Acid Soils Nitrogen Leaching and Buffering Capacity Mitigation Using Charcoal and Sago Bark Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling, Preparation, and Characterization
2.2. Characterization of Charcoal and Sago Bark Ash
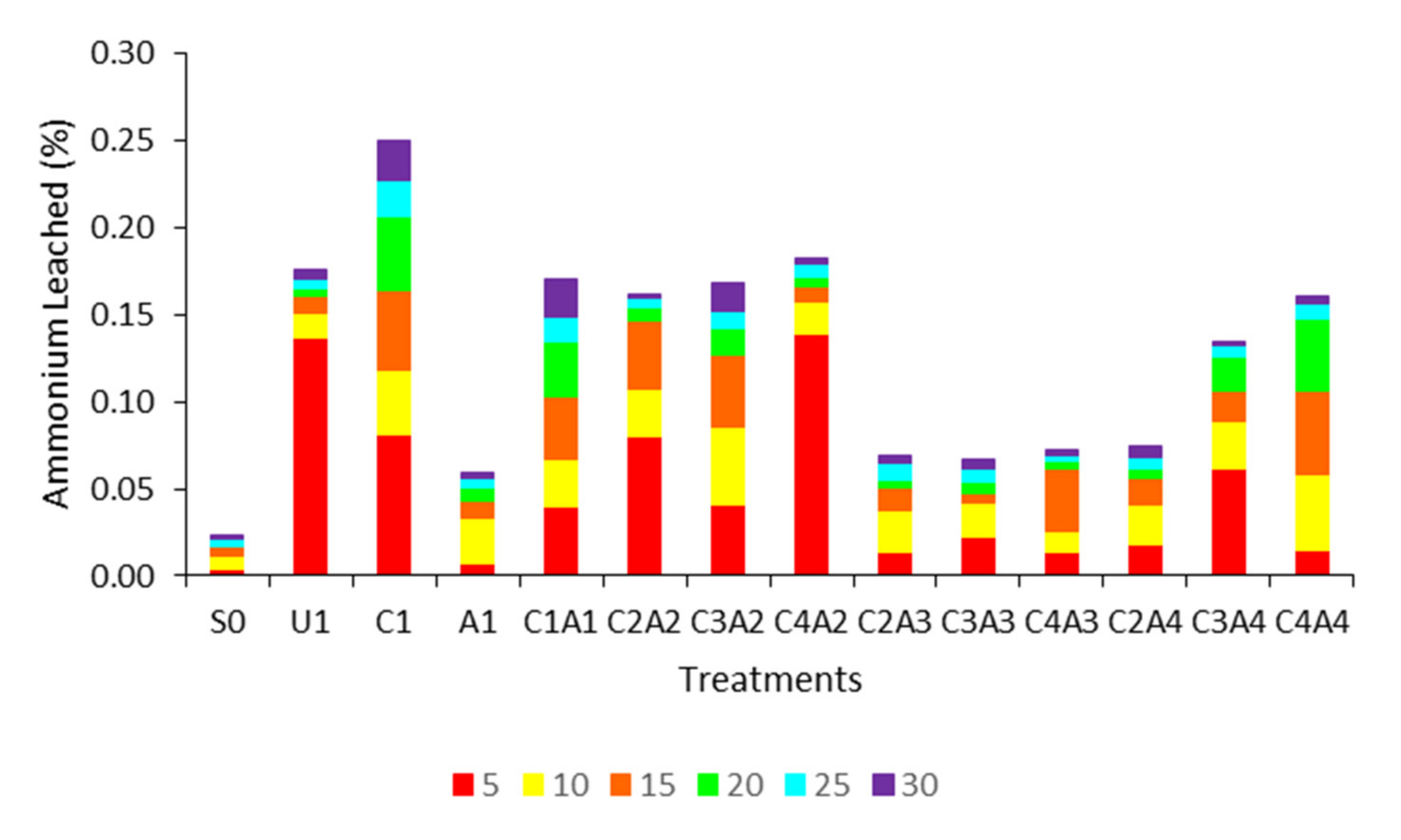
2.3. Leaching Study Set Up
rainfall in cm per month
container in cm2 × Volume of monthly rainfall)/area of rain gauge in cm2
- S0
- : soil only
- S0
- U1: 4.7 g urea only
- S0
- C1: soil + urea + 51.4 g charcoal
- S0
- A1: soil + urea + 25.7 g sago bark ash
- S0
- C1A1: soil + urea + 51.4 g charcoal + 25.7 g sago bark ash
- S0
- C2A2: soil + urea + 38.6 g charcoal + 19.3 g sago bark ash
- S0
- C3A2: soil + urea + 25.7 g charcoal + 19.3 g sago bark ash
- S0
- C4A2: soil + urea + 12.9 g charcoal + 19.3 g sago bark ash
- S0
- C2A3: soil + urea + 38.6 g charcoal + 12.9 g sago bark ash
- S0
- C3A3: soil + urea + 25.7 g charcoal + 12.9 g sago bark ash
- S0
- C4A3: soil + urea + 12.9 g charcoal + 12.9 g sago bark ash
- S0
- C2A4: soil + urea + 38.6 g charcoal + 6.4 g sago bark ash
- S0
- C3A4: soil + urea + 25.7 g charcoal + 6.4 g sago bark ash
- S0
- C4A4: soil + urea + 12.9 g charcoal + 6.4 g sago bark ash
2.4. Buffering Capacity Experiment Set Up
- T1
- : 300 g soil only
- T1
- T2: 300 g charcoal only
- T1
- T3: 300 g sago bark ash only
- T1
- T4: 300 g soil + 15.42 g charcoal
- T1
- T5: 300 g soil + 7.71 g sago bark ash
- T1
- T6: 300 g soil + 15.42 g charcoal + 7.71 g sago bark ash
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Treatments Effects on pH, Ammonium, and Nitrate on Leachate Solution at Five Days Interval
3.2. Treatments Effects on Selected Soil Chemical Properties after Thirty Days of Leaching
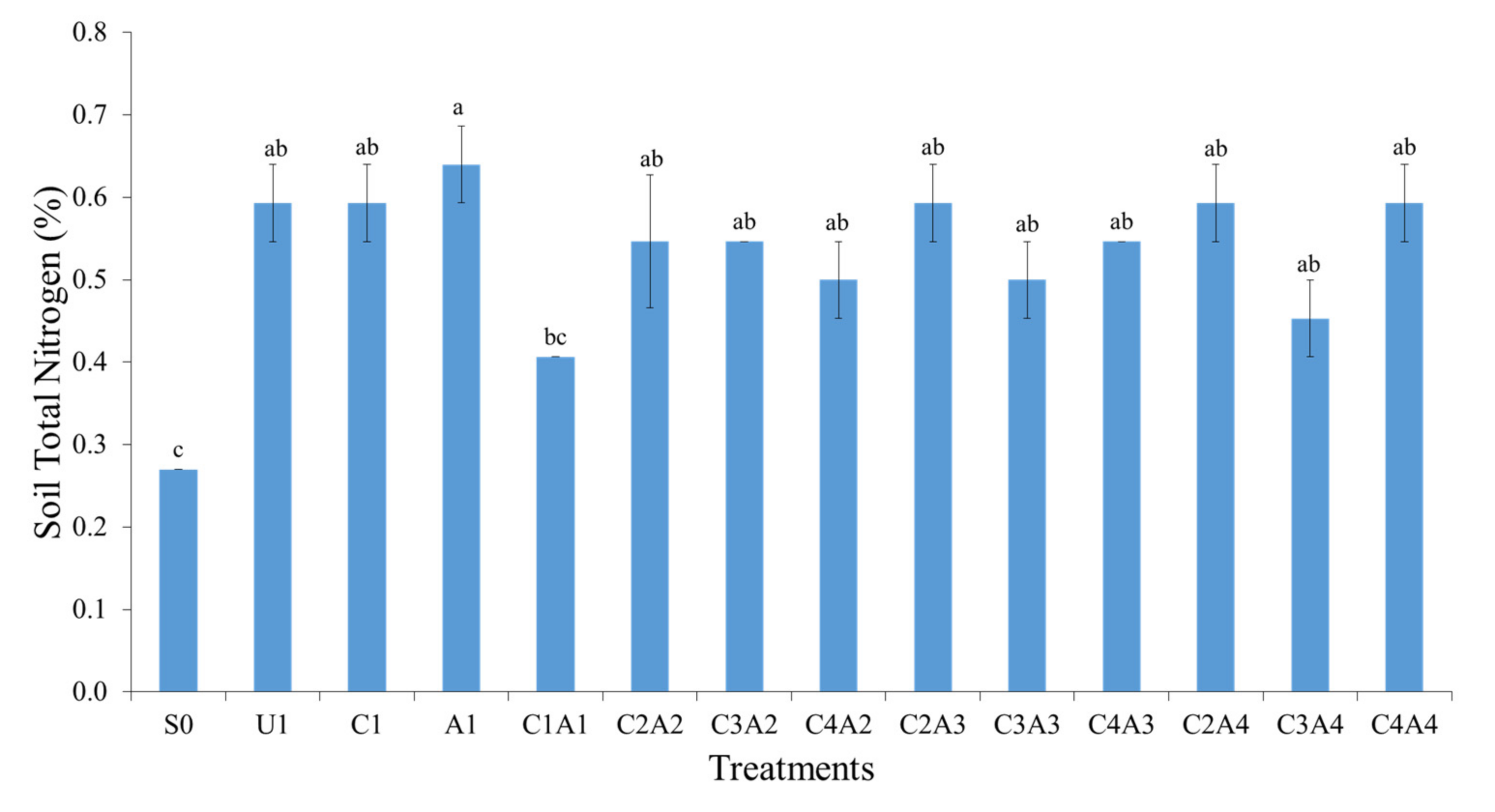
3.3. Treatments Effects on Soil Total Nitrogen, Exchangeable Ammonium, and Available Nitrate after Thirty Days of Leaching
3.4. Charcoal and Sago Bark Ash Effects on Soil pH Buffering Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Managing Living Soils; Global Soil Partnership International Technical Workshop: Rome, Italy, 2012; pp. 20–25. Available online: http://www.fao.org/documents/card/en/c/d018fe5b-59af-454e-8a27-8c75b671ba37/ (accessed on 15 October 2021).
- Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’Ng, H.Y. Soil Nitrogen Sorption Using Charcoal and Wood Ash. J. Agron. 2021, 11, 1801. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Tsiknia, M.; Tzanakakis, V.A.; Oikonomidis, D.; Paranychianakis, N.V.; Nikolaidis, N. Effects of olive mill wastewater on soil carbon and nitrogen cycling. Appl. Microbiol. Biotechnol. 2014, 98, 2739–2749. [Google Scholar] [CrossRef]
- Lu, X.K.; Mao, Q.G.; Gilliam, F.S.; Luo, Y.Q.; Mo, J.M. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ji, C.; Ma, W.; Wang, S.; Wang, S.; Han, W.; Mohammat, A.; Robinson, D.; Smith, P. Significant soil acidification across northern China’s grasslands during 1980s–2000s. Glob. Chang. Biol. 2012, 18, 2292–2300. [Google Scholar] [CrossRef]
- Yoo, G.; Kim, H.; Chen, J.; Kim, Y. Effects of biochar addition on nitrogen leaching and soil structure following fertilizer ap-plication to rice paddy soil. Soil Sci. Soc. Am. J. 2014, 78, 852–860. [Google Scholar] [CrossRef]
- Ulrich, B. Soil acidity and its relations to acid deposition. In Effects of Accumulation of Air Pollutants in Forest Ecosystems; Ulrich, B., Pankrath, J., Eds.; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1983; pp. 127–146. [Google Scholar]
- Vet, R.; Artz, R.S.; Carou, S.; Shaw, M.; Ro, C.-U.; Aas, W.; Baker, A.; Bowersox, V.C.; Dentener, F.; Galy-Lacaux, C.; et al. A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 2014, 93, 3–100. [Google Scholar] [CrossRef]
- Wong, M.T.F.; Webb, M.J.; Wittwer, K. Development of buffer methods and evaluation of pedotransfer functions to estimate pH buffer capacity of highly weathered soils. Soil Use Manag. 2013, 29, 30–38. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Mo, J.; Gilliam, F.S.; Zhou, G.; Luo, Y.; Zhang, W.; Huang, J. Divergent Responses of Soil Buffering Capacity to Long-Term N Deposition in Three Typical Tropical Forests with Different Land-Use History. Environ. Sci. Technol. 2015, 49, 4072–4080. [Google Scholar] [CrossRef]
- Tang, C.; Weligama, C.; Sale, P.W.G. Subsurface Soil Acidification in Farming Systems: Its Possible Causes and Management Options. In Molecular Environmental Soil Science; Springer: Amsterdam, The Netherlands, 2013; pp. 389–412. [Google Scholar]
- Lieb, A.M.; Darrouzet-Nardi, A.; Bowman, W.D. Nitrogen deposition decreases acid buffering capacity of alpine soils in the southern Rocky Mountains. Geoderma 2011, 164, 220–224. [Google Scholar] [CrossRef]
- Jian, J.; Steele, M.K.; Thomas, R.Q.; Day, S.; Hodges, S.C. Constraining estimates of global soil respiration by quantifying sources of variability. Glob. Chang. Biol. 2018, 24, 4143–4159. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Tran, T.T.H. The Contribution of Various Components to pH Buffering Capacity of Acrisols in Southeastern Vietnam. Commun. Soil Sci. Plant Anal. 2019, 50, 1170–1177. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Yu, M.; Li, K.; Shao, Y.; Yan, J. Responses of soil buffering capacity to acid treatment in three typical subtropical forests. Sci. Total. Environ. 2016, 563, 1068–1077. [Google Scholar] [CrossRef]
- Guelfi, D. Stabilized nitrogen fertilizers, slow or controlled release. Inf. Agronômicas 2017, 157, 1–14. [Google Scholar]
- Ariyaratne, R.M. Integrated Plant Nutrition Systems (IPNS) training manual (Sri Lanka). Fertil. Advis. Dev. Inf. Netw. Asia Pac. (FADINAP) 2000, 2000, 140. [Google Scholar]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Marichelvam, M.; Azhagurajan, A. Removal of mercury from effluent solution by using banana corm and neem leaves activated charcoal. Environ. Nanotechnol. Monit. Manag. 2018, 10, 360–365. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, X.; Liu, M.; Yang, L.; Wu, Z.; Xia, S.; Zhao, J. Adsorption of Pb(II) in aqueous solutions by bamboo charcoal modified with KMnO4 via microwave irradiation. Colloids Surf. 2012, 414, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Y.; Xia, S.; Chen, L.; Zhang, Y.; Zhao, J. Pb(II) removal from water using Fe-coated charcoal with the assistance of microwaves. Res. J. Environ. Sci. 2013, 25, 1044–1053. [Google Scholar] [CrossRef]
- Lv, D.; Liu, Y.; Zhou, J.; Yang, K.; Lou, Z.; Baig, S.A.; Xu, X. Application of EDTA-functionalized bamboo activated carbon (BAC) for Pb(II) and Cu(II) removal from aqueous solutions. Appl. Surf. Sci. 2018, 428, 648–658. [Google Scholar] [CrossRef]
- Etiégni, L.; Campbell, A. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Ohno, T. Neutralization of Soil Acidity and Release of Phosphorus and Potassium by Wood Ash. J. Environ. Qual. 1992, 21, 433–438. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Field and Laboratory Methods Manual. Soil Survey Investigations Report no. 51, Version 2.0.; Burt, R., Staff, S.S., Eds.; U.S. Department of Agriculture, Natural Resources Conservation Service: Lincoln, NE, USA, 2014.
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils 1. J. Agron. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Peech, M. Hydrogen-Ion Activity. In Agronomy Monographs; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 9, pp. 914–926. [Google Scholar]
- Rimmer, D.L.; Rodwell, D.L. Soil Science: Methods and Application. J. Ecol. 1995, 83, 352. [Google Scholar] [CrossRef]
- Chefetz, B.; Hatcher, P.G.; Hadar, Y.; Chen, Y. Chemical and Biological Characterization of Organic Matter during Composting of Municipal Solid Waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Cotennie, A. Soil and plant testing as a basis of fertilizer recommendation. FAO Soil Bull. 1980. (No. 38/2). [Google Scholar]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Norman, A.G., Ed.; Inc. Publisher: Madison, WI, USA, 1965; Volume 9, pp. 1149–1178. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties 9.2.2, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1983; Volume 9, pp. 643–698. [Google Scholar] [CrossRef]
- Paramananthan, S. Soils of Malaysia: Their Characteristics and Identification; Academy of Sciences Malaysia: Lumpur, Malaysia, 2000; Volume 1. [Google Scholar]
- Malaysian Agricultural Research and Development Institute (MARDI). Jagung Manis Baru, Masmadu (New Sweet Corn, Masmadu); Malaysian Agricultural Research and Development: Serdang, Malaysia, 1993; pp. 3–5.
- Costello, R.C.; Sullivan, D.M. Determining the pH Buffering Capacity of Compost Via Titration with Dilute Sulfuric Acid. Waste Biomass Valorization 2014, 5, 505–513. [Google Scholar] [CrossRef]
- Xu, J.M.; Tang, C.; Chen, Z.L. The role of plant residues in pH change of acid soils differing in initial pH. Soil Biol. Biochem. 2006, 38, 709–719. [Google Scholar] [CrossRef]
- Demeyer, A.; Nkana, J.V.; Verloo, M. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ball, B. Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob. Chang. Biol. 2014, 21, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- De Datta, S.K. Nitrogen transformation processes in relation to improved cultural practices for lowland rice. Plant Soil 1987, 100, 47–69. [Google Scholar] [CrossRef]
- Pitman, R.M. Wood ash use in forestry—A review of the environmental impacts. For. Int. J. For. Res. 2006, 79, 563–588. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.-L.; Lee, C.-H.; Jien, S.-H. Reduction of Nutrient Leaching Potential in Coarse-Textured Soil by Using Biochar. Water 2020, 12, 2012. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.; Nkedi-Kizza, P.; Gao, B. Catechol and Humic Acid Sorption onto a Range of Laboratory-Produced Black Carbons (Biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef]
- Omar, L.; Ahmed, O.H.; Majid, N.M.A. Improving Ammonium and Nitrate Release from Urea Using Clinoptilolite Zeolite and Compost Produced from Agricultural Wastes. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.W.; Hargrove, W.L. The Chemistry of Soil Acidity. Agron. Monogr. 2015, 12, 3–56. [Google Scholar] [CrossRef]
- Ritchie, G.S.P. Role of dissolution and precipitation of minerals in controlling soluble aluminium in acidic soils. Adv. Agron. 642 1994, 53, 47–83. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Xu, M.; Zhang, L.; Yang, Y.; Wang, B.; Wen, S.; Misselbrook, T.H.; Carswell, A.M.; Duan, Y.; Gao, S. Decarboxylation of organic anions to alleviate acidification of red soils from urea application. J. Soils Sediments 2020, 20, 3124–3135. [Google Scholar] [CrossRef]
- Demirbas, A.; Ahmad, W.; AlAmoudi, R.; Sheikh, M. Sustainable charcoal production from biomass. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 1882–1889. [Google Scholar] [CrossRef]
- Kleber, M.; Sollins, P.; Sutton, R. A conceptual model of organo-mineral interactions in soils: Self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 2007, 85, 9–24. [Google Scholar] [CrossRef]
- Binkley, D.; Bell, R.; Sollins, P. Comparison of methods for estimating soil nitrogen transformations in adjacent conifer and alder-conifer forests. Can. J. For. Res. 1992, 22, 858–863. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Zhang, D.; Wu, M.; Pan, B. Limited role of biochars in nitrogen fixation through nitrate adsorption. Sci. Total. Environ. 2017, 592, 758–765. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, X.; Chen, C.; Tao, P.; Han, Z.; Zhang, X. Biochar impact on nitrate leaching in upland red soil, China. Environ. Earth Sci. 2016, 75, 1–10. [Google Scholar] [CrossRef]
- Tang, S.; Cheng, W.; Hu, R.; Nakajima, M.; Guigue, J.; Kimani, S.M.; Sato, S.; Tawaraya, K.; Xu, X. Decomposition of soil organic carbon influenced by soil temperature and moisture in Andisol and Inceptisol paddy soils in a cold temperate region of Japan. J. Soils Sediments 2017, 17, 1843–1851. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Muller, C.W. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.T.; Nelson, P.N.; Li, M.-H.; Cai, J.P.; Zhang, Y.Y.; Yang, S.; Wang, R.Z.; Wang, Z.W.; Wu, Y.N.; Han, X.G.; et al. Contrasting pH buffering patterns in neutral-alkaline soils along a 3600 km transect in northern China. Biogeosciences 2015, 12, 7047–7056. [Google Scholar] [CrossRef] [Green Version]
- Bloom, P.R. Soil pH and pH buffering. In Handbook of Soil Science; Sumner, M.E., Huang, P.M., Li, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 333–352. [Google Scholar]
- Nelson, P.N.; Su, N. Soil pH buffering capacity: A descriptive function and its application to some acidic tropical soils. Soil Res. 2010, 48, 201–207. [Google Scholar] [CrossRef]












| Property | Current Study |
|---|---|
| pH (H2O) | 4.61 |
| pH (KCl) | 3.95 |
| EC (µS cm−1) | 35.10 |
| Bulk density (g cm−3) | 1.25 |
| Total C (%) | 2.16 |
| Total N (%) | 0.08 |
| Exchangeable NH4+ (%) | 0.00049 |
| Available NO3− (%) | 0.00049 |
| CEC (cmol kg−1) | 4.67 |
| Exchangeable K+ (cmol kg−1) | 0.06 |
| Exchangeable Ca2+ (cmol kg1−) | 0.02 |
| Exchangeable Mg2+ (cmol kg−1) | 0.22 |
| Exchangeable Na+ (cmol kg−1) | 0.03 |
| Exchangeable Fe2+ (cmol kg−1) | 1.09 |
| Exchangeable Mn2+ (cmol kg−1) | 0.01 |
| Total titratable acidity (cmol kg−1) | 1.15 |
| Exchangeable H+ (cmol kg−1) | 0.13 |
| Exchangeable Al3+ (cmol kg−1) | 1.02 |
| Soil texture | Sand (%): 71.9 Silt (%): 13.5 Clay (%): 14.6 Sandy loam |
| Property | Charcoal | Sago Bark Ash |
|---|---|---|
| pH (H2O) | 7.74 | 9.99 |
| pH (KCl) | 7.31 | 9.66 |
| EC (dS m−1) | 0.27 | 5.75 |
| Total N (%) | 1.54 | 1.37 |
| Exchangeable NH4+ (%) | 0.00023 | 0.00026 |
| Available NO3− (%) | 0.00023 | 0.00026 |
| Exchangeable K+ (cmol kg−1) | 3.67 | 23.33 |
| Exchangeable Ca2+ (cmol kg−1) | 11.71 | 16.77 |
| Exchangeable Mg2+ (cmol kg−1) | 3.37 | 3.57 |
| Exchangeable Na+ (cmol kg−1) | 0.43 | 1.51 |
| Exchangeable Fe2+ (cmol kg−1) | 0.15 | 0.03 |
| Treatments | Initial pH | pH Buffering Capacity (mol H+ kg−1 Soil) | Regression Coefficient (R2) |
|---|---|---|---|
| T1 | 5.31 | 0.25 | 0.92 * |
| T2 | 7.76 | 0.29 | 0.90 * |
| T3 | 9.78 | 0.34 | 0.92 * |
| T4 | 6.51 | 0.26 | 0.97 * |
| T5 | 6.41 | 0.28 | 0.93 * |
| T6 | 6.65 | 0.29 | 0.92 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y.; Johan, P.D.; Paramisparam, P.; Jalloh, M.B. Acid Soils Nitrogen Leaching and Buffering Capacity Mitigation Using Charcoal and Sago Bark Ash. Sustainability 2021, 13, 11808. https://doi.org/10.3390/su132111808
Hamidi NH, Ahmed OH, Omar L, Ch’ng HY, Johan PD, Paramisparam P, Jalloh MB. Acid Soils Nitrogen Leaching and Buffering Capacity Mitigation Using Charcoal and Sago Bark Ash. Sustainability. 2021; 13(21):11808. https://doi.org/10.3390/su132111808
Chicago/Turabian StyleHamidi, Nur Hidayah, Osumanu Haruna Ahmed, Latifah Omar, Huck Ywih Ch’ng, Prisca Divra Johan, Puvan Paramisparam, and Mohamadu Boyie Jalloh. 2021. "Acid Soils Nitrogen Leaching and Buffering Capacity Mitigation Using Charcoal and Sago Bark Ash" Sustainability 13, no. 21: 11808. https://doi.org/10.3390/su132111808
APA StyleHamidi, N. H., Ahmed, O. H., Omar, L., Ch’ng, H. Y., Johan, P. D., Paramisparam, P., & Jalloh, M. B. (2021). Acid Soils Nitrogen Leaching and Buffering Capacity Mitigation Using Charcoal and Sago Bark Ash. Sustainability, 13(21), 11808. https://doi.org/10.3390/su132111808








