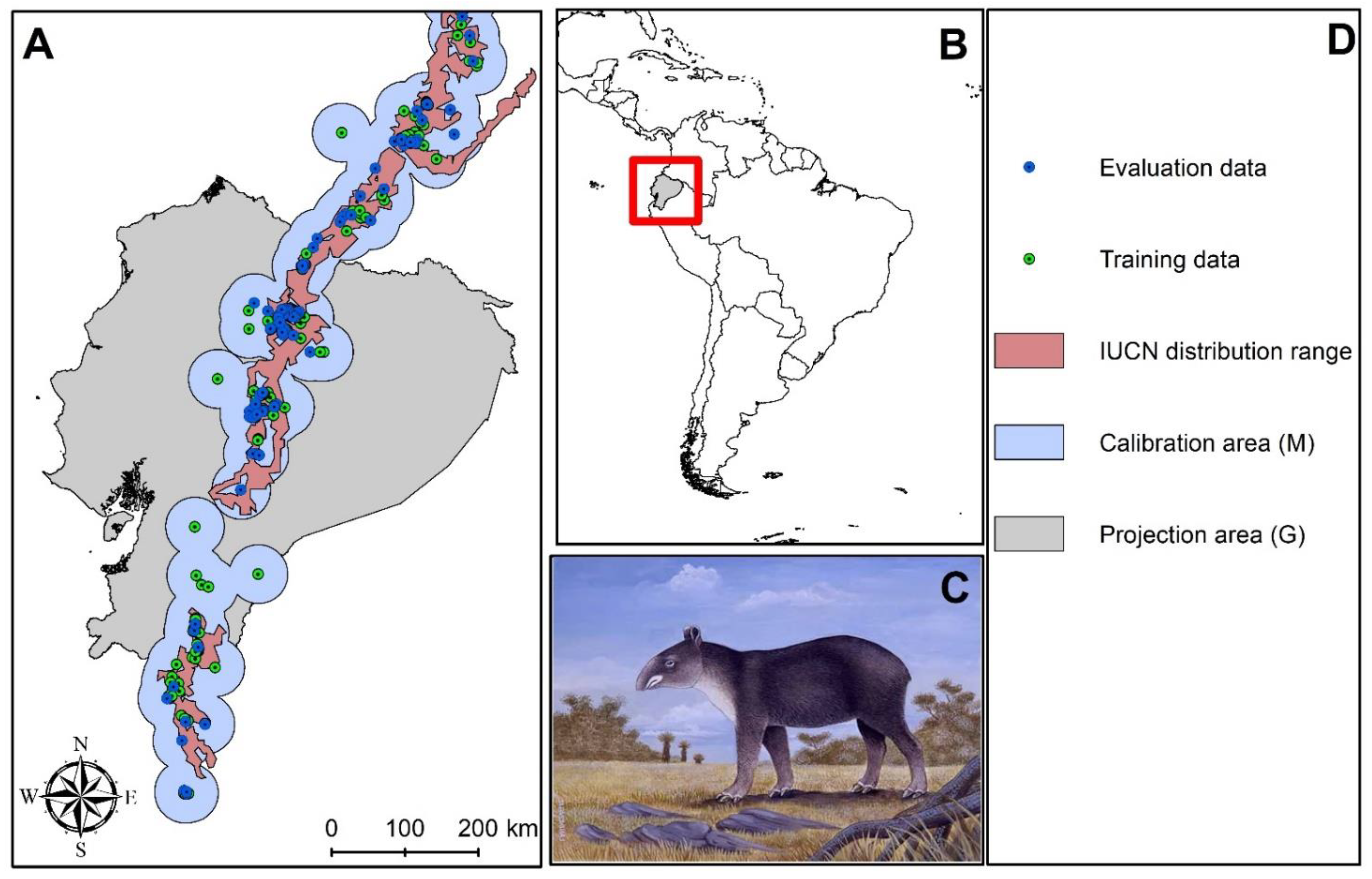
Occurrence Data
Environmental Data
Calibration Area
Model Calibration
Evaluation and Creation of Final Models
2.2.2. Representativeness of Predicted Suitable Areas in Ecuador’s SNAP
2.2.3. Habitat Changes in the Distribution
3. Results
3.1. Model Validation
3.2. Representativeness of Suitable Areas Predicted by the Models in the SNAP
3.2.1. Model 1: Current
3.2.2. Model 2: Future
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakamura, G.; Gonçalves, L.O.; da Silva Duarte, L. Revisiting the dimensionality of biological diversity. Ecography 2020, 43, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Dunn, C.P. Biological and cultural diversity in the context of botanic garden conservation strategies. Plant Divers. 2017, 39, 396–401. [Google Scholar] [CrossRef]
- Woodward, G.; Bohan, D.A. Ecosystem Services: From Biodiversity to Society; Part 2; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Balvanera, P.; Quijas, S.; Karp, D.S.; Ash, N.; Bennett, E.M.; Boumans, R.; Brown, C.; Chan, K.M.A.; Chaplin-Kramer, R.; Halpern, B.S. Ecosystem Services. In The GEO Handbook on Biodiversity Observation Networks; Walters, M., Scholes, R.J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 39–78. [Google Scholar]
- Vimal, R.; Navarro, L.M.; Jones, Y.; Wolf, F.; Le Moguédec, G.; Réjou-Méchain, M. The global distribution of protected areas management strategies and their complementarity for biodiversity conservation. Biol. Conserv. 2021, 256, 109014. [Google Scholar] [CrossRef]
- Wilson, M.; Liu, S. Evaluating the non-market value of ecosystem goods and services provided by coastal and nearshore marine systems. In Ecological Economics of the Oceans and Coasts; Edward Elgar Publishing: Cheltenham, UK, 2008. [Google Scholar]
- Hernández-Blanco, M.; Costanza, R.; Anderson, S.; Kubiszewski, I.; Sutton, P. Future scenarios for the value of ecosystem services in Latin America and the Caribbean to 2050. Curr. Res. Environ. Sustain. 2020, 2, 100008. [Google Scholar] [CrossRef]
- Soley, F.G.; Perfecto, I. A way forward for biodiversity conservation: High-quality landscapes. Trends Ecol. Evol. 2021, 36, 770–773. [Google Scholar] [CrossRef]
- Pielke, R.A.; Adegoke, J.; Hossain, F.; Niyogi, D. Environmental and Social Risks to Biodiversity and Ecosystem Health—A Bottom-Up, Resource-Focused Assessment Framework. Earth 2021, 2, 440–456. [Google Scholar] [CrossRef]
- Dueñas, M.-A.; Hemming, D.J.; Roberts, A.; Diaz-Soltero, H. The threat of invasive species to IUCN-listed critically endangered species: A systematic review. Glob. Ecol. Conserv. 2021, 26, e01476. [Google Scholar] [CrossRef]
- Mantyka-Pringle, C.S.; Visconti, P.; di Marco, M.; Martin, T.G.; Rondinini, C.; Rhodes, J.R. Climate change modifies risk of global biodiversity loss due to land-cover change. Biol. Conserv. 2015, 187, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Waldron, A.; Miller, D.C.; Redding, D.; Mooers, A.; Kuhn, T.S.; Nibbelink, N.; Roberts, J.T.; Tobias, J.A.; Gittleman, J.L. Reductions in global biodiversity loss predicted from conservation spending. Nature 2017, 551, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Githiru, M.; Njambuya, J.W. Globalization and Biodiversity Conservation Problems: Polycentric REDD+ Solutions. Land 2019, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Mori, A.S.; Dee, L.E.; Gonzalez, A.; Ohashi, H.; Cowles, J.; Wright, A.J.; Isbell, F. Biodiversity–productivity relationships are key to nature-based climate solutions. Nat. Clim. Chang. 2021, 11, 543–550. [Google Scholar] [CrossRef]
- Marta, S.; Brunetti, M.; Manenti, R.; Provenzale, A.; Ficetola, G.F. Climate and land-use changes drive biodiversity turnover in arthropod assemblages over 150 years. Nat. Ecol. Evol. 2021, 5, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, P.; Arriagada, R.; Echeverría, C. How much deforestation do protected areas avoid in tropical Andean landscapes? Environ. Sci. Policy 2016, 56, 56–66. [Google Scholar] [CrossRef]
- Schwartz, K.R.; Gusset, M.; Crosier, A.E.; Versteege, L.; Eyre, S.; Tiffin, A.; otzé, A. The Role of Zoos in Tree Kangaroo Conservation: Connecting Ex Situ and In Situ Conservation Action. In Biodiversity of World: Conservation from Genes to Landscapes; Dabek, L., Valentine, P., Blessington, J., Schwartz Krbt, T.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 22; pp. 329–361. [Google Scholar]
- O’Connor, B.; Bojinski, S.; Röösli, C.; Schaepman, M.E. Monitoring global changes in biodiversity and climate essential as ecological crisis intensifies. Ecol. Inform. 2020, 55, 101033. [Google Scholar] [CrossRef]
- Fei, S.; Jo, I.; Guo, Q.; Wardle, D.A.; Fang, J.; Chen, A.; Brockerhoff, E.G. Impacts of climate on the biodiversity-productivity relationship in natural forests. Nat. Commun. 2018, 9, 5436. [Google Scholar] [CrossRef]
- Cheng, H.; Sinha, A.; Cruz, F.W.; Wang, X.; Edwards, R.L.; d’Horta, F.M.; Auler, A.S. Climate change patterns in Amazonia and biodiversity. Nat. Commun. 2013, 4, 1411. [Google Scholar] [CrossRef]
- Visscher, A.M.; Vanek, S.; Meza, K.; de Goede, R.G.; Valverde, A.A.; Ccanto, R.; Fonte, S.J. Eucalyptus and alder field margins differ in their impact on ecosystem services and biodiversity within cropping fields of the Peruvian Andes. Agric. Ecosyst. Environ. 2020, 303, 107107. [Google Scholar] [CrossRef]
- Fastré, C.; Possingham, H.P.; Strubbe, D.; Matthysen, E. Identifying trade-offs between biodiversity conservation and ecosystem services delivery for land-use decisions. Sci. Rep. 2020, 10, 7971. [Google Scholar] [CrossRef]
- Cunalata García, A.; López Pumalema, J. Turismo de humedales en Ecuador: Análisis a los sitios RAMSAR. Green World J. 2020, 3, 1–12. Available online: https://www.greenworldjournal.com/doi-018-ac-2020 (accessed on 15 September 2021).
- Bax, V.; Francesconi, W. Conservation gaps and priorities in the Tropical Andes biodiversity hotspot: Implications for the expansion of protected areas. J. Environ. Manag. 2019, 232, 387–396. [Google Scholar] [CrossRef]
- Acha, S.; Linan, A.; MacDougal, J.; Edwards, C. The evolutionary history of vines in a neotropical biodiversity hotspot: Phylogenomics and biogeography of a large passion flower clade (Passiflora section Decaloba). Mol. Phylogenetics Evol. 2021, 164, 107260. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.S. Not the Same: Rethinking Chineseness in a Global Context Through Poster Design. In The Disappearance of Hong Kong in Comics, Advertising and Graphic Design; Wong, W.S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 215–239. [Google Scholar]
- Mestanza-Ramón, C.; Henkanaththegedara, S.M.; Vásconez Duchicela, P.; Vargas Tierras, Y.; Sánchez Capa, M.; Constante Mejía, D.; Jimenez Gutierrez, M.; Charco Guamán, M.; Mestanza Ramón, P. In-Situ and Ex-Situ Biodiversity Conservation in Ecuador: A Review of Policies, Actions and Challenges. Diversity 2020, 12, 315. [Google Scholar] [CrossRef]
- Graham, R.W.; Grady, F.; Ryan, T.M. Juvenile Pleistocene tapir skull from Russells Reserve Cave, Bath County, Virginia: Implications for cold climate adaptations. Quat. Int. 2019, 530–531, 35–41. [Google Scholar] [CrossRef]
- Lizcano, D.J.; Cavelier, J. Using GPS collars to study mountain tapirs (Tapirus pinchaque) in the Central Andes of Colombia. Tapir. Conserv. 2004, 13, 18–23. [Google Scholar]
- Heredia, A.; Achoa, O.; Sandoval-Cañas, L.; Iglesias, M. Reportes sobre la presencia del Tapir de Montaña (Tapirus pinchaque) en el Parque Nacional Llanganates, Ecuador. Tapir. Conserv. 2007, 19, 1813–2286. [Google Scholar]
- Ortega-Andrade, H.M.; Prieto-Torres, D.A.; Gómez-Lora, I.; Lizcano, D.J. Ecological and Geographical Analysis of the Distribution of the Mountain Tapir (Tapirus pinchaque) in Ecuador: Importance of Protected Areas in Future Scenarios of Global Warming. PLoS ONE 2015, 10, e0121137. [Google Scholar] [CrossRef] [Green Version]
- Downer, C.C. Observations on the diet and habitat of the mountain tapir (Tapirus pinchaque). J. Zool. 2001, 254, 279–291. [Google Scholar] [CrossRef]
- Lizcano, D.J.; Cavelier, J. Daily and seasonal activity of the mountain tapir (Tapirus pinchaque) in the Central Andes of Colombia. J. Zool. 2000, 252, 429–435. [Google Scholar] [CrossRef]
- Lizcano, D.J.; Cavelier, J. Densidad Poblacional y Disponibilidad de Híbitat de la Danta de Montaña (Tapirus pinchaque) en los Andes Centrales de Colombia. Biotropica 2000, 32, 165–173. [Google Scholar] [CrossRef]
- Paz Mena, Y.S. Contaminación marina por actividades antrópicas. Green World J. 2018, 1, 1. [Google Scholar]
- Castellanos-Peñafiel, A.X.; Brito, J.; Castellanos-Insuasti, F.X. Tapirus pinchaque in Southwestern Ecuador. Serie Zoológica 2020, 16, 1–4. [Google Scholar]
- Herrera-Feijoo, R.J.; de Decker, M.; Chicaiza-Ortiz, C.; Penafilel Arcos, P.; Garzon Ortega, C. Posibles cambios en el rango de distribución de Tapirus pinchaque bajo escenarios de cambio climático. Green World J. 2021, 4, 1–19. [Google Scholar] [CrossRef]
- Alzate, A.A.; Downer, C.C.; Delgado, C.A.; Sánchez-Londoño, J.D. Un registro de tapir de montaña (Tapirus Pinchaque) en el norte de la Cordillera Occidental de Colombia. Mastozoología Neotropical 2010, 17, 111–116. [Google Scholar]
- Willis, S.G.; Hole, D.G.; Huntley, B. Climate Change and Conservation. In Trade-offs in Conservation: Deciding What to Save; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 329–348. [Google Scholar]
- Branco, V.V.; Cardoso, P. An expert-based assessment of global threats and conservation measures for spiders. Glob. Ecol. Conserv. 2020, 24, e01290. [Google Scholar] [CrossRef]
- Baker-Médard, M.; Gantt, C.; White, E.R. Classed conservation: Socio-economic drivers of participation in marine resource management. Environ. Sci. Policy 2021, 124, 156–162. [Google Scholar] [CrossRef]
- Fonseca, C.R.; Paterno, G.B.; Guadagnin, D.L.; Venticinque, E.M.; Overbeck, G.E.; Ganade, G.; Metzger, J.P.; Kollmann, J.; Sauer, J.; Cardoso, M.Z. Conservation biology: Four decades of problem- and solution-based research. Perspect. Ecol. Conserv. 2021, 19, 121–130. [Google Scholar] [CrossRef]
- Loaiza, P.J.A.; Cordova, P.A.A.; Valverde, F.M.V.; Ordóñez, D.A.T.; Torres, A.C.E.; Moretta, P.Y. El Tapir de montaña (Tapirus pinchaque), como especie bandera en los Andes del sur del Ecuador. INNOVA Res. J. 2017, 2, 86–103. [Google Scholar] [CrossRef]
- Simoes, M.; Romero-Alvarez, D.; Nuñez-Penichet, C.; Jiménez, L.; Cobos, M.E. General theory and good practices in ecological niche modeling: A basic guide. Biodivers. Inform. 2020, 15, 67–68. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [Green Version]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Zizka, A.; Antonelli, A.; Silvestro, D. Sampbias, a method for quantifying geographic sampling biases in species distribution data. Ecography 2021, 44, 25–32. [Google Scholar] [CrossRef]
- Gábor, L.; Moudrý, V.; Barták, V.; Lecours, V. How do species and data characteristics affect species distribution models and when to use environmental filtering? Int. J. Geogr. Inf. Sci. 2020, 34, 1567–1584. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Lobo, J.M. Debemos fiarnos de los modelos de distribución de especies? In Los Bosques y la Biodiversidad Frente al Cambio Climático: Impactos, Vulnerabilidad y Adaptación en España; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2015; pp. 407–417. [Google Scholar]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. J. R. Meteorol. Soc. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Escobar, L.E.; Lira-Noriega, A.; Medina-Vogel, G.; Peterson, A.T. Potential for spread of the white-nose fungus (Pseudogymnoascus destructans) in the Americas: Use of Maxent and NicheA to assure strict model transference. Geospat. Health 2014, 9, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Flato, G.; Marotzke, J.; Abiodun, B.; Braconnot, P.; Chou, S.C.; Collins, W.; Cox, P.; Driouech, F.; Emori, S.; Eyring, V. Evaluation of climate models. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 741–866. [Google Scholar]
- Zappa, G.; Shepherd, T.G. Storylines of atmospheric circulation change for European regional climate impact assessment. J. Clim. 2017, 30, 6561–6577. [Google Scholar] [CrossRef] [Green Version]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Osorio-Olvera, L.; Lira-Noriega, A.; Soberon, J.; Peterson, A.T.; Falconi, M.; Contreras-Díaz, R.G.; Martínez-Meyer, E.; Barve, V.; Barve, N. ntbox: An R package with graphical user interface for modelling and evaluating multidimensional ecological niches. Methods Ecol. Evol. 2020, 11, 1199–1206. [Google Scholar] [CrossRef]
- Mota-Vargas, C.; Encarnación-Luévano, A.; Ortega-Andrade, H.M.; Prieto-Torres, D.A.; Peña-Peniche, A.; Rojas-Soto, O.R. Una breve introducción a los modelos de nicho ecológico. In La Biodiversidad en un Mundo Cambiante: Fundamentos Teóricos y Metodológicos Para su Estudio Universidad Autónoma del Estado de Hidalgo/Libermex, Ciudad de México; Universidad Autónoma del Estado de Hidalgo: Pachuca, Mexico, 2019; pp. 39–63. [Google Scholar]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Mendes, P.; Velazco, S.J.E.; de Andrade, A.F.A.; Júnior, P.D.M. Dealing with overprediction in species distribution models: How adding distance constraints can improve model accuracy. Ecol. Model. 2020, 431, 109180. [Google Scholar] [CrossRef]
- Mena, J.L.; Yagui, H.; La Rosa, F.; Pastor, P.; Rivero, J.; Appleton, R. Topography and disturbance explain mountain tapir (Tapirus pinchaque) occupancy at its southernmost global range. Topography and disturbance explain mountain tapir (Tapirus pinchaque) occupancy at its southernmost global range. Mamm. Biol. 2020, 100, 231–239. [Google Scholar] [CrossRef]
- Padilla, M.; Dowler, R.C.; Downer, C.C. Tapirus pinchaque (Perissodactyla: Tapiridae). Mamm. Species 2010, 42, 166–182. [Google Scholar] [CrossRef]
- Tirira, D.G.; Urgilés-Verdugo, C.A.; Tapia, A.; Cajas-Bermeo, C.A.; Izurieta, X.; Zapata-Ríos, G. Tropical Ungulates of Ecuador: An Update of the State of Knowledge. In Ecology and Conservation of Tropical Ungulates in Latin America; Springer: Berlin/Heidelberg, Germany, 2019; pp. 217–271. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallgren, W.; Santana, F.; Low-Choy, S.; Zhao, Y.; Mackey, B. Species distribution models can be highly sensitive to algorithm configuration. Ecol. Model. 2019, 408, 108719. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Anderson, R.P.; Lew, D.; Peterson, A.T. Evaluating predictive models of species’ distributions: Criteria for selecting optimal models. Ecol. Model. 2003, 162, 211–232. [Google Scholar] [CrossRef]
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140135. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Hoorn, C.; Guayasamin, J.M.; Ortega-Andrade, H.M.; Linder, P.; Bonaccorso, E. Celebrating Alexander von Humboldt’s 250th anniversary: Exploring bio-and geodiversity in the Andes (IBS Quito 2019). Front. Biogeogr. 2019, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Saura, S.; Bertzky, B.; Bastin, L.; Battistella, L.; Mandrici, A.; Dubois, G. Protected area connectivity: Shortfalls in global targets and country-level priorities. Biol. Conserv. 2018, 219, 53–67. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef]
- Loor, D.K.B. Dieta del tapir de montaña (Tapirus pinchaque) en tres localidades del corredor ecológico Llangantes–Sangay. Boletín Técnico Serie Zoológica 2019, 10, 1–11. [Google Scholar]
- Puig, C.R.; Alvear, G.R. Diversidad en formícidos y plantas vasculares en el Parque Nacional Yasuní, Ecuador. Boletín Técnico Serie Zoológica 2019, 12, 10–11. [Google Scholar]
- Arias-Gutiérrez, R.; Reyes-Puig, J.; Tapia, A.; Terán, A.; Rodríguez, X.; Bermudez, D.; López de Vargas-Machuca, K. Desarrollo local y conservación en la vertiente oriental andina: Corredor ecológico Llanganates-Sangay-valle del Anzu. Revista Amazónica Ciencia y Tecnología 2016, 5, 52–68. [Google Scholar]
- Freeman, B.G.; Song, Y.; Feeley, K.J.; Zhu, K. Montane species and communities track recent warming more closely in the tropics. bioRxiv 2020. [Google Scholar] [CrossRef]
- Carnicer, C.; Eisenlohr, P.V.; de A. Jácomo, A.T.; Silveira, L.; Alves, G.B.; Tôrres, N.M.; de Melo, F.R. Running to the mountains: Mammal species will find potentially suitable areas on the Andes. Biodivers. Conserv. 2020, 29, 1855–1869. [Google Scholar] [CrossRef]
- Cuesta, F.; Peralvo, M.; Merino-Viteri, A.; Bustamante, M.; Baquero, F.; Freile, J.F.; Muriel, P.; Torres-Carvajal, O. Priority areas for biodiversity conservation in mainland Ecuador. Neotrop. Biodivers. 2017, 3, 93–106. [Google Scholar] [CrossRef]
- Herzog, S.K.; Tiessen, H. Climate Change and Biodiversity in the Tropical Andes; Fundacion Ambiente y Recursos Naturales: Capital Federal, Argentina, 2017. [Google Scholar]
- Báez, S.; Jaramillo, L.; Cuesta, F.; Donoso, D.A. Effects of climate change on Andean biodiversity: A synthesis of studies published until 2015. Neotrop. Biodivers. 2016, 2, 181–194. [Google Scholar] [CrossRef]
- Peters, T.; Drobnik, T.; Meyer, H.; Rankl, M.; Richter, M.; Rollenbeck, R.; Thies, B.; Bendix, J. Environmental changes affecting the Andes of Ecuador. In Ecosystem Services, Biodiversity and Environmental Change in a Tropical Mountain Ecosystem of South Ecuador; Springer: Berlin/Heidelberg, Germany, 2013; pp. 19–29. [Google Scholar]

| Model | AUC Ratio | OR | AICc | ∆AICc | RM | FC |
|---|---|---|---|---|---|---|
| 1 | 1.246 | 0.045 | 8443.803 | 0.000 | 0.7 | qp |
| 2 | 1.173 | 0.045 | 8445.106 | 1.303 | 0.8 | qp |
| 3 | 1.162 | 0.045 | 8445.185 | 1.382 | 0.6 | qp |
| Name of Protected Area | Design Type | Current (km2) | RCP 4.5 (km2) | RCP 8.5 (km2) |
|---|---|---|---|---|
| Antisana | ER | 1392 | 1406 (+1.01%) | 1408 (+1.15%) |
| Bellavista | PPA | 3 | 3 (0%) | 3 (0%) |
| Cajas | NP | 350 | 350 (0%) | 350 (0%) |
| Cayambe Coca | NP | 4305 | 4235 (−1.63%) | 4300 (−0.12%) |
| Cerro Plateado | BR | 224 | 235 (+4.91%) | 238 (+6.25%) |
| Chimborazo | WPR | 388 | 613 (+57.99%) | 621 (+60.05%) |
| Cofan Bermejo | ER | 133 | 129 (−3.01%) | 127 (−4.51%) |
| Colonso Chalupas | BR | 976 | 969 (−0.72%) | 980 (+0.41%) |
| Cordillera Oriental Del Carchi | DAPA | 231 | 231 (0%) | 231 (0%) |
| Cotacachi Cayapas | NP | 694 | 757 (+9.08%) | 807 (+16.28%) |
| Cotopaxi | NP | 350 | 374 (+6.86%) | 376 (+7.43%) |
| El Ángel | ER | 187 | 187 (0%) | 187 (0%) |
| El Boliche | NRA | 6 | 6 (0%) | 6 (0%) |
| El Quimi | BR | 102 | 100 (−1.96%) | 98 (0%) |
| El Zarza | WR | 6 | 0 (−100.00%) | 0 (0%) |
| Ichubamba Yasepan | PPA | 54 | 54 (0%) | 54 (0%) |
| La Bonita | MCA | 621 | 621 (0%) | 621 (0%) |
| Llanganates | NP | 2487 | 2473 (−0.56%) | 2479 (−0.32%) |
| Los Ilinizas | ER | 745 | 957 (+28.46%) | 1055 (+41.61%) |
| Marcos Pérez De Castilla | CPA | 99 | 99 (0%) | 99 (0%) |
| Pasochoa | WR | 4 | 4 (0%) | 4 (0%) |
| Podocarpus | NP | 1383 | 1432 (+3.54%) | 1443 (+4.34%) |
| Pululahua | GR | 37 | 38 (+2.70%) | 38 (+2.70%) |
| Quimsacocha | NRA | 32 | 32 (0%) | 32 (0%) |
| Rio Negro Sopladora | NP | 386 | 386 (0%) | 385 (−0.26%) |
| Sangay | NP | 4920 | 4852 (−1.38%) | 4865 (−1.12%) |
| Siete Iglesias | MCA | 175 | 174 (−0.57%) | 171 (−2.29%) |
| Sumaco Napo-Galeras | NP | 1760 | 1722 (−2.16%) | 1750 (−0.57%) |
| Tambillo | CPA | 21 | 21 (0%) | 21 (0%) |
| Yacuambi | MCA | 307 | 307 (0%) | 307 (0%) |
| Yacuri | NP | 494 | 496 (+0.40%) | 497 (+0.61%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mestanza-Ramón, C.; Herrera Feijoo, R.J.; Chicaiza-Ortiz, C.; Gaibor, I.D.; Mateo, R.G. Estimation of Current and Future Suitable Areas for Tapirus pinchaque in Ecuador. Sustainability 2021, 13, 11486. https://doi.org/10.3390/su132011486
Mestanza-Ramón C, Herrera Feijoo RJ, Chicaiza-Ortiz C, Gaibor ID, Mateo RG. Estimation of Current and Future Suitable Areas for Tapirus pinchaque in Ecuador. Sustainability. 2021; 13(20):11486. https://doi.org/10.3390/su132011486
Chicago/Turabian StyleMestanza-Ramón, Carlos, Robinson J. Herrera Feijoo, Cristhian Chicaiza-Ortiz, Isabel Domínguez Gaibor, and Rubén G. Mateo. 2021. "Estimation of Current and Future Suitable Areas for Tapirus pinchaque in Ecuador" Sustainability 13, no. 20: 11486. https://doi.org/10.3390/su132011486
APA StyleMestanza-Ramón, C., Herrera Feijoo, R. J., Chicaiza-Ortiz, C., Gaibor, I. D., & Mateo, R. G. (2021). Estimation of Current and Future Suitable Areas for Tapirus pinchaque in Ecuador. Sustainability, 13(20), 11486. https://doi.org/10.3390/su132011486









