Advances in the Application of Nanocatalysts in Photocatalytic Processes for the Treatment of Food Dyes: A Review
Abstract
:1. Introduction
2. Dyes in the Food Industry
3. Advanced Oxidation Process
4. Advances in Heterogeneous Advanced Oxidation Processes
4.1. Catalysts in AOPs for Removal of Food Dyes
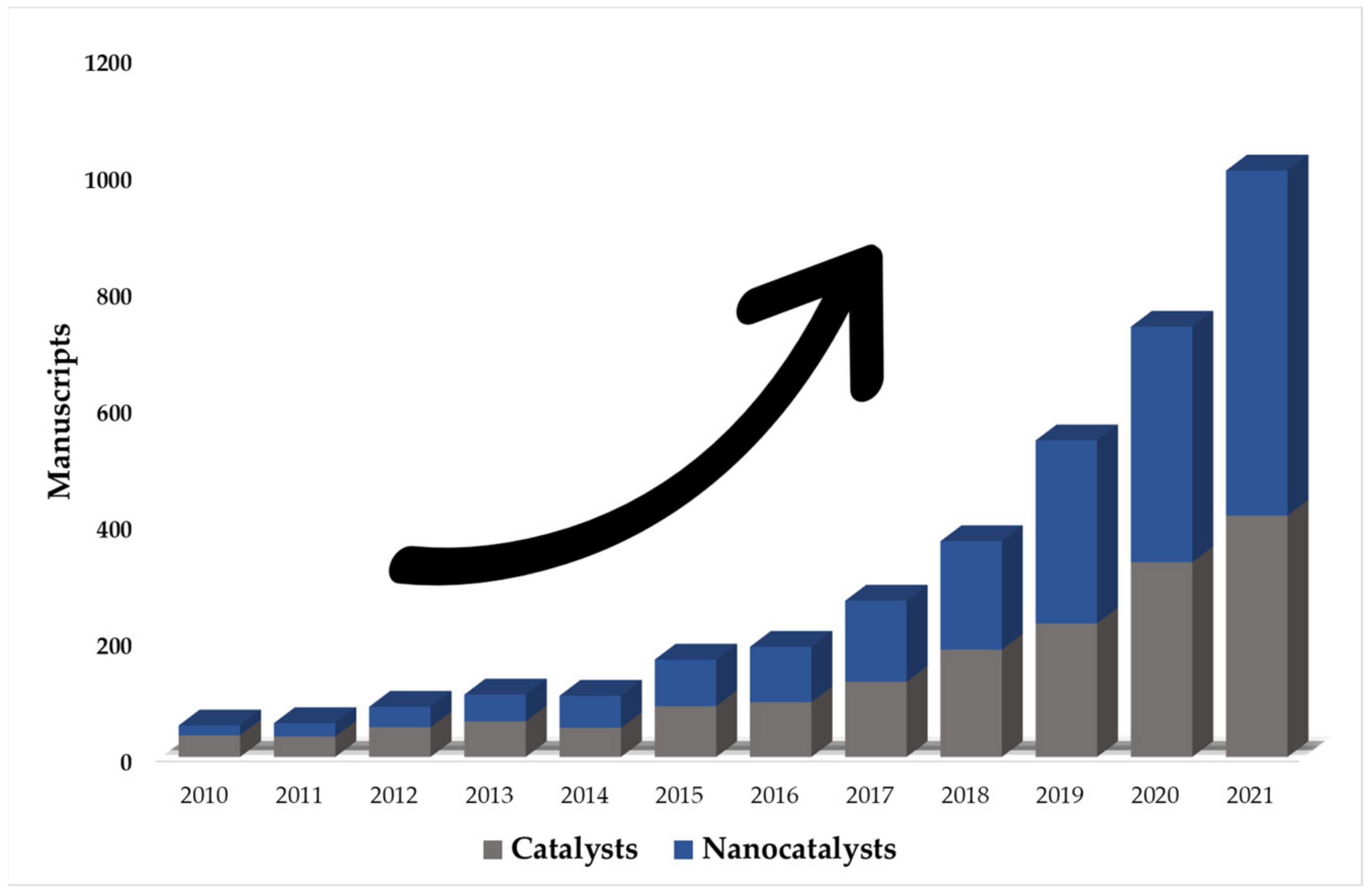
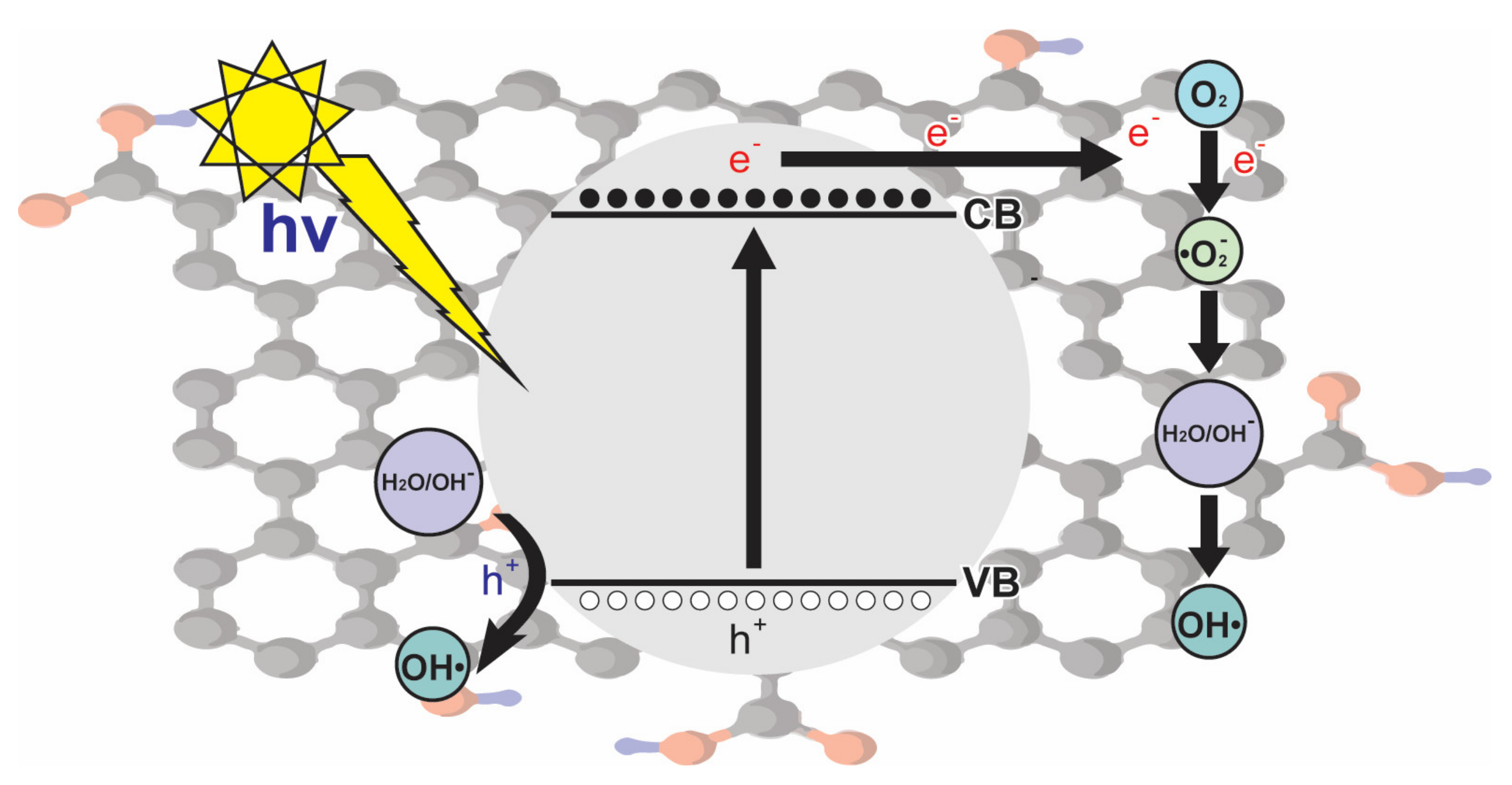
4.1.1. Nanocatalysts
4.1.2. Metal Oxide-Based Nanocatalysts
4.1.3. Graphene-Based Nanocatalysts
4.1.4. Metal–Organic Frameworks Synthesized with Nanocomposites
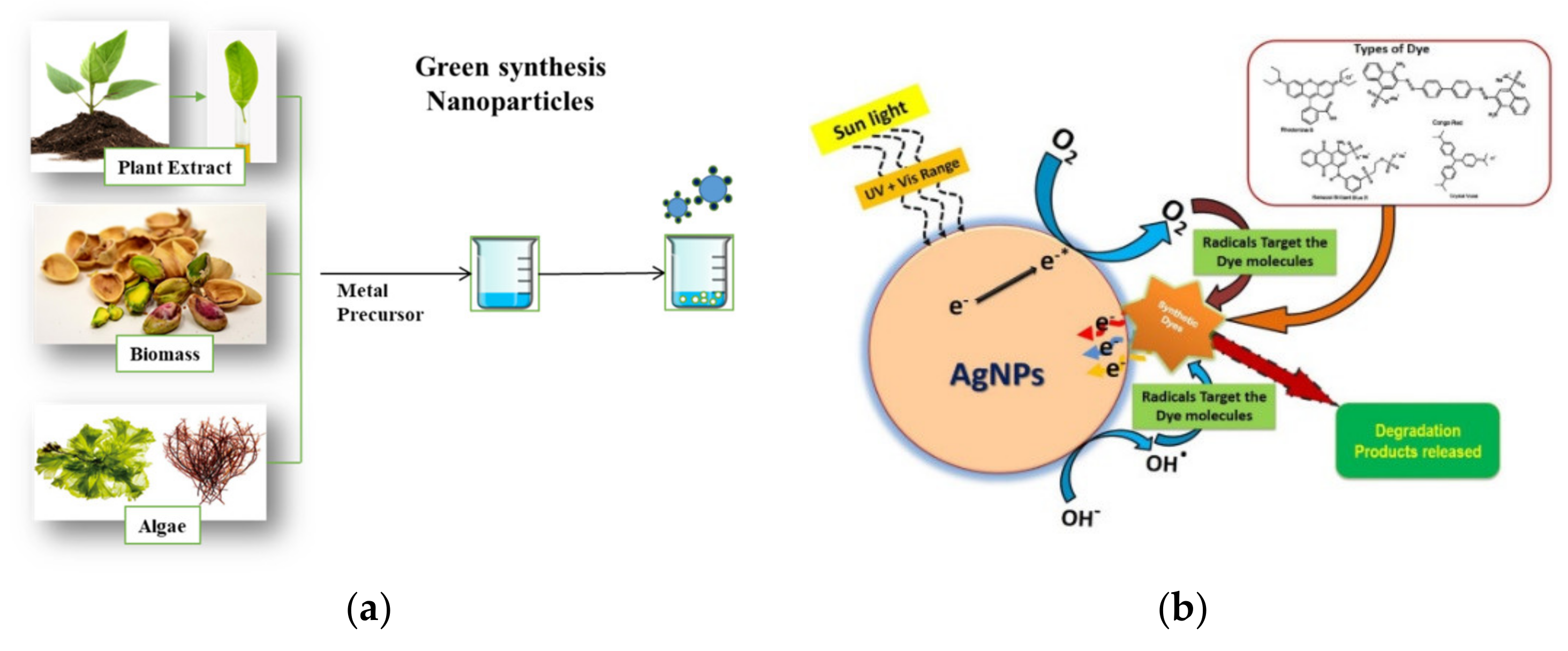
4.1.5. Nanomaterials Synthesized by Green Technologies
5. Discussion
6. Conclusions and Prospects
- (i)
- Reducing the evaluation of the degradation of food dyes from synthetic waters, with more attention on the analysis of real water systems, since several factors that are not considered notably affect the ecosystem or the application of the nanocatalyst in real remediation systems;
- (ii)
- Measuring the toxicity mechanisms of the intermediates generated by the use of nanomaterials for the removal of pollutants in wastewater, and to couple such procedures to advanced analytical chromatography techniques to control the various reaction steps given during the treatment; and
- (iii)
- Controlling and evaluating the type of surface coating, particle size, and residues also generated by the nanomaterials in their different reaction cycles, since even low concentrations of nanoparticles could alter natural processes or cycles and, consequently, affect the microbial community and the ecosystem in general.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, S.; Khan, N.; Gul, S.; Khan, S.; Khan, H. Contamination of water resources by food dyes and its removal technologies. In Water Chemistry; Intech Open: London, UK, 2019. [Google Scholar]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Tantawy, H.R.; Elsayed, M.A.; Bechelany, M.; Elmowafy, M.E. Elaboration of nano titania-magnetic reduced graphene oxide for degradation of tartrazine dye in aqueous solution. Solid State Sci. 2018, 78, 116–125. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collivignarelli, M.C.; Pedrazzani, R.; Sorlini, S.; Abbà, A.; Bertanza, G. H2O2 based oxidation processes for the treatment of real high strength aqueous wastes. Sustainability 2017, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Hassaan, M.A.; el Nemr, A.; Madkour, F.F. Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. Egypt. J. Aquat. Res. 2017, 43, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef]
- Khan, S.; Malik, A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ. Sci. Pollut. Res. 2018, 25, 4446–4458. [Google Scholar] [CrossRef]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process.-Process Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J. Hazard. Mater. 2020, 401, 123401. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Divyapriya, G.; Nambi, I.M.; Senthilnathan, J. Nanocatalysts in Fenton based advanced oxidation process for water and wastewater treatment. J. Bionanosci. 2016, 10, 356–368. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S.; Hamid, S.B.A. Recent advances in heterogeneous photocatalytic decolorization of synthetic dyes. Sci. World J. 2014, 2014, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.E.; Decker, E.A.; Ferruzzi, M.; Giusti, M.M.; Mejia, C.D.; Goldschmidt, M.; Talcott, S.T. Establishing standards on colors from natural sources. J. Food Sci. 2017, 82, 2539–2553. [Google Scholar] [CrossRef]
- Scotter, M. COLORANTS (COLOURANTS)|Properties and Determinants of Synthetic Pigments. In Encyclopedia of Food Sciences and Nutrition (Second Edition); Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 1556–1567. [Google Scholar]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the re-evaluation of Brown FK (E 154) as a food additive. EFSA J. 2010, 8. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products and Allergies. Scientific Opinion on the appropriateness of the food azo-colours Tartrazine (E 102), Sunset Yellow FCF (E 110), Carmoisine (E 122), Amaranth (E 123), Ponceau 4R (E 124), Allura Red AC (E 129), Brilliant Black BN (E 151), Brown FK (E 154), Brown HT (E 155) and Litholrubine BK (E 180) for inclusion in the list of food ingredients set up in Annex IIIa of Directive 2000/13/EC. EFSA J. 2010, 8, 1778. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Reconsideration of the temporary ADI and refined exposure assessment for Sunset Yellow FCF (E 110). EFSA J. 2014, 12, 3765. [Google Scholar] [CrossRef]
- European Food Safety Authority. Refined exposure assessment for Azorubine/Carmoisine (E 122). EFSA J. 2015, 13, 4072. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Amaranth (E 123) as a food additive. EFSA J. 2010, 8, 1649. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Allura Red AC (E 129) as a food additive. EFSA J. 2009, 7, 1327. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Litholrubine BK (E 180) as a food additive. EFSA J. 2010, 8, 1586. [Google Scholar] [CrossRef]
- Kobylewski, S.; Jacobson, M.F. Toxicology of food dyes. Int. J. Occup. Environ. Health 2012, 18, 220–246. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Brilliant Black BN (E 151) as a food additive. EFSA J. 2010, 8, 1540. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources (ANS). Scientific Opinion on the re-evaluation of Brown HT (E 155) as a food additive. EFSA J. 2010, 8, 1536. [Google Scholar] [CrossRef]
- Abbas, J.R.; Al-Hamadawi, H.A. Effect of chocolate brown HT E155 on some hormones in male albino rats. Eur. Asian J. BioSci. 2019, 13, 485–489. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Patent Blue V (E 131) as a food additive. EFSA J. 2013, 11, 2818. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Brilliant Blue FCF (E 133) as a food additive. EFSA J. 2010, 8, 1853. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Singh, A.; Sharma, D.K.; Kishore, K. Toxicity of food additives. In Food Safety and Human Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–98. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Green S (E 142) as a food additive. EFSA J. 2010, 8, 1851. [Google Scholar] [CrossRef] [Green Version]
- Villaño, D.; Cristina, G.-V.; Mena, P. Colors: Health Effects. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; Volume 12, p. 31. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Erythrosine (E 127) as a food additive. EFSA J. 2011, 9, 1854. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Quinoline Yellow (E 104) as a food additive. EFSA J. 2009, 7, 1329. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Indigo Carmine (E 132) as a food additive. EFSA J. 2014, 12, 3768. [Google Scholar] [CrossRef]
- Sun, W.; Chen, L.; Tian, J.; Wang, J.; He, S. Degradation of a monoazo dye Alizarin Yellow GG in aqueous solutions by gamma irradiation: Decolorization and biodegradability enhancement. Radiat. Phys. Chem. 2013, 83, 86–89. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; Elharfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L.; Sannino, D. Advanced oxidation processes for the removal of food dyes in wastewater. Curr. Org. Chem. 2017, 21, 1068–1073. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Graphene-based materials supported advanced oxidation processes for water and wastewater treatment: A review. Environ. Sci. Pollut. Res. 2017, 24, 27047–27069. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.; Kamal, T.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, D.; Palacios-Antón, M.E.; Santana, R.M.D.R.; Quiroz-Fernández, L.S.; Gómez-Salcedo, Y.; De Lucena, A.L.A.; Napoleão, D.C.; Rodriguez-Diaz, J.M. Comparative Study of the Degradation of the Diclofenac Drug Using Photo-Peroxidation and Heterogeneous Photocatalysis with UV-C and Solar Radiation. Water Air Soil Pollut. 2020, 231, 1–12. [Google Scholar] [CrossRef]
- Giler-Molina, J.M.; Zambrano-Intriago, L.A.; Quiroz-Fernández, L.S.; Napoleão, D.C.; dos Santos Vieira, J.; Simões Oliveira, N.; Rodríguez-Díaz, J.M. Degradation of Oxytetracycline in Aqueous Solutions: Application of Homogeneous and Heterogeneous Advanced Oxidative Processes. Sustainability 2020, 12, 8807. [Google Scholar] [CrossRef]
- Zaidan, L.E.M.C.; Sales, R.V.D.L.; Salgado, J.B.D.A.; Da Silva, A.M.R.B.; Napoleão, D.C.; Rodríguez-Díaz, J.M.; Marques, O.M.; Benachour, M.; Da Silva, V.L. Photodegradation applied to the treatment of phenol and derived substances catalyzed by TiO2/BiPO4 and biological toxicity analysis. Environ. Sci. Pollut. Res. 2017, 24, 6002–6012. [Google Scholar] [CrossRef]
- Nascimento, G.E.D.; Cavalcanti, V.O.M.; Santana, R.M.R.; Sales, D.C.S.; Rodríguez-Díaz, J.M.; Napoleão, D.; Duarte, M. Degradation of a Sunset Yellow and Tartrazine Dye Mixture: Optimization Using Statistical Design and Empirical Mathematical Modeling. Water Air Soil Pollut. 2020, 231, 1–17. [Google Scholar] [CrossRef]
- Nascimento, G.E.D.; Oliveira, M.A.S.; Santana, R.M.D.R.; Ribeiro, B.G.; Sales, D.C.S.; Rodríguez-Díaz, J.M.; Napoleão, D.; Sobrinho, M.A.D.M.; Duarte, M.M.M.B. Investigation of paracetamol degradation using LED and UV-C photo-reactors. Water Sci. Technol. 2020, 81, 2545–2558. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; van Halem, D.; Liu, G.; Lekkerkerker-Teunissen, K.; van der Hoek, J.P. Effect of residual H2O2 from advanced oxidation processes on subsequent biological water treatment: A laboratory batch study. Chemosphere 2017, 185, 637–646. [Google Scholar] [CrossRef]
- Muruganandham, M.; Suri, R.P.S.; Jafari, S.; Sillanpää, M.; Lee, G.-J.; Wu, J.; Swaminathan, M. Recent developments in homogeneous advanced oxidation processes for water and wastewater treatment. Int. J. Photoenergy 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere 2004, 55, 1235–1243. [Google Scholar] [CrossRef]
- Kumar, A.; Paliwal, M.; Ameta, R.; Ameta, S. Oxidation of fast green FCF by the solar photo-Fenton process. J. Iran. Chem. Soc. 2008, 5, 346–351. [Google Scholar] [CrossRef]
- Benincá, C.; Peralta-Zamora, P.; Camargo, R.C.; Tavares, C.R.G.; Zanoelo, E.F.; Igarashi-Mafra, L. Kinetics of oxidation of ponceau 4R in aqueous solutions by Fenton and photo-Fenton processes. React. Kinet. Mech. Catal. 2012, 105, 293–306. [Google Scholar] [CrossRef]
- Oancea, P.; Meltzer, V. Photo-Fenton process for the degradation of Tartrazine (E102) in aqueous medium. J. Taiwan Inst. Chem. Eng. 2013, 44, 990–994. [Google Scholar] [CrossRef]
- Benincá, C.; Peralta-Zamora, P.; Tavares, C.R.G.; Igarashi-Mafra, L. Degradation of an azo dye (Ponceau 4R) and treatment of wastewater from a food industry by ozonation. Ozone Sci. Eng. 2013, 35, 295–301. [Google Scholar] [CrossRef]
- Ruhul, A.; Kalam, M.; Masjuki, H.; Fattah, I.R.; Reham, S.; Rashed, M. State of the art of biodiesel production processes: A review of the heterogeneous catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Rosu, M.-C.; Coros, M.; Pogacean, F.; Magerusan, L.; Socaci, C.; Turza, A.; Pruneanu, S.M. Azo dyes degradation using TiO2-Pt/graphene oxide and TiO2-Pt/reduced graphene oxide photocatalysts under UV and natural sunlight irradiation. Solid State Sci. 2017, 70, 13–20. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Samoila, P.; Airinei, A.; Olaru, N.; Rusu, D.; Rosca, I.; Suchea, M. Photocatalytic and antimicrobial activity of electrospun ZnO: Ag nanostructures. J. Alloys Compd. 2020, 834, 155144. [Google Scholar] [CrossRef]
- Roşu, M.-C.; Socaci, C.; Floare-Avram, V.; Borodi, G.; Pogăcean, F.; Coros, M.; Măgeruşan, L.; Pruneanu, S.M. Photocatalytic performance of graphene/TiO2-Ag composites on amaranth dye degradation. Mater. Chem. Phys. 2016, 179, 232–241. [Google Scholar] [CrossRef]
- Mahdiani, M.; Soofivand, F.; Ansari, F.; Salavati-Niasari, M. Grafting of CuFe12O19 nanoparticles on CNT and graphene: Eco-friendly synthesis, characterization and photocatalytic activity. J. Clean. Prod. 2018, 176, 1185–1197. [Google Scholar] [CrossRef]
- Medina-Ramírez, I.; Hernández-Ramírez, A.; Maya-Trevino, M.L. Synthesis Methods for Photocatalytic Materials. In Photocatalytic Semiconductors; Springer: Berlin/Heidelberg, Germany, 2015; pp. 69–102. [Google Scholar]
- Azimi, S. Sol-gel synthesis and structural characterization of nano-thiamine hydrochloride structure. Int. Sch. Res. Not. 2013, 2013, 815071. [Google Scholar] [CrossRef] [Green Version]
- Khiabani, P.S.; Soeriyadi, A.; Nam, E.V.; Peterson, J.R.; Webb, J.E.; Thordarson, P.; Donald, W.; Gooding, J.J. Understanding the performance of a paper-based UV exposure sensor: The photodegradation mechanism of brilliant blue FCF in the presence of TiO2 photocatalysts in both the solid state and solution. Rapid Commun. Mass Spectrom. 2019, 33, 1076–1083. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Dhatshanamurthi, P.; Shanthi, M. Preparation and characterization of SeO2/TiO2 composite photocatalyst with excellent performance for sunset yellow azo dye degradation under natural sunlight illumination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 489–498. [Google Scholar] [CrossRef]
- Costa, L.L.; Prado, A.G. TiO2 nanotubes as recyclable catalyst for efficient photocatalytic degradation of indigo carmine dye. J. Photochem. Photobiol. A Chem. 2009, 201, 45–49. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Osugi, M.; Chanmanee, W.; Chenthamarakshan, C.; Zanoni, M.V.B.; Kajitvichyanukul, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 171–192. [Google Scholar] [CrossRef]
- Sudrajat, H.; Babel, S.; Sakai, H.; Takizawa, S. Rapid enhanced photocatalytic degradation of dyes using novel N-doped ZrO2. J. Environ. Manag. 2016, 165, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, K.; Suo, H.; Arshad, T.; Shah, Z.; Khan, S.A.; Khan, S.B.; Khan, M.N.; Liu, M.; Ma, L.; Cui, J.; et al. Metal oxides nanomaterials for the photocatalytic mineralization of toxic water wastes under solar light illumination. J. Water Process Eng. 2020, 34, 101138. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.-M.; Lu, G.Q.M. Titania-based photocatalysts—Crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.V. Catalyst Handbook; Routledge: Abingdon, UK, 2018. [Google Scholar]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Tang, L. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, N.; Zhang, Y.; Zhang, L. Novel low-cost Fenton-like layered Fe-titanate catalyst: Preparation, characterization and application for degradation of organic colorants. J. Colloid Interface Sci. 2014, 422, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kiss, F.E.; Jovanović, M.; Bošković, G.C. Economic and ecological aspects of biodiesel production over homogeneous and heterogeneous catalysts. Fuel Process. Technol. 2010, 91, 1316–1320. [Google Scholar] [CrossRef]
- Teh, C.M.; Mohamed, A.R. Roles of titanium dioxide and ion-doped titanium dioxide on photocatalytic degradation of organic pollutants (phenolic compounds and dyes) in aqueous solutions: A review. J. Alloys Compd. 2011, 509, 1648–1660. [Google Scholar] [CrossRef]
- Tekin, G.; Ersöz, G.; Atalay, S. Visible light assisted Fenton oxidation of tartrazine using metal doped bismuth oxyhalides as novel photocatalysts. J. Environ. Manag. 2018, 228, 441–450. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. Fast-growing field of magnetically recyclable nanocatalysts. Chem. Rev. 2014, 114, 6949–6985. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef] [Green Version]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [Green Version]
- Aoudjit, L.; Martins, P.; Madjene, F.; Petrovykh, D.; Lanceros-Mendez, S. Photocatalytic reusable membranes for the effective degradation of tartrazine with a solar photoreactor. J. Hazard. Mater. 2018, 344, 408–416. [Google Scholar] [CrossRef]
- Homaeigohar, S. The nanosized dye adsorbents for water treatment. Nanomaterials 2020, 10, 295. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.; Sharma, G.; Ahamad, T.; Ghfar, A.A.; Pathania, D.; Naushad, M. Efficient photocatalytic degradation of toxic dyes from aqueous environment using gelatin-Zr (IV) phosphate nanocomposite and its antimicrobial activity. Colloids Surf. B Biointerfaces 2017, 157, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Karkmaz, M.; Puzenat, E.; Guillard, C.; Herrmann, J. Photocatalytic degradation of the alimentary azo dye amaranth: Mineralization of the azo group to nitrogen. Appl. Catal. B Environ. 2004, 51, 183–194. [Google Scholar] [CrossRef]
- Orooji, Y.; Mohassel, R.; Amiri, O.; Sobhani, A.; Salavati-Niasari, M. Gd2ZnMnO6/ZnO nanocomposites: Green sol-gel auto-combustion synthesis, characterization and photocatalytic degradation of different dye pollutants in water. J. Alloys Compd. 2020, 835, 155240. [Google Scholar] [CrossRef]
- Taylor, M.G.; Austin, N.; Gounaris, C.E.; Mpourmpakis, G. Catalyst design based on morphology-and environment-dependent adsorption on metal nanoparticles. ACS Catal. 2015, 5, 6296–6301. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W. Morphology-dependent nanocatalysts: Rod-shaped oxides. Chem. Soc. Rev. 2014, 43, 1543–1574. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Mori, K.; Kuwahara, Y.; Raja, R.; Yamashita, H. Plasmonic nanocatalysts for visible-NIR light induced hydrogen generation from storage materials. Mater. Adv. 2021, 2, 880–906. [Google Scholar] [CrossRef]
- Bahadori, E.; Vaiano, V.; Esposito, S.; Armandi, M.; Sannino, D.; Bonelli, B. Photo-activated degradation of tartrazine by H2O2 as catalyzed by both bare and Fe-doped methyl-imogolite nanotubes. Catal. Today 2018, 304, 199–207. [Google Scholar] [CrossRef]
- Darwish, M.; Mohammadi, A.; Assi, N. Microwave-assisted polyol synthesis and characterization of pvp-capped cds nanoparticles for the photocatalytic degradation of tartrazine. Mater. Res. Bull. 2016, 74, 387–396. [Google Scholar] [CrossRef]
- Nazim, M.; Khan, A.A.P.; Asiri, A.M.; Kim, J.H. Exploring Rapid Photocatalytic Degradation of Organic Pollutants with Porous CuO Nanosheets: Synthesis, Dye Removal, and Kinetic Studies at Room Temperature. ACS Omega 2021, 6, 2601–2612. [Google Scholar] [CrossRef]
- Zhang, T.; Souza, I.P.; Xu, J.; Almeida, V.C.; Asefa, T. Mesoporous graphitic carbon nitrides decorated with cu nanoparticles: Efficient photocatalysts for degradation of tartrazine yellow dye. Nanomaterials 2018, 8, 636. [Google Scholar] [CrossRef] [Green Version]
- Alcantara-Cobos, A.; Gutiérrez-Segura, E.; Solache-Ríos, M.; Amaya-Chávez, A.; Solís-Casados, D. Tartrazine removal by ZnO nanoparticles and a zeolite-ZnO nanoparticles composite and the phytotoxicity of ZnO nanoparticles. Microporous Mesoporous Mater. 2020, 302, 110212. [Google Scholar] [CrossRef]
- Rao, M.P.; Wu, J.J.; Asiri, A.M.; Anandan, S. Photocatalytic degradation of tartrazine dye using CuO straw-sheaf-like nanostructures. Water Sci. Technol. 2017, 75, 1421–1430. [Google Scholar] [CrossRef] [Green Version]
- Türkyılmaz, Ş.Ş.; Güy, N.; Özacar, M. Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals. J. Photochem. Photobiol. A Chem. 2017, 341, 39–50. [Google Scholar] [CrossRef]
- Balu, S.; Velmurugan, S.; Palanisamy, S.; Chen, S.-W.; Velusamy, V.; Yang, T.C.; El-Shafey, E.-S.I. Synthesis of α-Fe2O3 decorated g-C3N4/ZnO ternary Z-scheme photocatalyst for degradation of tartrazine dye in aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 99, 258–267. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, Y.; Dai, W.; Luo, X. Highly Efficient Degradation of Tartrazine with a Benzoic Acid/TiO2 System. ACS Omega 2019, 4, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.d.; Semkiw, A.W.; Monteiro, F.C.; Weinert, P.L.; Monteiro, J.F.H.L.; Fujiwara, S.T. Synthesis of CuCr2O4 by self-combustion method and photocatalytic activity in the degradation of Azo Dye with visible light. J. Photochem. Photobiol. A Chem. 2020, 401, 112797. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Green synthesis of biogenic silver nanoparticles for efficient catalytic removal of harmful organic dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, A.; Blasco, A.; Johnston, P.F.; Miranda, M.A.; Marin, M.L. Enhanced photodegradation of synthetic dyes mediated by Ag3PO4-based semiconductors under visible light irradiation. Catalysts 2020, 10, 774. [Google Scholar] [CrossRef]
- Marković, M.; Marinović, S.; Mudrinić, T.; Ajduković, M.; Jović-Jovičić, N.; Mojović, Z.; Orlić, J.; Milutinović-Nikolić, A.; Bankovic, P. Co (II) impregnated Al (III)-pillared montmorillonite–Synthesis, characterization and catalytic properties in Oxone® activation for dye degradation. Appl. Clay Sci. 2019, 182, 105276. [Google Scholar] [CrossRef]
- Vu, A.-T.; Xuan, T.N.; Lee, C.-H. Preparation of mesoporous Fe2O3• SiO2 composite from rice husk as an efficient heterogeneous Fenton-like catalyst for degradation of organic dyes. J. Water Process Eng. 2019, 28, 169–180. [Google Scholar] [CrossRef]
- Ajduković, M.; Stojadinović, S.; Marinović, S.; Milutinović-Nikolić, A.; Dojčinović, B.; Banković, P. Activation of Oxone® with plasma deposited mixed cobalt and alumina oxide for the dye degradation. Appl. Surf. Sci. 2020, 503, 144144. [Google Scholar] [CrossRef]
- Souza, I.P.; Crespo, L.H.; Spessato, L.; Melo, S.A.; Martins, A.F.; Cazetta, A.L.; Almeida, V.C. Optimization of thermal conditions of sol-gel method for synthesis of TiO2 using RSM and its influence on photodegradation of tartrazine yellow dye. J. Environ. Chem. Eng. 2021, 9, 104753. [Google Scholar] [CrossRef]
- Guo, R.; Wang, Y.; Li, J.; Cheng, X.; Dionysiou, D.D. Sulfamethoxazole degradation by visible light assisted peroxymonosulfate process based on nanohybrid manganese dioxide incorporating ferric oxide. Appl. Catal. B Environ. 2020, 278, 119297. [Google Scholar] [CrossRef]
- Khorasanizadeh, M.H.; Ghiyasiyan-Arani, M.; Monsef, R.; Salavati-Niasari, M.; Moayedi, H. Ultrasound-accelerated synthesis of uniform DyVO4 nanoparticles as high activity visible-light-driven photocatalyst. Ultrason. Sonochem. 2019, 59, 104719. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Morassaei, M.S.; Salavati-Niasari, M. Eco-friendly synthesis of Nd2Sn2O7–based nanostructure materials using grape juice as green fuel as photocatalyst for the degradation of erythrosine. Compos. Part B Eng. 2019, 167, 643–653. [Google Scholar] [CrossRef]
- Mazloom, F.; Ghiyasiyan-Arani, M.; Monsef, R.; Salavati-Niasari, M. Photocatalytic degradation of diverse organic dyes by sol–gel synthesized Cd 2 V 2 O 7 nanostructures. J. Mater. Sci. Mater. Electron. 2018, 29, 18120–18127. [Google Scholar] [CrossRef]
- Sakir, M.; Salem, S.; TapanSanduvacae, S.; Sahmetliogluaf, E.; Sarp, G.; SerdarOnsesac, M.; Yilmazabd, E. Photocatalytic green fabrication of Au nanoparticles on ZnO nanorods modified membrane as flexible and photocatalytic active reusable SERS substrates. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124088. [Google Scholar] [CrossRef]
- Soofivand, F.; Esmaeili, E.; Sabet, M.; Salavati-Niasari, M. Simple synthesis, characterization and investigation of photocatalytic activity of NiS 2 nanoparticles using new precursors by hydrothermal method. J. Mater. Sci. Mater. Electron. 2018, 29, 858–865. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Salehi, Z.; Salavati-Niasari, M. Green synthesis of Dy2Ce2O7 ceramic nanostructures using juice of Punica granatum and their efficient application as photocatalytic degradation of organic contaminants under visible light. Ceram. Int. 2018, 44, 3873–3883. [Google Scholar] [CrossRef]
- Shirzadi-Ahodashti, M.; Ebrahimzadeh, M.A.; Amiri, O.; Naghizadeh, A.; Mortazavi-Derazkola, S. Novel NiFe/Si/Au magnetic nanocatalyst: Biogenic synthesis, efficient and reusable catalyst with enhanced visible light photocatalytic degradation and antibacterial activity. Appl. Organomet. Chem. 2020, 34, e5467. [Google Scholar] [CrossRef]
- Hunge, Y.; Yadav, A.; Mathe, V. Ultrasound assisted synthesis of WO3-ZnO nanocomposites for brilliant blue dye degradation. Ultrason. Sonochem. 2018, 45, 116–122. [Google Scholar] [CrossRef]
- Nikravesh, B.; Shomalnasab, A.; Nayyer, A.; Aghababaei, N.; Zarebi, R.; Ghanbari, F. UV/Chlorine process for dye degradation in aqueous solution: Mechanism, affecting factors and toxicity evaluation for textile wastewater. J. Environ. Chem. Eng. 2020, 8, 104244. [Google Scholar] [CrossRef]
- Naik, A.P.; Mittal, H.; Wadi, V.K.S.; Sane, L.; Raj, A.; Alhassan, S.; Al Alili, A.; Bhosale, S.V.; Morajkar, P.P. Super porous TiO2 photocatalyst: Tailoring the agglomerate porosity into robust structural mesoporosity with enhanced surface area for efficient remediation of azo dye polluted waste water. J. Environ. Manag. 2020, 258, 110029. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.; Kansal, S.K. Enhanced visible light driven photocatalytic application of Ag2O decorated ZnO nanorods heterostructures. Sep. Purif. Technol. 2017, 183, 341–349. [Google Scholar] [CrossRef]
- Zhang, M.-W.; Lin, K.-Y.A.; Huang, C.-F.; Tong, S. Enhanced degradation of toxic azo dye, amaranth, in water using Oxone catalyzed by MIL-101-NH2 under visible light irradiation. Sep. Purif. Technol. 2019, 227, 115632. [Google Scholar] [CrossRef]
- Zhang, M.-W.; Yang, M.-T.; Tong, S.; Lin, K.-Y.A. Ferrocene-modified iron-based metal-organic frameworks as an enhanced catalyst for activating oxone to degrade pollutants in water. Chemosphere 2018, 213, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-H.; Lin, K.-Y.A.; Yang, M.-T.; Thanh, B.X.; Tsang, D.C. Prussian Blue Analogue-derived co/fe bimetallic nanoparticles immobilized on S/N-doped carbon sheet as a magnetic heterogeneous catalyst for activating peroxymonosulfate in water. Chemosphere 2020, 244, 125444. [Google Scholar] [CrossRef] [PubMed]
- Balu, S.; Chen, Y.-L.; Yang, T.C.-K.; Chen, J.-N.; Chen, S.-W. Effect of ultrasound-induced hydroxylation and exfoliation on P90–TiO2/g-C3N4 hybrids with enhanced optoelectronic properties for visible-light photocatalysis and electrochemical sensing. Ceram. Int. 2020, 46, 18002–18018. [Google Scholar] [CrossRef]
- Feizi, R.; Ahmad, M.; Jorfi, S.; Ghanbari, F. Sunset yellow degradation by ultrasound/peroxymonosulfate/CuFe2O4: Influential factors and degradation processes. Korean J. Chem. Eng. 2019, 36, 886–893. [Google Scholar] [CrossRef]
- Deepika, S.; Harishkumar, R.; Dinesh, M.; Abarna, R.; Anbalagan, M.; Roopan, S.M.; Selvaraj, C.I. Photocatalytic degradation of synthetic food dye, sunset yellow FCF (FD&C yellow no. 6) by Ailanthus excelsa Roxb. possessing antioxidant and cytotoxic activity. J. Photochem. Photobiol. B Biol. 2017, 177, 44–55. [Google Scholar] [CrossRef]
- Hassanien, R.; Abed-Elmageed, A.A.; Husein, D.Z. Eco-Friendly Approach to Synthesize Selenium Nanoparticles: Photocatalytic Degradation of Sunset Yellow Azo Dye and Anticancer Activity. ChemistrySelect 2019, 4, 9018–9026. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Bernardino, T.S.; Junior, F.E.; Lanza, M.R.; Barros, W.R. Enhanced electrodegradation of the Sunset Yellow dye in acid media by heterogeneous Photoelectro-Fenton process using Fe3O4 nanoparticles as a catalyst. J. Environ. Chem. Eng. 2020, 8, 103621. [Google Scholar] [CrossRef]
- Xia, L.; Jiang, X.; Cheng, Z.; Liao, Y.; Wang, Z.; Pu, Q.; Duan, M. Synthesis of Pp-16@ Ag/AgCl of high performance photocatalyst particles for decomposition of Rhodamine B and fast green dyes. Mater. Chem. Phys. 2018, 218, 98–107. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Al-Saeedi, S.I.; Al-Senani, G.M.; Nafady, A.; Ahamad, T.; Naushad, M.; Stadler, F.J. Fabrication of oxidized graphite supported La2O3/ZrO2 nanocomposite for the photoremediation of toxic fast green dye. J. Mol. Liq. 2019, 277, 738–748. [Google Scholar] [CrossRef]
- Muhambihai, P.; Rama, V.; Subramaniam, P. Photocatalytic degradation of aniline blue, brilliant green and direct red 80 using NiO/CuO, CuO/ZnO and ZnO/NiO nanocomposites. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100360. [Google Scholar] [CrossRef]
- Sharma, G.; Bhogal, S.; Gupta, V.K.; Agarwal, S.; Kumar, A.; Pathania, D.; Mola, G.T.; Stadler, F.J. Algal biochar reinforced trimetallic nanocomposite as adsorptional/photocatalyst for remediation of malachite green from aqueous medium. J. Mol. Liq. 2019, 275, 499–509. [Google Scholar] [CrossRef]
- Rane, Y.; Shende, D.; Raghuwanshi, M.; Koli, R.; Gosavi, S.; Deshpande, N. Visible-light assisted CdO nanowires photocatalyst for toxic dye degradation studies. Optik 2019, 179, 535–544. [Google Scholar] [CrossRef]
- Agorku, E.S.; Mamo, M.A.; Mamba, B.B.; Pandey, A.C.; Mishra, A.K. Palladium-decorated zinc sulfide/reduced graphene oxide nanocomposites for enhanced visible light-driven photodegradation of indigo carmine. Mater. Sci. Semicond. Process. 2015, 33, 119–126. [Google Scholar] [CrossRef]
- Rekhila, G.; Saidani, A.; Hocine, F.; Hariz, S.H.B.; Trari, M. Characterization of the hetero-system ZnCo2O4/ZnO prepared by sol gel: Application to the degradation of Ponceau 4R under solar light. Appl. Phys. A 2020, 126, 1–8. [Google Scholar] [CrossRef]
- Mossmann, A.; Dotto, G.L.; Hotza, D.; Jahn, S.L.; Foletto, E.L. Preparation of polyethylene–supported zero–valent iron buoyant catalyst and its performance for Ponceau 4R decolorization by photo–Fenton process. J. Environ. Chem. Eng. 2019, 7, 102963. [Google Scholar] [CrossRef]
- Barrera, H.; Cruz-Olivares, J.; Frontana-Uribe, B.A.; Gómez-Díaz, A.; Reyes-Romero, P.G.; Barrera-Diaz, C.E. Electro-Oxidation–Plasma Treatment for Azo Dye Carmoisine (Acid Red 14) in an Aqueous Solution. Materials 2020, 13, 1463. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.K.; Chattopadhyaya, M.C. Chapter 6—Sorption of Dyes on Graphene-Based Nanocomposites. In Nanomaterials for Wastewater Remediation; Gautam, R.K., Chattopadhyaya, M.C., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2016; pp. 111–138. [Google Scholar]
- Wang, C.; Astruc, D. Recent developments of metallic nanoparticle-graphene nanocatalysts. Prog. Mater. Sci. 2018, 94, 306–383. [Google Scholar] [CrossRef]
- Gan, Z.; Wu, X.; Meng, M.; Zhu, X.; Yang, L.; Chu, P.K. Photothermal contribution to enhanced photocatalytic performance of graphene-based nanocomposites. ACS Nano 2014, 8, 9304–9310. [Google Scholar] [CrossRef]
- Singh, P.; Shandilya, P.; Raizada, P.; Sudhaik, A.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A. Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab. J. Chem. 2020, 13, 3498–3520. [Google Scholar] [CrossRef]
- Viciano-Chumillas, M.; Mon, M.; Ferrando-Soria, J.; Corma, A.; Leyva-Pérez, A.; Armentano, D.; Pardo, E. Metal–organic frameworks as chemical nanoreactors: Synthesis and stabilization of catalytically active metal species in confined spaces. Acc. Chem. Res. 2020, 53, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Zhou, J.; Xie, Z.; Kuang, Q.; Zheng, L. A nano-reactor based on PtNi@ metal–organic framework composites loaded with polyoxometalates for hydrogenation–esterification tandem reactions. Nanoscale 2019, 11, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazardous Mater. 2020, 404, 124082. [Google Scholar] [CrossRef]
- Gautam, P.K.; Singh, A.; Misra, K.; Sahoo, A.K.; Samanta, S.K. Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment. J. Environ. Manag. 2019, 231, 734–748. [Google Scholar] [CrossRef]
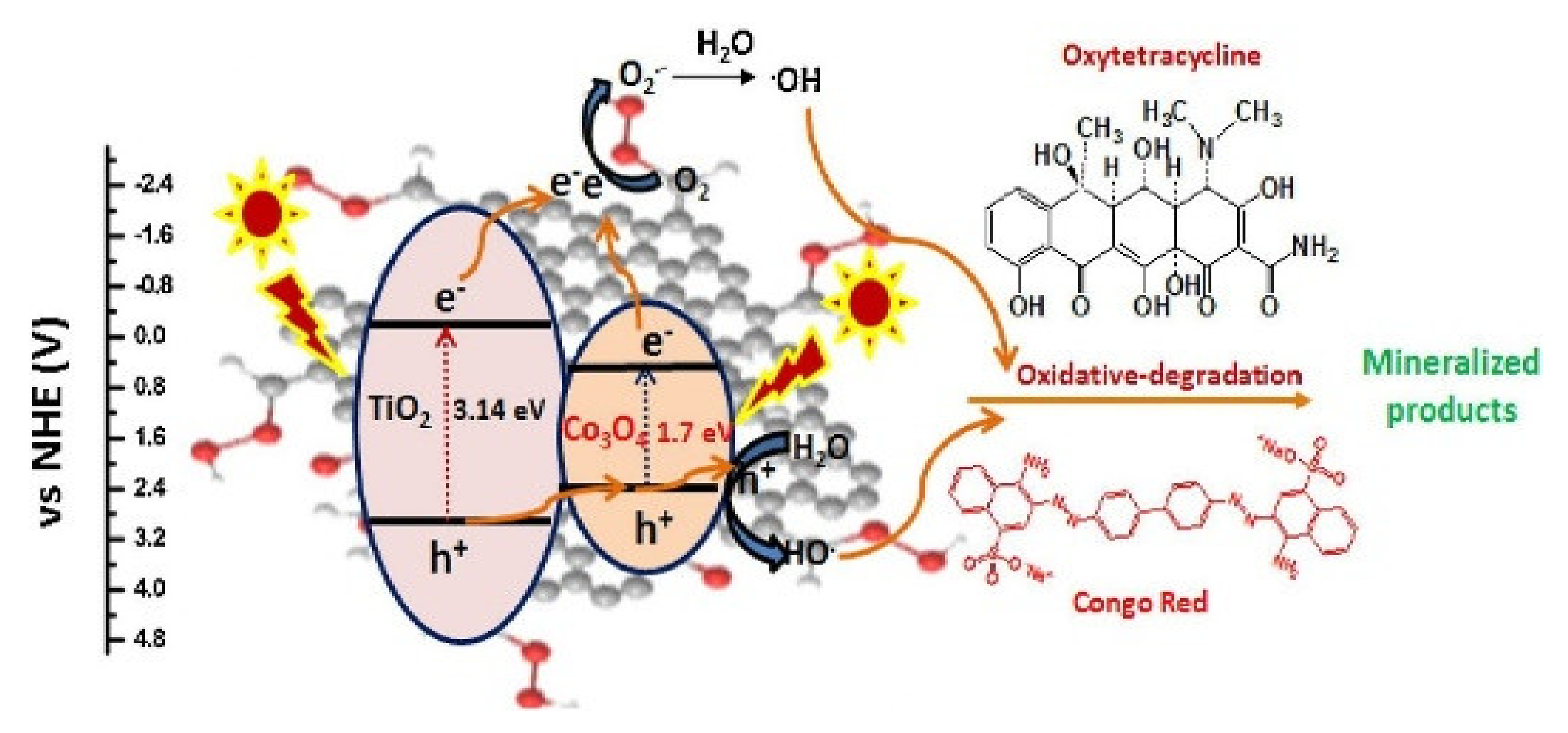
- Jo, W.-K.; Kumar, S.; Isaacs, M.A.; Lee, A.F.; Karthikeyan, S. Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl. Catal. B Environ. 2017, 201, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Xu, P.; Wang, H.; Zeng, G.; Huang, D.; Chen, M.; Lai, C.; Zhang, C.; Wan, J.; Xue, W. Strategies to improve metal organic frameworks photocatalyst’s performance for degradation of organic pollutants. Coord. Chem. Rev. 2018, 376, 449–466. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, D.; Shen, T.; Hou, X.; Zhu, M.; Liu, S.; Hu, Q. Titanium dioxide/magnetic metal-organic framework preparation for organic pollutants removal from water under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124484. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Wang, N.; Guo, P.; Muhler, M.; Wang, Y.; Lin, S.; Chen, Z.; Yang, G. Highly efficient photocatalytic degradation of dyes by a copper–triazolate metal–organic framework. Chem.–A Eur. J. 2018, 24, 16804–16813. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I.; Sultan, M.; Ali, M.; Abbasi, I.A.; Islam, T.; Ye, T. Overview of microbes based fabricated biogenic nanoparticles for water and wastewater treatment. J. Environ. Manag. 2019, 230, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Trimm, D. The regeneration or disposal of deactivated heterogeneous catalysts. Appl. Catal. A Gen. 2001, 212, 153–160. [Google Scholar] [CrossRef]
- Lu, S.; Liu, L.; Demissie, H.; An, G.; Wang, D. Design and application of metal-organic frameworks and derivatives as heterogeneous Fenton-like catalysts for organic wastewater treatment: A review. Environ. Int. 2021, 146, 106273. [Google Scholar] [CrossRef] [PubMed]



| Type of Structure | Name | 2D Chemical Structure | Color | E-Number | * ADI | FD&C | Application | Hazardous Effect | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Azo | Brown FK | 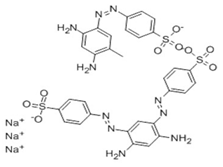 | Orange-brown | E 154 | 0.14 | None | Single-use for coloring herring | Azo dyes, in general, can produce hyperactivity, restlessness, and sleep disturbances in children; when the dyes are combined with other food additives, such as sodium benzoate, it could lead to possible neurodevelopmental impairment and carcinogenic properties. | [21,22] |
| Monoazo | Sunset Yellow FCF |  | Yellow-orange | E 110 | 4 | Yellow No. 6 | Soft drinks, confectionery, jams, jellies, cookies, ice cream, potato chips, instant pasta, food decorations, and coatings. | [22,23] | |
| Carmoisine |  | Red to maroon | E 122 | 4 | None | Non-alcoholic flavored beverages. Confectionery products. Yogurt. | [22,24] | ||
| Amaranth |  | Red | E 123 | 0.15 | None | Alcoholic beverages. Candied fruits. | [22,25] | ||
| Ponceau 4R |  | Red-orange | E 124 | 0.7 | None | Soft drinks. Confectionery. Coatings. Cookies and ice cream. Fine bakery products. | [22] | ||
| Allura Red AC |  | Yellowish red | E 129 | 7 | Red No. 40 | Potato chips. Cookies, candies, ice cream, and jams. Non-alcoholic flavored drinks and yogurts. | [22,26] | ||
| Lithol Rubine BK |  | Red-blue | E 180 | 1.5 | Prohibited | Cheese surface coating. | [27] | ||
| Citrus red | 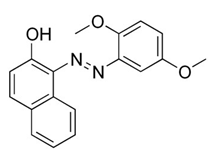 | Scarlet red | Prohibited | - | Red No. 2 | Orange peel coating. | [28] | ||
| Diazo | Brilliant Black BN |  | Blue-black | E 151 | 1–5 | None | Confectionery products. Decorations and coatings. Fine bakery products. Ice cream and desserts. Soft drinks, spirits, fruit wines, cider, among others. | Clastogenic and/or aneugenic properties, damage DNA. | [22,29] |
| Brown HT |  | Dark brown | E 155 | 1.5 | None | Possible reduction of body weight and cholesterol (HDL). Increase of hepatic enzymes in the blood. Negative effects on hypothalamic-pituitary-testicular axis function. | [22,30,31] | ||
| Triarylmethane | Patent Blue V | 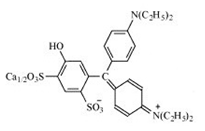 | Violet blue | E 131 | 5 | None | Surface coating of cheese and edible casings. Alcoholic beverages, soft drinks, and other food products. | Anaphylactic reactions Alterations of hematological parameters. | [32] |
| Brilliant Blue FCF | 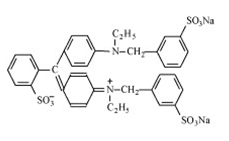 | Greenish blue | E 133 | 6 | Blue No.1 | Dairy products. Sweets. Beverages. | Carcinogenic effect. | [33,34] | |
| Green S | 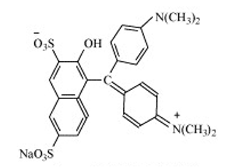 | Blue-green | E 142 | 5 | None | Confectionery products. Decorations and coatings. Ice cream, desserts. Processed peas. | Mild anemia (transient), increased cecal weight, thyroid degeneration, enlarged lymph nodes in the intestinal wall. | [35] | |
| Fast Green FCF |  | Blue-green | Prohibited | - | Green No. 3 | Peas. Vegetables. Fish. Desserts, dry bakery mixes, and sauces. | Chromosomal aberration Inhibition of neurotransmitter release. | [34,36] | |
| Azopyrazolone | Tartrazine |  | Yellow | E 102 | 7.5 | Yellow No.5 | Desserts and sweets. French fries. Muesli, corn flakes, noodles, tartar sauce, mustard, and bouillon cubes. Energy drinks | Migraine. Asthma. Skin conditions (urticaria). | [22,36] |
| Xanthene | Erythrosine | 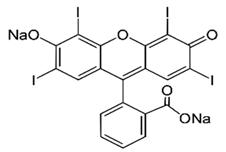 | Blue pink | E 127 | 0.1 | Red No.3 | Cocktails. Candied cherries. | Oncogenic effect on the rat thyroid gland. | [28,37] |
| Quinoline | Quinoline Yellow WS | 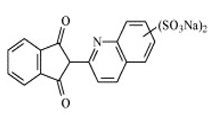 | Yellow-green | E 104 | 0.5 | None | Sports and energy drinks. Ice cream and confectionery. | Urticaria. Rhinitis. | [36,38] |
| Indigo | Indigo carmine | 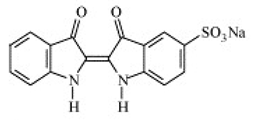 | Deep blue | E 132 | 5 | Blue No. 2 | Tablets and capsules. Coatings. Ice cream, cookies, candies. Confectionery. | Asthma. | [39] |
| AOP | Source of Radicals OH | Advantages | Disadvantages |
|---|---|---|---|
| Electrochemical oxidation | Electricity, 2–20 A (water electrolysis) | High-energy efficiency. Operates at ambient conditions. | Selective |
| Sonochemical oxidation | Ultrasound 20 kHz–2 MHz (water sonolysis) | Does not require the addition of chemicals or catalysts. Operates at ambient conditions. | It needs to be combined with other oxidative processes to improve its degradation performance. |
| Sonoelectrchemistry | Electricity, 2–20A Ultrasound 20 kHz–2 MHz | Fast reaction rate. Guaranteed degradation efficiency. Increased energy efficiency. | Greater emphasis on the frequency and acoustic power, electrode positioning, cell geometry, and the distance between the tip of the ultrasonic horn and the electrode. |
| Ozonation | O3 O3/UV O3/H2O2 O3/H2O2/UV | Increased color removal. No sludge or hazardous by-products. In situ O3 generation. Effective at basic pH (lower environmental risk). Treatment is more efficient when combined with H2O2 and/or UV. Complete mineralization with O3/H2O2 system. | High equipment cost and energy requirements. Mass transfer is the limiting factor of the process. Formation of bromate (carcinogen) by effluents with an excess of bromide ions. |
| Processes based on H2O2 | H2O2/UV | The formation of bromate is suppressed. | Lower mineralization rate. |
| H2O2/Fe2+ (Fenton) | Lower energy consumption than ozone. Does not form bromates. | Expensive. The strict control of H2O2/Fe2+ ratio. Continuous addition of ferrous ions (high sludge deposition). An acidic medium is required for OH radical generation. pH 3 is the optimum operating pH (Fe(OH)3 precipitation at pH > 4. | |
| H2O2/Fe3+ (Fenton-like) H2O2/Fe3+/UV (photo-Fenton-like) | Using Fe3+ ion is less expensive than Fe2+. | Slow process. Limited applicability in environmental technologies. | |
| H2O2/Fe2+/UV (photo-Fenton) | The addition of UV light improves the degradation rate. Cyclic process, regeneration of ferrous ion (Fe2+) by photo-reduction of ferric ions. Sludge formation is minimal. | The efficiency of the reaction depends on the lamp power and type of light. | |
| Electro-Fenton | Efficient for complete degradation and mineralization of synthetic and real wastewaters. | Optimal at strong acid pH (pH 2.8–3.5). Non-recyclability of the catalyst used. | |
| Photoelectro-Fenton | |||
| Heterogeneous photocatalysis | TiO2/ZnO/CdS + UV TiO2/UV/H2O2 | More efficient than homogeneous catalysis. High availability and cost-effectiveness of photocatalysts. Non-selective catalytic activity. Applicability to a wide pH range. Does not generate sludge or toxic compounds. | Limited application to inorganic environments. Low chemical stability of the photocatalyst. High-energy consumption. Rapid deactivation/poisoning of the surface-active site. Spent photocatalyst is disposed of as secondary solid waste. |
| O3/catalyst/light (Photocatalytic Ozonation) |
| Process | Dye | C0 | Catalyst | Dose C | Preparation Method | t | R | D | R.C | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Fenton photooxidation | Tartrazine | 50 | Cu-(BiOCl) with H2O2 | 0.5 | Coprecipitation | 90 | Visible | 91 | 1 | [87] |
| Photocatalytic | Tartrazine | 10 | 8% TiO2 (PVDF-TrFE) | 0.9 | - | 300 | Solar | 78 | 2 | [91] |
| Photocatalytic | Tartrazine | 50 | MRG-TiO2 50 | 0.2 | Coprecipitation | 180 | Visible | 95 | 4 | [3] |
| Photocatalytic | Tartrazine | 10 | - (mpg-C3N4) | 0.5 | Pyrolysis | 240 | UV | 81.9 | - | [102] |
| Adsorption-photocatalysis | Tartrazine | 10 | Ze-nanZnO | 0.1 | Chemical precipitation | 900 | UV | 87 | - | [103] |
| Photocatalytic | Tartrazine | 16 | CuO | 0.25 | Chemical precipitation | 240 | Visible | 90 | - | [104] |
| Photo-Fenton | Tartrazine | 40 | MeIMO-Fe 0.025 with H2O2 | 0.25 | Ion exchange | 240 | UV | 100 | 5 | [99] |
| Photocatalytic | Tartrazine | 25 | ZnO doped Ni | 0.1 | Hydrothermal | 60 | UV | 98.2 | - | [105] |
| Photocatalytic | Tartrazine | 10 | C3N4/ZnO @ α-Fe2O3 | 0.5 | Direct pyrolysis and sol–gel | 35 | UV | 99.34 | 3 | [106] |
| Photocatalytic | Tartrazine | 20 | Benzoic acid/TiO2 | 0.1 | - | 70 | Visible | 99.08 | 4 | [107] |
| Photocatalytic | Tartrazine | 50 | CuCr2O4 | 0.5 | Auto-combustion | 120 | Visible | 99.6 | 4 | [108] |
| Catalytic | Tartrazine | 100 | AgNP | 60 ug | Green route | 15 | - | 26 | 6 | [109] |
| Photocatalytic | Tartrazine | 25 | semiconductors in Ag3PO4 | 0.025 | - | 120 | Visible | 100 | - | [110] |
| Catalytic | Tartrazine | 50 | Co(II) impregnated Al(III)- | 0.1 | Incipient wetness (CoAP) | 10 | - | 100 | - | [111] |
| Heterogeneous Fenton | Tartrazine | 50 | Fe2O3 · SiO2 | 0.5 | Easy impregnation | 80 | - | 98.5 | 5 | [112] |
| Catalytic | Tartrazine | 25 | Al2O3 doped with cobalt | 0.025 | Electrolytic oxidation by plasma | 180 | - | 100 | 5 | [113] |
| Photocatalytic | Tartrazine | 10 | TPt-GO | 0.2 | Chemical–thermal | 180 | Solar | 96.23 | - | [67] |
| Photocatalyst | Tartrazine | 50mg/L | NP TiO2 | 0.5 | Sol–gel | 220 | UV | 83 | 1 | [114] |
| Photocatalyst | Tartrazine | 10 | Fe2O3 (MF) | 0.6 | 25 | Visible | 97.1 | 5 | [115] | |
| Photocatalytic | Erythrosine | 5 | DyVO4 | 0.05 | Sonochemical | 180 | UV | 88 | 5 | [116] |
| Photocatalytic | Erythrosine | 20 | Graphene -NCs | 0.02 | Ecological sol–gel | 50 | UV | 88.2 | - | [70] |
| Photocatalytic | Erythrosine | 1.2 | Nd2Sn2O7- Nd2O3 | 0.05 | Ecological synthesis | 60 | UV | 96 | 8 | [117] |
| Photocatalytic | Erythrosine | 45 | Cd2V2O7 | 0.05 | Sol–gel | 90 | UV | 67 | 3 | [118] |
| Photocatalytic | Erythrosine | 10 | AuNPs @ ZnO | 0.05 | Chemical hydrothermal | 30 | UV | 100 | 6 | [119] |
| Photocatalytic | Erythrosine | 20 | NiS2 | 0.02 | Hydrothermal | 75 | UV | 57 | - | [120] |
| Photocatalytic | Erythrosine | 1.5 | Dy2Ce2O7 | 0.06 | Green Synthesis | 70 | Visible | 91.4 | 8 | [121] |
| Photocatalytic | Erythrosine | 8 | NiFe2O4/SiO2/Au (NiFe/Si/Au) | 0.2 | Sonochemical | 80 | UV and Visible | 97.3 | 7 | [122] |
| Sonocatalytic | Brilliant blue | 396 | WO3-ZnO | 0.2 | Sonochemical | 40 | Ultrasonic | 90 | 6 | [123] |
| Catalytic | Brilliant blue | 100 | AgNP Viburnum opulus L. | 60 ug | Green Synthesis | 15 | - | 97 | 6 | [109] |
| Photocatalytic | Brilliant blue | 25 | Chlorine | 0.6 Mm | - | 30 | UV | 95 | - | [124] |
| Photocatalytic | Brilliant blue | 25 | semiconductors in Ag3PO4 | 2.5 | - | 120 | Visible | 90 | - | [110] |
| Photocatalytic | Amaranth | 25 | ZnO: Ag 1% | 0.16 | Electrospinning | 600 | Visible | 98.4 | - | [68] |
| Photocatalytic | Amaranth | 25 | ZnO: Ag 1% | 0.16 | Electrospinning | 600 | UV | 95.9 | - | [68] |
| Photocatalytic | Amaranth | 30 | superporous TiO2 | 0.3 | Sol–gel | 15 | UV | 95.6 | 5 | [125] |
| Photocatalytic | Amaranth | 25 | ZnO decorated with Ag2O | 1 | Ultrasound-assisted precipitation | 30 | Visible | 94 | - | [126] |
| Photocatalytic | Amaranth | 12 | TPt-GO | 0.2 | Chemical-thermal | 180 | Solar | 99.56 | - | [67] |
| Photocatalytic | Amaranth | 50 | MIL-N catalyzed oxone | 0.1 | - | 30 | Visible | 100 | - | [127] |
| Catalytic | Amaranth | 50 | (MOF) Fe, MIL-101 | 0.2 | Schiff’s base reaction | 60 | - | 100 | 5 | [128] |
| Catalytic | Amaranth | 50 | Bimetallic Co/Fe nanoparticles | 0.05 | Carbonization | 20 | - | 100 | 5 | [129] |
| Photocatalytic | Sunset Yellow | 9 | TPt-GO | 0.2 | Chemical-thermal | 180 | Solar | 99.15 | - | [67] |
| Photocatalytic | Sunset Yellow | 10 | P90/CN at 40% | 0.1 | Sonication | 5 | Solar simulated | 98.8 | - | [130] |
| Sonochemical oxidation | Sunset Yellow | 50 | peroxymonosulfate/CuFe2O4 | 0.025 | Coprecipitation | 30 | Ultrasonic | 95.8 | - | [131] |
| Photocatalytic | Sunset Yellow | 10 | Ailanthus excelsa Roxb | 7 | Green synthesis | 30 | UV | 55 | - | [132] |
| Photocatalytic | Sunset Yellow | 40 | Selenium NP-Drumstick | 0.3 | Green synthesis | 180 | Solar | 83.8 | - | [133] |
| photoelectron-Fenton | Sunset Yellow | 100 | NP de Fe3O4 | 0.25 | Coprecipitation | 90 | Solar | 100 | 8 | [134] |
| Photocatalytic | Fast green | 10 | Pp-16 @ Ag/AgCl(1:40) | 0.1 | In situ polymerization | 20 | UV | 99 | 4 | [135] |
| Photocatalytic | Fast green | 30 | OG/La2O3/ZrO2 | 0.05 | Coprecipitation | 90 | Visible | 89 | 5 | [136] |
| Photocatalytic | Brilliant green | 50 | NiO/CuO | 0.09 | - | 60 | Solar | 82 | 3 | [137] |
| Photocatalytic | Malachite green | 0.73 | AlBc @La/Cu/Zr | 0.1 | Microwave | 240 | Solar | 94 | 5 | [138] |
| Photocatalytic | Indigo carmine | 10 | CdO nanowires | - | Mild chemical pathway | 270 | Visible | 30 | - | [139] |
| Photocatalytic | Indigo carmine | 20 | Pd-ZnS/rGO | 1.0 | Co-precipitation | 210 | UV | 100 | - | [140] |
| Photocatalytic | Ponceau 4R | 10 | CdO nanowires | - | Mild chemical pathway | 270 | Visible | 39 | - | [139] |
| Photoelectrochemical | Ponceau 4R | 15 | ZnCo2O4/ZnO | 0.125 | Sol–gel | 220 | Solar | 70 | - | [141] |
| photo-Fenton | Ponceau 4R | 50 | Floating catalyst ZVI supported with LDPE | 0.072 | Thermal fixation | 15 | UV | 100 | 4 | [142] |
| Catalytic | Carmoisine | 100 | (AgNP) | 60 ug | Green route | 15 | - | 48 | 6 | [109] |
| Electrochemical | Carmoisine | 5 | electro-oxidation-plasma electrode BBD | 0.045 Mm | Electro-oxidation-plasma | 60 | - | 100 | - | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navia-Mendoza, J.M.; Filho, O.A.E.; Zambrano-Intriago, L.A.; Maddela, N.R.; Duarte, M.M.M.B.; Quiroz-Fernández, L.S.; Baquerizo-Crespo, R.J.; Rodríguez-Díaz, J.M. Advances in the Application of Nanocatalysts in Photocatalytic Processes for the Treatment of Food Dyes: A Review. Sustainability 2021, 13, 11676. https://doi.org/10.3390/su132111676
Navia-Mendoza JM, Filho OAE, Zambrano-Intriago LA, Maddela NR, Duarte MMMB, Quiroz-Fernández LS, Baquerizo-Crespo RJ, Rodríguez-Díaz JM. Advances in the Application of Nanocatalysts in Photocatalytic Processes for the Treatment of Food Dyes: A Review. Sustainability. 2021; 13(21):11676. https://doi.org/10.3390/su132111676
Chicago/Turabian StyleNavia-Mendoza, Jennifer María, Otoniel Anacleto Estrela Filho, Luis Angel Zambrano-Intriago, Naga Raju Maddela, Marta Maria Menezes Bezerra Duarte, Luis Santiago Quiroz-Fernández, Ricardo José Baquerizo-Crespo, and Joan Manuel Rodríguez-Díaz. 2021. "Advances in the Application of Nanocatalysts in Photocatalytic Processes for the Treatment of Food Dyes: A Review" Sustainability 13, no. 21: 11676. https://doi.org/10.3390/su132111676
APA StyleNavia-Mendoza, J. M., Filho, O. A. E., Zambrano-Intriago, L. A., Maddela, N. R., Duarte, M. M. M. B., Quiroz-Fernández, L. S., Baquerizo-Crespo, R. J., & Rodríguez-Díaz, J. M. (2021). Advances in the Application of Nanocatalysts in Photocatalytic Processes for the Treatment of Food Dyes: A Review. Sustainability, 13(21), 11676. https://doi.org/10.3390/su132111676












