Nephelium lappaceum Extract as an Organic Inhibitor to Control the Corrosion of Carbon Steel Weldment in the Acidic Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. NP Extract Preparation
2.2. Weldment Preparation
2.3. Corrosion Testing
3. Results
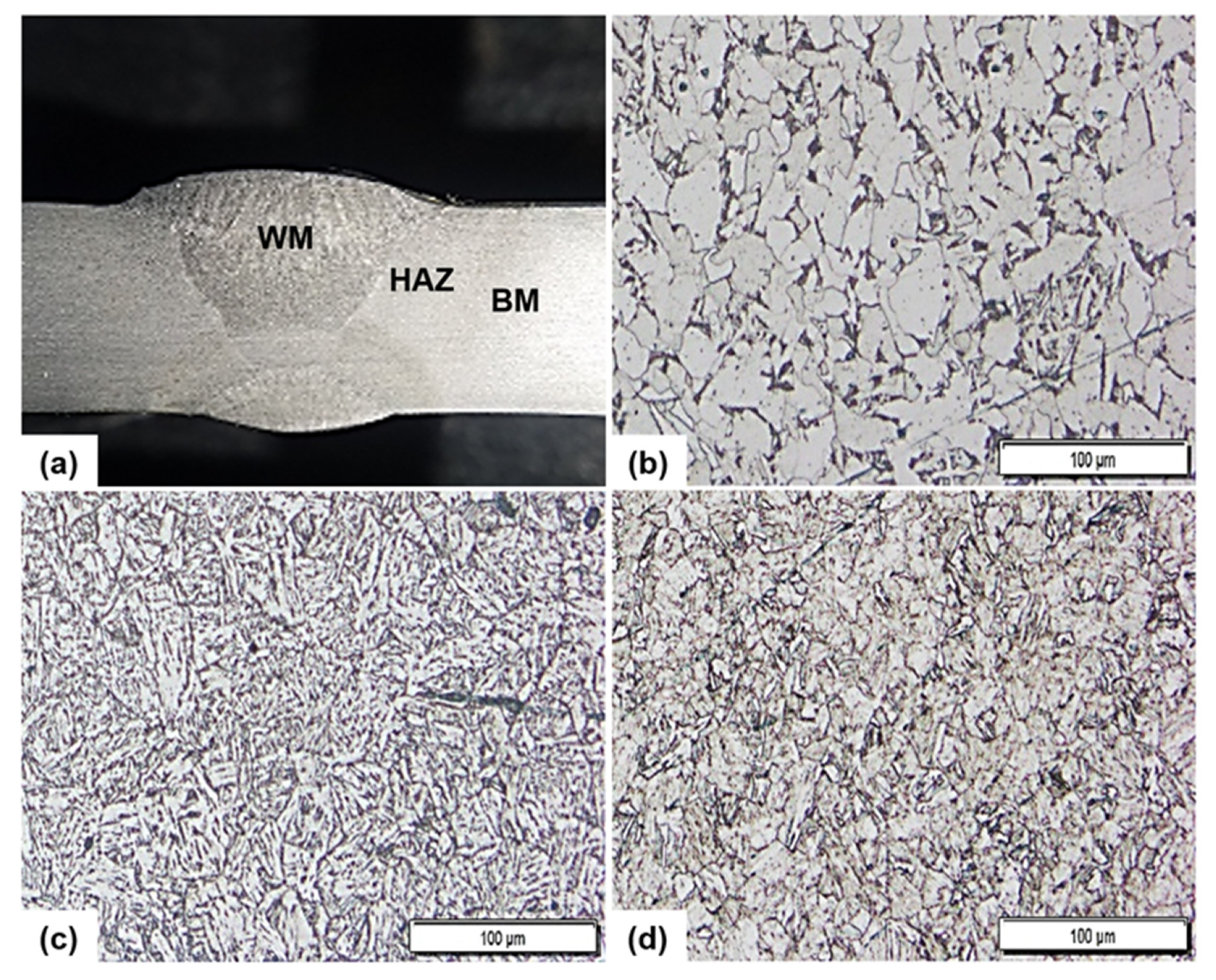
3.1. Weldment Structure and Hardness
3.2. NP Extract Characteristics
3.3. Potentiodynamic Polarization
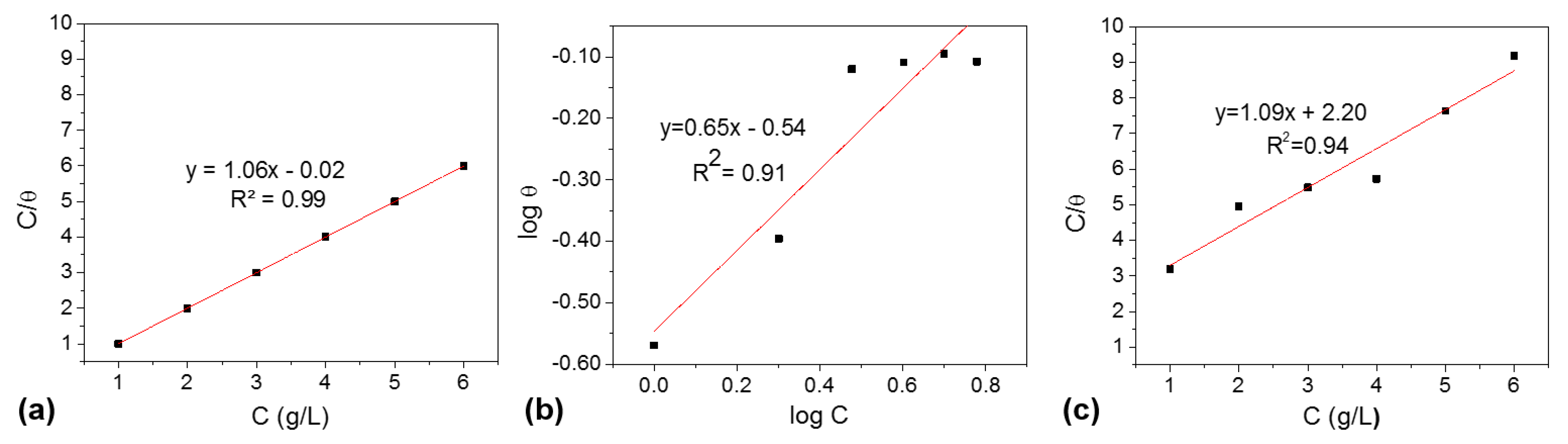
3.4. Adsorption Isotherms Plots of NP Extracts Adsorption on Carbon Steel Surface
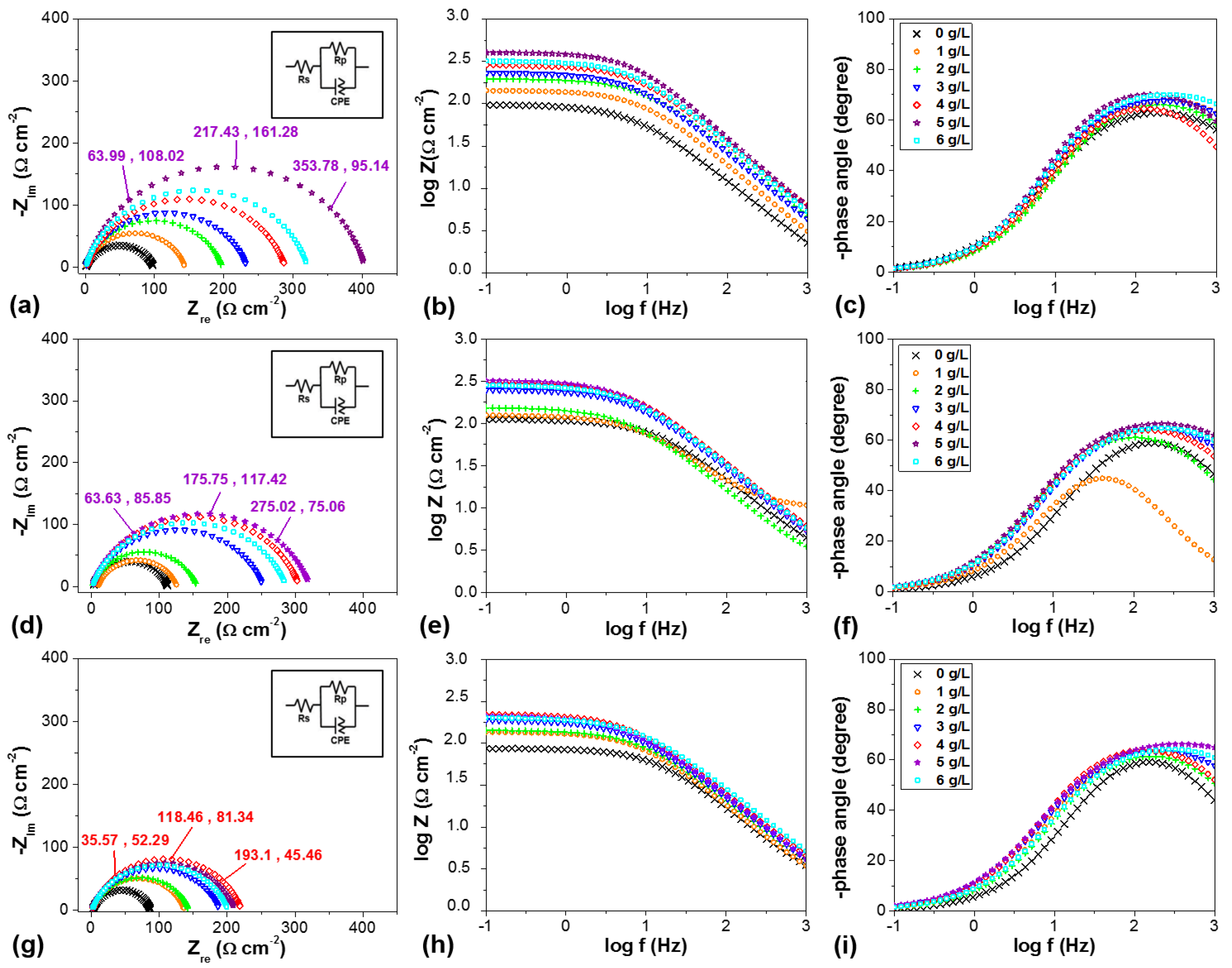
3.5. Electrochemical Impedance Spectroscopy
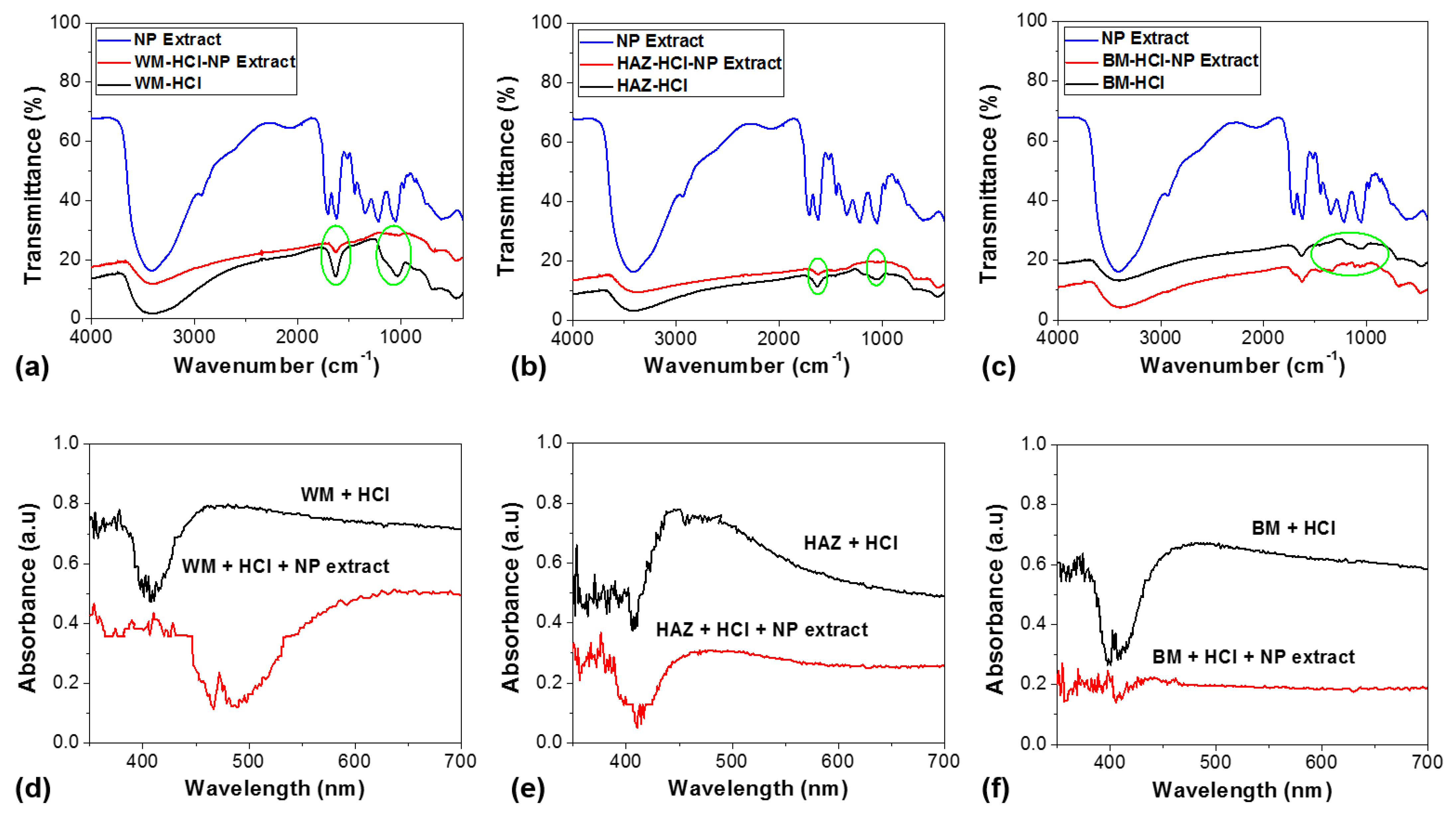
3.6. SEM/EDS Observation
4. Discussion
4.1. Effectiveness of NP Extract as Corrosion Inhibitor
4.2. Proposed Mechanism of Inhibition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Natarajan, S.; Babu, S.K. Corrosion and its inhibition in SA213-T22 TIG weldments used in power plants under neutral and alkaline environments. Mater. Sci. Eng. A 2006, 432, 47–51. [Google Scholar] [CrossRef]
- Nishimura, T.; Katayama, H.; Noda, K.; Kodama, T. Electrochemical Behavior of Rust Formed on Carbon Steel in a Wet/Dry Environment Containing Chloride Ions. Corrosion 2000, 56, 935–941. [Google Scholar] [CrossRef]
- Davis, J.R. Corrosion of Weldment; Davis, J.R., Ed.; ASM International: Materials Park, OH, USA, 2006; Chapter 1; pp. 1–10. [Google Scholar]
- Gollapudi, S. Grain size distribution effects on the corrosion behaviour of materials. Corros. Sci. 2012, 62, 90–94. [Google Scholar] [CrossRef]
- Fattahi, M.; Nabhani, N.; Vaezi, M.R.; Rahimi, E. Improvement of impact toughness of AWS E6010 weld metal by adding TiO2 nanoparticles to the electrode coating. Mater. Sci. Eng. A 2011, 528, 8031–8039. [Google Scholar] [CrossRef]
- Tristijanto, H.; Ilman, M.N.; Tri Iswanto, P. Corrosion Inhibition of Welded of X – 52 Steel Pipelines by Sodium Molybdate in 3.5% NaCl Solution. Egypt. J. Pet. 2020, 29, 155–162. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A. Recent advances in metallic corrosion inhibition: A review. J. Mol. Liq. 2021, 322, 114862. [Google Scholar] [CrossRef]
- Sastri, V.S. Adsorption in Corrosion Inhibition. In Green Corrosion Inhibitors: Theory and Practice; Sastri, V.S., Ed.; Wiley: Hoboken, NJ, USA, 2011; Chapter 3; pp. 103–108. [Google Scholar]
- Abdallah, M. Rhodanine azosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in hydrochloric acid solution. Corros. Sci. 2002, 44, 717–728. [Google Scholar] [CrossRef]
- Fouda, A.S.; Elmorsi, M.A.; Elmekkawy, A. Eco-friendly chalcones derivatives as corrosion inhibitors for carbon steel in hydrochloric acid solution. Afr. J. Pure Appl. Chem. 2013, 7, 337–349. [Google Scholar] [CrossRef]
- Abiola, O.K.; Odin, E.M.; Olowoyo, D.N.; Adeloye, T.A. Gossipium hirsutum L. extract as green corrosion inhibitor for aluminum in HCl solution. Bull. Chem. Soc. Ethiop. 2011, 25, 475–480. [Google Scholar] [CrossRef]
- Oguzie, E. Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract. Corros. Sci. 2007, 49, 1527–1539. [Google Scholar] [CrossRef]
- Verma, C.; Haque, J.; Quraishi, M.A.; Ebenso, E.E. Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: A review. J. Mol. Liq. 2019, 275, 18–40. [Google Scholar] [CrossRef]
- El-Etre, A.Y. Inhibition of aluminum corrosion using Opuntia extract. Corros. Sci. 2003, 45, 2485–2495. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Flores-Gallegos, A.C.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J.A. Rambutan(Nephelium lappaceum L.):Nutritional and functional properties. Trends Food Sci. Technol. 2019, 85, 201–210. [Google Scholar] [CrossRef]
- Thitilertdecha, N.; Teerawutgulrag, A.; Rakariyatham, N. Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT-Food Sci. Technol. 2008, 41, 2029–2035. [Google Scholar] [CrossRef]
- Thirumalairaj, B.; Jaganathan, M. Corrosion protection of mild steel by a new binary inhibitor system in hydrochloric acid solution. Egypt. J. Pet. 2016, 25, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Gapsari, F.; Madurani, K.A.; Simanjuntak, F.M.; Andoko, A.; Wijaya, H.; Kurniawan, F. Corrosion Inhibition of Honeycomb Waste Extracts for 304 Stainless Steel in Sulfuric Acid Solution. Materials 2019, 12, 2120. [Google Scholar] [CrossRef] [Green Version]
- Gapsari, F.; Soenoko, R.; Suprapto, A.; Suprapto, W. Bee Wax Propolis Extract as Eco-Friendly Corrosion Inhibitors for 304SS in Sulfuric Acid. Int. J. Corros. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Maayta, A.K.; Al-Rawashdeh, N.A.F. Inhibition of acidic corrosion of pure aluminum by some organic compounds. Corros. Sci. 2004, 46, 1129–1140. [Google Scholar] [CrossRef]
- Kurniawan, F.; Madurani, K.A. Electrochemical and optical microscopy study of red pepper seed oil corrosion inhibition by self-assembled monolayers (SAM) on 304 SS. Prog. Org. Coat. 2015, 88, 256–262. [Google Scholar] [CrossRef]
- Iroha, N.B.; Madueke, N.A.; Mkpenie, V.; Ogunyemi, B.T.; Nnanna, L.A.; Singh, S.; Akpan, E.D.; Ebenso, E.E. Experimental, adsorption, quantum chemical and molecular dynamics simulation studies on the corrosion inhibition performance of Vincamine on J55 steel in acidic medium. J. Mol. Struct. 2020, 1227, 129533. [Google Scholar] [CrossRef]
- Umoren, S.A.; Obot, I.B.; Ebenso, E.E.; Obi-Egbedi, N.O. The Inhibition of aluminium corrosion in hydrochloric acid solution by exudate gum from Raphia hookeri. Desalination 2009, 247, 561–572. [Google Scholar] [CrossRef]
- Hussin, M.H.; Kassim, M.J. The corrosion inhibition and adsorption behavior of Uncaria gambir extract on mild steel in 1M HCl. Mater. Chem. Phys. 2011, 125, 461–468. [Google Scholar] [CrossRef]
- Awad, M.I. Eco friendly corrosion inhibitors: Inhibitive action of quinine for corrosion of low carbon steel in 1 m HCl. J. Appl. Electrochem. 2006, 36, 1163–1168. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Zhang, J.; Cheng, J.; Wang, X. Double-layered surface decoration of flaky aluminum pigments with zinc aluminum phosphate and phytic acid–aluminum complexes for high-performance waterborne coatings. Powder Technol. 2020, 362, 462–473. [Google Scholar] [CrossRef]
- Cruz, J.; Pandiyan, T.; García-Ochoa, E. A new inhibitor for mild carbon steel: Electrochemical and DFT studies. J. Electroanal. Chem. 2005, 583, 8–16. [Google Scholar] [CrossRef]
- Satapathy, A.K.; Gunasekaran, G.; Sahoo, S.C.; Amit, K.; Rodrigues, P.V. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 2009, 51, 2848–2856. [Google Scholar] [CrossRef]
- Medina, L.Z.; da Costa, M.V.T.; Paschalidou, E.M.; Lindwall, G.; Riekehr, L.; Korvela, M.; Fritze, S.; Kolozsvári, S.; Gamstedt, E.K.; Nyholm, L.; et al. Enhancing corrosion resistance, hardness, and crack resistance in magnetron sputtered high entropy CoCrFeMnNi coatings by adding carbon. Mater. Des. 2021, 205, 109711. [Google Scholar] [CrossRef]
- Weng, S.; Huang, Y.H.; Xuan, F.Z.; Luo, L.H. Correlation between Microstructure, Hardness and Corrosion of Welded Joints of Disc Rotors. Procedia Eng. 2015, 130, 1761–1769. [Google Scholar] [CrossRef] [Green Version]
- Septimio, R.S.; Arenas, M.A.; Conde, A.; Garcia, A.; Cheung, N.; De Damborenea, J. Correlation between microstructure and corrosion behaviour of Bi-Zn solder alloys. Corros. Eng. Sci. Technol. 2019, 54, 362–368. [Google Scholar] [CrossRef]
- Ashari, R.; Eslami, A.; Shamanian, M.; Asghari, S. Effect of weld heat input on corrosion of dissimilar welded pipeline steels under simulated coating disbondment protected by cathodic protection. J. Mater. Res. Technol. 2020, 9, 2136–2145. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Y.; Yang, J.; Sheng, Y.; Yi, Y.; Zeng, X.; Chen, L.; Yin, F.; Su, J.; Zhang, T.; et al. Effect of grain refinement induced by wire and arc additive manufacture (WAAM) on the corrosion behaviors of AZ31 magnesium alloy in NaCl solution. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Huda; Shoeb, M.; Murmu, M.; Banerjee, P. Proline nitrate ionic liquid as high temperature acid corrosion inhibitor for mild steel: Experimental and molecular-level insights. J. Ind. Eng. Chem. 2021, 100, 333–350. [Google Scholar] [CrossRef]
- Sastri, V.S. Corrosion Inhibition Mechanisms. In Green Corrosion Inhibitors: Theory and Practice; Sastri, V.S., Ed.; Wiley: Hoboken, NJ, USA, 2011; Chapter 5; pp. 167–211. [Google Scholar]
- Al-Sabagh, A.M.; Abd-El-Bary, H.M.; El-Ghazawy, R.A.; Mishrif, M.R.; Hussein, B.M. Corrosion inhibition efficiency of heavy alkyl benzene derivatives for carbon steel pipelines in 1M HCl. Egypt. J. Pet. 2012, 21, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Trung, D.C.; Pham, T.T.; Minh, Q.B.P.; Panaitescu, C.; Tran, N.Q.; Anh, H.T.; Bach, L.X.; Dang, N.N. The use of Piper Betle leaf extract for forming a barrier layer on steel surface in hydrochloric acid solution. Prog. Org. Coat. 2021, 158, 106340. [Google Scholar] [CrossRef]



| Peak | R Time | Area% | Name | Structural Formula | Molecular Weight |
|---|---|---|---|---|---|
| 1 | 9.003 | 3.12 | 1,2-Benzenediol | 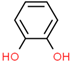 | 110 |
| 2 | 9.280 | 2.22 | Unidentified | ||
| 3 | 9.880 | 1.83 | 1,2,3-Benzenetriol |  | 126 |
| 4 | 10.377 | 3.85 | Unidentified | ||
| 5 | 11.015 | 2.44 | Mome Inositol | 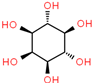 | 180 |
| 6 | 11.263 | 25.02 | Mome Inositol | ||
| 7 | 11.570 | 35.74 | Mome Inositol | ||
| 8 | 11.784 | 3.97 | Unidentified | ||
| 9 | 12.373 | 4.38 | Unidentified | ||
| 10 | 12.660 | 17.43 | Heptadecene-(8)-carbonic acid-(1) |  | 268.4 |
| NP Extract (g/L) | βa (V/dec) | −βc (V/dec) | Ecorr (V) | icorr (A/cm2) | Corrosion Rate (mm/year) | IE (%) |
|---|---|---|---|---|---|---|
| WM | ||||||
| 0 | 0.096 | 0.111 | −0.414 | 1.64 × 10−3 | 19.3 | 0 |
| 1 | 0.101 | 0.105 | −0.438 | 1.81 × 10−4 | 2.13 | 89 |
| 2 | 0.081 | 0.081 | −0.442 | 9.10 × 10−5 | 1.07 | 94 |
| 3 | 0.075 | 0.066 | −0.437 | 8.06 × 10−5 | 0.95 | 95 |
| 4 | 0.068 | 0.052 | −0.439 | 6.52 × 10−5 | 0.77 | 96 |
| 5 | 0.094 | 0.079 | −0.438 | 5.72 × 10−5 | 0.67 | 97 |
| 6 | 0.137 | 0.101 | −0.434 | 1.41 × 10−4 | 1.66 | 91 |
| HAZ | ||||||
| 0 | 0.204 | 0.143 | −0.450 | 3.41 × 10−4 | 4.01 | 0 |
| 1 | 0.105 | 0.102 | −0.441 | 2.49 × 10−4 | 2.93 | 27 |
| 2 | 0.117 | 0.085 | −0.440 | 2.04 × 10−4 | 2.40 | 40 |
| 3 | 0.096 | 0.071 | −0.436 | 8.23 × 10−4 | 0.97 | 76 |
| 4 | 0.084 | 0.076 | −0.447 | 7.54 × 10−4 | 0.89 | 78 |
| 5 | 0.086 | 0.058 | −0.436 | 6.69 × 10−4 | 0.78 | 80 |
| 6 | 0.097 | 0.076 | −0.440 | 7.49 × 10−4 | 0.88 | 78 |
| BM | ||||||
| 0 | 0.126 | 0.145 | −0.432 | 3.46 × 10−4 | 4.08 | 0 |
| 1 | 0.120 | 0.118 | −0.427 | 2.38 × 10−4 | 2.80 | 31 |
| 2 | 0.132 | 0.098 | −0.432 | 2.07 × 10−4 | 2.43 | 40 |
| 3 | 0.140 | 0.067 | −0.421 | 1.57 × 10−4 | 1.85 | 56 |
| 4 | 0.091 | 0.065 | −0.430 | 1.04 × 10−4 | 1.23 | 70 |
| 5 | 0.112 | 0.077 | −0.427 | 1.2 × 10−4 | 1.40 | 65 |
| 6 | 0.103 | 0.078 | −0.422 | 1.2 × 10−4 | 1.41 | 65 |
| Weldment | Kads | ∆Gads |
|---|---|---|
| WM | 45.87 | −19.42 |
| HAZ | 3.51 | −13.06 |
| BM | 0.45 | −25.21 |
| NP Extract (g/L) | Rs (Ω.cm−2) | Rp (Ω.cm−2) | CPE (µF.cm−2) | n | Χ2 | IE (%) |
|---|---|---|---|---|---|---|
| WM | ||||||
| 0 | 0.58 | 98 | 491 | 0.789 | 0.285 | 0 |
| 1 | 0.61 | 143 | 243 | 0.829 | 0.327 | 31 |
| 2 | 1.26 | 196 | 169 | 0.83 | 0.156 | 50 |
| 3 | 0.84 | 232 | 183 | 0.824 | 0.099 | 58 |
| 4 | 2.77 | 286 | 134 | 0.834 | 0.169 | 66 |
| 5 | 1.89 | 402 | 97 | 0.862 | 0.035 | 76 |
| 6 | 0.80 | 320 | 128 | 0.839 | 0.122 | 69 |
| HAZ | ||||||
| 0 | 1.96 | 110 | 245 | 0.808 | 0.089 | 0 |
| 1 | 1.85 | 129 | 350 | 0.804 | 0.087 | 17 |
| 2 | 1.65 | 153 | 355 | 0.8 | 0.103 | 28 |
| 3 | 1.3 | 251 | 189 | 0.801 | 0.072 | 56 |
| 4 | 2.02 | 304 | 161 | 0.808 | 0.111 | 64 |
| 5 | 0.97 | 321 | 171 | 0.804 | 0.07 | 66 |
| 6 | 1.12 | 286 | 180 | 0.794 | 0.066 | 62 |
| BM | ||||||
| 0 | 1.77 | 85 | 285 | 0.825 | 0.148 | 0 |
| 1 | 1.23 | 136 | 279 | 0.815 | 0.222 | 37 |
| 2 | 1.52 | 142 | 260 | 0.803 | 0.128 | 40 |
| 3 | 0.92 | 188 | 273 | 0.786 | 0.066 | 55 |
| 4 | 1.66 | 219 | 212 | 0.814 | 0.054 | 61 |
| 5 | 0.37 | 211 | 257 | 0.785 | 0.047 | 60 |
| 6 | 0.84 | 201 | 206 | 0.789 | 0.036 | 58 |
| Specimen | Element (wt %) | ||||
|---|---|---|---|---|---|
| C | O | Na | Cl | Fe | |
| WM in HCl | 1.63 | 1.63 | 0.43 | 0.25 | 96.07 |
| WM in HCl+NP extract | 1.84 | 29.04 | 0.21 | 0.19 | 68.72 |
| HAZ in HCl | 1.84 | 1.24 | 0.55 | 0.16 | 96.06 |
| HAZ in HCl+NP extract | 1.91 | 27.87 | 0.16 | 0.34 | 69.71 |
| BM in HCl | 3.82 | 3.65 | 0.71 | 0.36 | 91.45 |
| BM in HCl+NP extract | 2.29 | 26.76 | 0.32 | 0.91 | 69.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gapsari, F.; Darmadi, D.B.; Setyarini, P.H.; Izzuddin, H.; Madurani, K.A.; Tanji, A.; Hermawan, H. Nephelium lappaceum Extract as an Organic Inhibitor to Control the Corrosion of Carbon Steel Weldment in the Acidic Environment. Sustainability 2021, 13, 12135. https://doi.org/10.3390/su132112135
Gapsari F, Darmadi DB, Setyarini PH, Izzuddin H, Madurani KA, Tanji A, Hermawan H. Nephelium lappaceum Extract as an Organic Inhibitor to Control the Corrosion of Carbon Steel Weldment in the Acidic Environment. Sustainability. 2021; 13(21):12135. https://doi.org/10.3390/su132112135
Chicago/Turabian StyleGapsari, Femiana, Djarot B. Darmadi, Putu H. Setyarini, Hubby Izzuddin, Kartika A. Madurani, Ayoub Tanji, and Hendra Hermawan. 2021. "Nephelium lappaceum Extract as an Organic Inhibitor to Control the Corrosion of Carbon Steel Weldment in the Acidic Environment" Sustainability 13, no. 21: 12135. https://doi.org/10.3390/su132112135








