Removal of Toxic Heavy Metals from Contaminated Aqueous Solutions Using Seaweeds: A Review
Abstract
1. Introduction
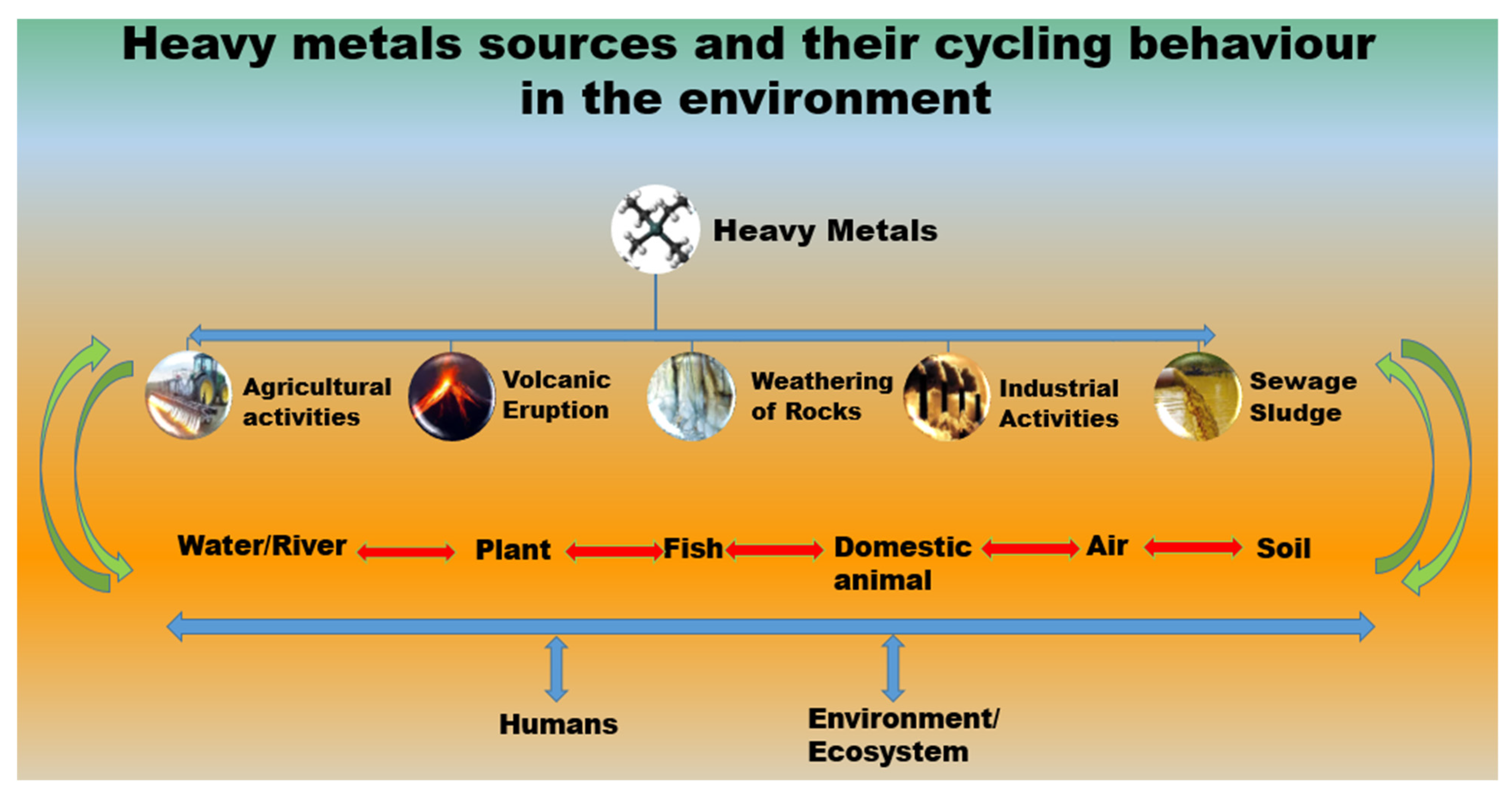
2. Heavy Metal Contamination in Water
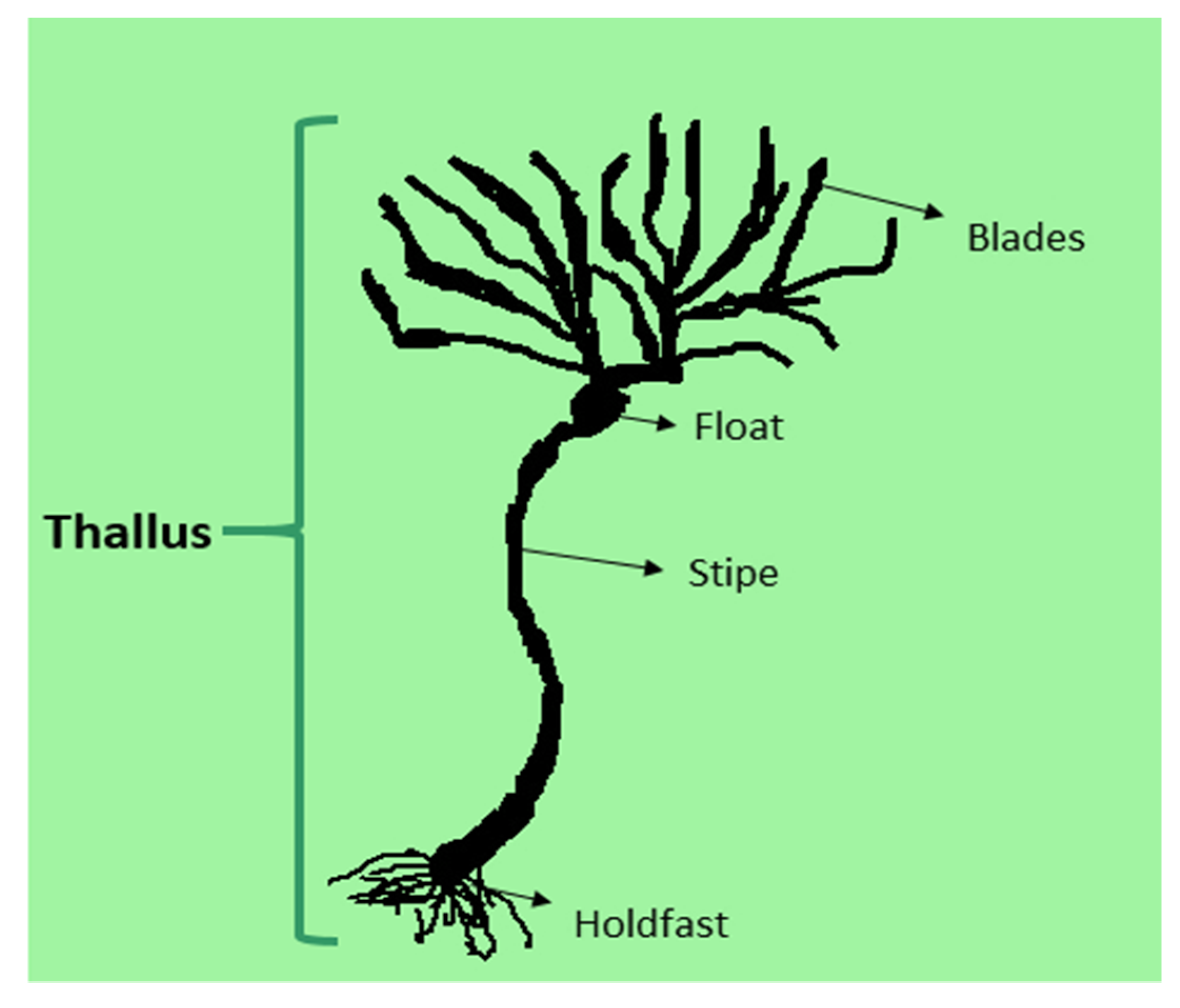
3. Structure and Classification of Seaweed
3.1. Seaweed: Metal Ion Biosorption Material
3.2. Various Natural Materials Used for Sorption
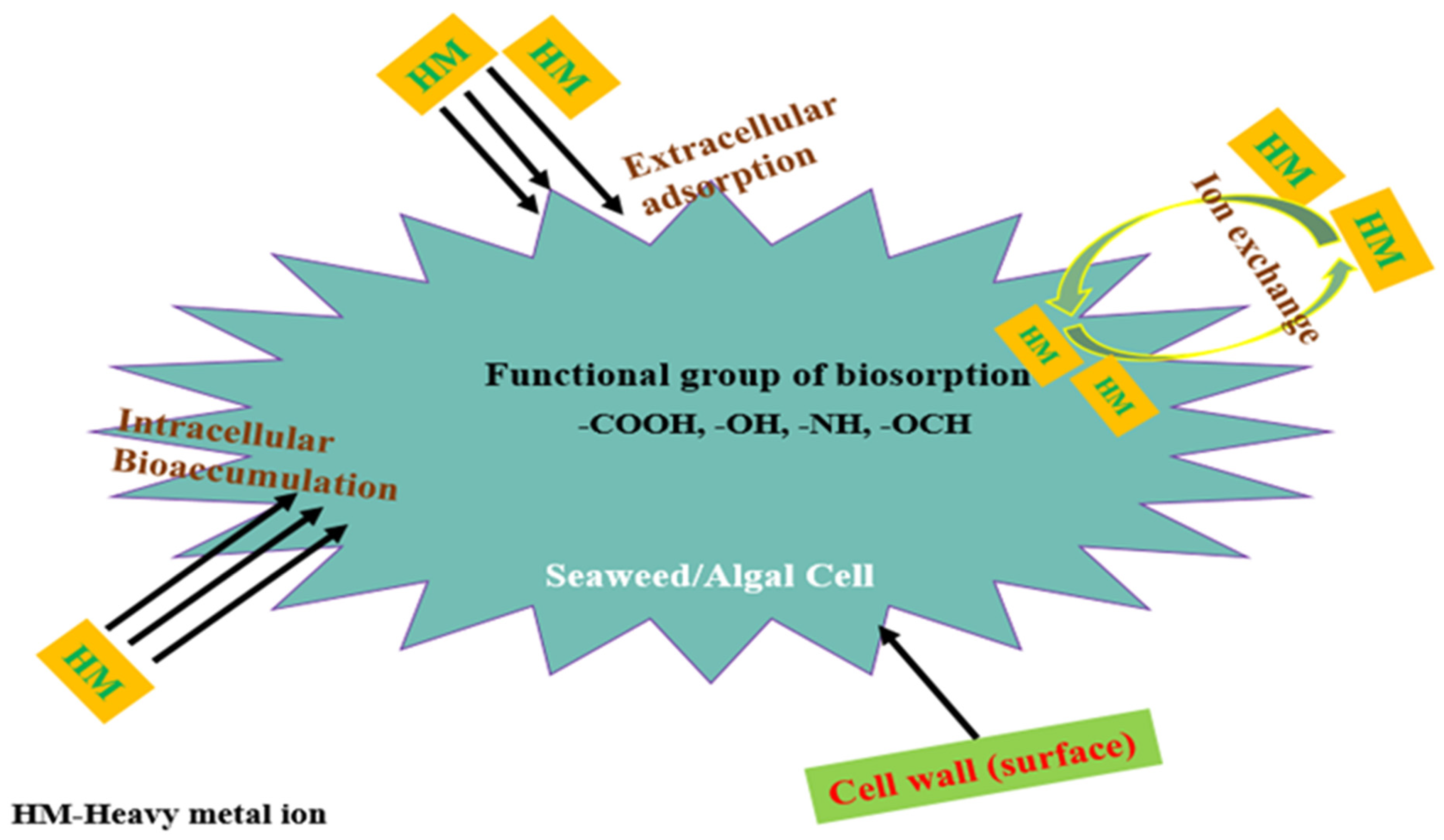
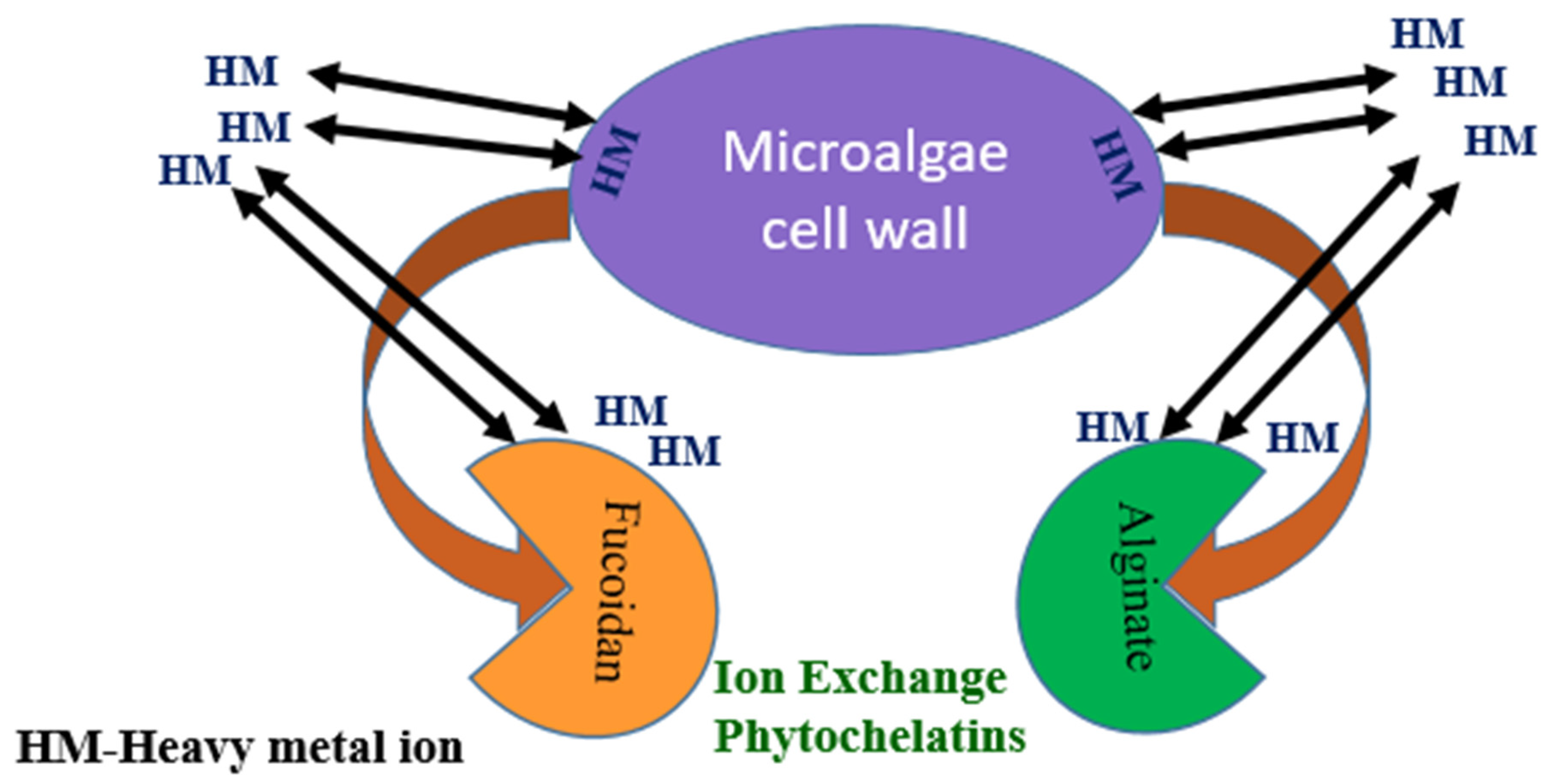
4. Sorption Mechanism of Seaweed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajjabi, L.C.; Chouba, L. Biosorption of Cu2+ and Zn2+ from aqueous solutions by dried marine green macroalga Chaetomorpha linum. J. Environ. Manag. 2009, 90, 3485–3489. [Google Scholar] [CrossRef]
- Bulut, Y.; Baysal, Z. Removal of Pb(II) from wastewater using wheat bran. J. Environ. Manag. 2006, 78, 107–113. [Google Scholar] [CrossRef]
- Al-Rub, F.; El-Naas, M.; Benyahia, F.; Ashour, I. Biosorption of nickel on blank alginate beads, free and immobilized algal cells. Process. Biochem. 2004, 39, 1767–1773. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Romera, D.E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Biosorption with Algae: A Statistical Review. Crit. Rev. Biotechnol. 2006, 26, 223–235. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Abrar, M.; Hussain, Z.; Akif, M.; Sok, K.; Muhammad, A.; Khan, A.; Khan, M. Textile effluents and their contribution towards aquatic pollution in the Kabul River (Pakistan). J. Chem. Soc. Pak. 2011, 24, 106. [Google Scholar]
- Afzal, M.S.; Ashraf, A.; Nabeel, M. Characterization of industrial effluents and groundwater of Hattar industrial estate, Haripur. Adv. Agric. Environ. Sci. Open Access (AAEOA) 2018, 1, 70–77. [Google Scholar]
- Yang, X.E.; Jin, X.F.; Feng, Y.; Islam, E. Molecular mechanisms and genetic basis of heavy metal toler-ance/hyperaccumulation in plants. J. Integr. Plant Biol. 2005, 47, 1025–1035. [Google Scholar] [CrossRef]
- Nriagu, J.O. A global assessment of natural sources of atmospheric trace metals. Nat. Cell Biol. 1989, 338, 47–49. [Google Scholar] [CrossRef]
- Yang, J.; Wei, W.; Pi, S.; Ma, F.; Li, A.; Wu, D.; Xing, J. Competitive adsorption of heavy metals by extracellular polymeric substances extracted from Klebsiella sp. J1. Bioresour. Technol. 2015, 196, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.; Egwurugwu, J. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Bravo, S.; Amorós, J.; Pérez-de-los-Reyes, C.; García, F.; Moreno, M.; Sánchez-Ormeño, M.; Higueras, P. Influence of the soil pH in the uptake and bioaccumulation of heavy metals (Fe, Zn, Cu, Pb and Mn) and other elements (Ca, K, Al, Sr and Ba) in vine leaves, Castilla-La Mancha (Spain). J. Geochem. Explor. 2017, 174, 79–83. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Prasher, P.; Mudila, H.; Sharma, M. Biosorption and Bioaccumulation of Pollutants for Environmental Remediation. In Microorganisms for Sustainability; Springer International Publishing: Singapore, 2021; pp. 379–405. [Google Scholar]
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Swizerland, 1996. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, First Addendum to the Fourth Edition; World Health Organization: Geneva, Swizerland, 2017. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Code of Federal Regulations-2003, Title 40-PART 141—NATIONAL PRIMARY DRINKING WATER REGULATIONS, Subpart B—Maximum Contaminant Levels. Available online: https://www.epa.gov/sites/default/files/2015-11/documents/howepargulates_cfr-2003-title40-vol20-part141_0.pdf (accessed on 30 October 2021).
- European Union. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union 2020, 435, 1–62. [Google Scholar]
- The National Standards of the People’s Republic of China. Environmental Quality Standards for Surface Water. Available online: http://english.mee.gov.cn/SOE/soechina1997/water/standard.htm (accessed on 30 October 2021).
- Water, E.A.W. The Water Supply (Water Quality) Regulations 2016, PART 13 Amendments and Revocations. Available online: https://www.legislation.gov.uk/uksi/2016/614/pdfs/uksi_20160614_en.pdf (accessed on 30 October 2021).
- Hayashi, K.; Rivai, I.F.; Herawati, N.; Suzuki, S.; Koyama, H. Cadmium, Copper, and Zinc Levels in Rice and Soil of Japan, Indonesia, and China by Soil Type. Bull. Environ. Contam. Toxicol. 2000, 64, 33–39. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Mahadevan, K. Seaweeds: A sustainable food source. In Seaweed Sustainability; Elsevier BV: Manchester, UK, 2015; pp. 347–364. [Google Scholar]
- Collins, K.G. An investigation of the prebiotic potential and gut health benefits of Irish seaweeds. Univ. Coll. Cork 2017, 371, 31–35. [Google Scholar]
- Gade, R.; Tulasi, M.S.; Bhai, V.A. Seaweeds: A novel biomaterial. Int. J. Pharm. Pharm. Sci. 2013, 5, 975–1491. [Google Scholar]
- Harbo, J.R.; Harris, J.W. Heritability in Honey Bees (Hymenoptera: Apidae) of Characteristics Associated with Resistance to Varroa jacobsoni(Mesostigmata: Varroidae). J. Econ. Entomol. 1999, 92, 261–265. [Google Scholar] [CrossRef][Green Version]
- Bittner, L.; Payri, C.; Couloux, A.; Cruaud, C.; De Reviers, B.; Rousseau, F. Molecular phylogeny of the Dictyotales and their position within the Phaeophyceae, based on nuclear, plastid and mitochondrial DNA sequence data. Mol. Phylogenetics Evol. 2008, 49, 211–226. [Google Scholar] [CrossRef]
- Yalçın, S.; Sezer, S.; Apak, R. Characterization and lead (II), cadmium (II), nickel (II) biosorption of dried marine brown macro algae Cystoseira barbata. Environ. Sci. Pollut. Res. 2012, 19, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Sheng, P.X.; Ting, Y.-P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Adamu, C.; Nganje, T.; Edet, A. Heavy metal contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Environ. Nanotechnol. Monit. Manag. 2015, 3, 10–21. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Tay, W.J.D.; Hidajat, K.; Uddin, M.S. Fe3O4/cyclodextrin polymer nanocom-posites for selective heavy metals removal from industrial wastewater. Carbohydr. Polymers 2013, 91, 322–332. [Google Scholar] [CrossRef]
- Turan, N.G.; Mesci, B. Use of Pistachio Shells as an Adsorbent for the Removal of Zinc(II) Ion. CLEAN—Soil Air Water 2011, 39, 475–481. [Google Scholar] [CrossRef]
- Pozdniakova, T.A.; Mazur, L.P.; Boaventura, R.A.; Vilar, V.J. Brown macro-algae as natural cation exchangers for the treatment of zinc containing wastewaters generated in the galvanizing process. J. Clean. Prod. 2016, 119, 38–49. [Google Scholar] [CrossRef]
- Mata, Y.; Blázquez, M.; Ballester, A.; González, F.; Muñoz, J.A. Characterization of the biosorption of cadmium, lead and copper with the brown alga Fucus vesiculosus. J. Hazard. Mater. 2008, 158, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Interactions of metal cations with anionic groups on the cell wall of the macroalga Vaucheria sp. Eng. Life Sci. 2010, 10, 209–217. [Google Scholar] [CrossRef]
- Gupta, V.; Rastogi, A. Biosorption of lead from aqueous solutions by green algae Spirogyra species: Kinetics and equilibrium studies. J. Hazard. Mater. 2008, 152, 407–414. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions From Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Errasquín, E.L.; Vázquez, C. Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere 2003, 50, 137–143. [Google Scholar] [CrossRef]
- Bishnoi, N.R.; Kumar, R.; Kumar, S.; Rani, S. Biosorption of Cr(III) from aqueous solution using algal biomass spirogyra spp. J. Hazard. Mater. 2007, 145, 142–147. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Shojaosadati, S.; Ranaie, S.; Mousavi, S. Optimization and evaluation of acetylcholine esterase immobilization on ceramic packing using response surface methodology. Process. Biochem. 2010, 45, 81–87. [Google Scholar] [CrossRef]
- Jalali, R.; Ghafourian, H.; Asef, Y.; Davarpanah, S.; Sepehr, S. Removal and recovery of lead using nonliving biomass of marine algae. J. Hazard. Mater. 2002, 92, 253–262. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.; Munoz, J. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chavan, S.L.; Sapkale, P.H. Heavy Metal Concentrations in Water, Sediments and Body Tissues of Red Worm (Tubifex spp.) Collected from Natural Habitats in Mumbai, India. Environ. Monit. Assess. 2006, 129, 471–481. [Google Scholar] [CrossRef]
- Pavasant, P.; Apiratikul, R.; Sungkhum, V.; Suthiparinyanont, P.; Wattanachira, S.; Marhaba, T.F. Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour. Technol. 2006, 97, 2321–2329. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chang, S.-P. The biosorption of heavy metals from aqueous solution by Spirogyra and Cladophora filamentous macroalgae. Bioresour. Technol. 2011, 102, 5297–5304. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Balasubramanian, R.; Iyer, C. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour. Technol. 2007, 98, 452–455. [Google Scholar] [CrossRef]
- Rajfur, M.; Kłos, A.; Wacławek, M. Sorption of copper(II) ions in the biomass of alga Spirogyra sp. Bioelectrochemistry 2012, 87, 65–70. [Google Scholar] [CrossRef]
- Hashim, M.; Chu, K. Biosorption of cadmium by brown, green, and red seaweeds. Chem. Eng. J. 2004, 97, 249–255. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Rastogi, A. Equilibrium and kinetic modelling of cadmium (II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J. Hazard. Mater. 2008, 153, 759–766. [Google Scholar] [CrossRef]
- Rajfur, M.; Kłos, A.; Wacławek, M. Sorption properties of algae Spirogyra sp. and their use for determination of heavy metal ions concentrations in surface water. Bioelectrochemistry 2010, 80, 81–86. [Google Scholar] [CrossRef]
- Zakhama, S.; Dhaouadi, H.; M’Henni, F. Nonlinear modelisation of heavy metal removal from aqueous solution using Ulva lactuca algae. Bioresour. Technol. 2011, 102, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.M. Biosorption of heavy metal ions from aqueous solution by red macroalgae. J. Hazard. Mater. 2011, 192, 1827–1835. [Google Scholar] [CrossRef]
- Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Copper removal by algae Gelidium, agar extraction algal waste and granu-lated algal waste: Kinetics and equilibrium. Bioresour. Technol. 2008, 99, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.; Tuzen, M. Biosorption of cadmium(II) from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 157, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Lodeiro, P.; García-Casal, L.J.; Vilariño, T.; Rey-Castro, C.; David, C.; Rodríguez, P. Full description of copper uptake by algal biomass combining an equilibrium NICA model with a kinetic intraparticle diffusion driving force approach. Bioresour. Technol. 2011, 102, 2990–2997. [Google Scholar] [CrossRef]
- Rathinam, A.; Maharshi, B.; Janardhanan, S.K.; Jonnalagadda, R.R.; Nair, B.U. Biosorption of cadmium metal ion from simulated wastewaters using Hypnea valentiae biomass: A kinetic and thermodynamic study. Bioresour. Technol. 2010, 101, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Murphy, V.; Hughes, H.; McLoughlin, P. Comparative study of chromium biosorption by red, green and brown seaweed biomass. Chemosphere 2008, 70, 1128–1134. [Google Scholar] [CrossRef]
- Holan, Z.R.; Volesky, B. Biosorption of lead and nickel by biomass of marine algae. Biotechnol. Bioeng. 1994, 43, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.; Volesky, B.; Vieira, R. Sargassum seaweed as biosorbent for heavy metals. Water Res. 2000, 34, 4270–4278. [Google Scholar] [CrossRef]
- Kleinübing, S.; Silva, E.; da Silva, M.G.C.; Guibal, E. Equilibrium of Cu(II) and Ni(II) biosorption by marine alga Sargassum filipendula in a dynamic system: Competitiveness and selectivity. Bioresour. Technol. 2011, 102, 4610–4617. [Google Scholar] [CrossRef]
- Ahmady-Asbchin, S.; Andres, Y.; Gerente, C.; Le Cloirec, P. Biosorption of Cu(II) from aqueous solution by Fucus serratus: Surface characterization and sorption mechanisms. Bioresour. Technol. 2008, 99, 6150–6155. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.; Costa, A.; Da Costa, A.C.A.; Henriques, C. Competitive biosorption of cadmium(II) and zinc(II) ions from binary systems by Sargassum filipendula. Bioresour. Technol. 2010, 101, 5104–5111. [Google Scholar] [CrossRef]
- Lodeiro, P.; Cordero, B.; Barriada, J.L.; Herrero, R.; de Vicente, M.S. Biosorption of cadmium by biomass of brown marine macroalgae. Bioresour. Technol. 2005, 96, 1796–1803. [Google Scholar] [CrossRef]
- Cazón, J.P.; Bernardelli, C.; Viera, M.; Donati, E.; Guibal, E. Zinc and cadmium biosorption by untreated and calci-um-treated Macrocystis pyrifera in a batch system. Bioresour. Technol. 2012, 116, 195–203. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Keshtkar, A.; Safdari, J.; Abadi, Z. Biosorption of nickel(II) from aqueous solution by brown algae: Equilibrium, dynamic and thermodynamic studies. J. Hazard. Mater. 2010, 175, 304–310. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.P. Biosorption of hexavalent chromium onto raw and chemically modified Sargassum sp. Bioresour. Technol. 2008, 99, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, Y.G.; Rico, I.L.R.; Guibal, E.; de Hoces, M.C.; Martín-Lara, M.Á. Biosorption of hexavalent chromium from aqueous solution by Sargassum muticum brown alga. Application of statistical design for process optimization. Chem. Eng. J. 2012, 183, 68–76. [Google Scholar] [CrossRef]
- Guo, D.; Mitchell, R.J.; Withington, J.M.; Fan, P.-P.; Hendricks, J.J. Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: Root branch order predominates. J. Ecol. 2008, 96, 737–745. [Google Scholar] [CrossRef]
- Pathe, P.P.; Nandy, T.; Kaul, S.N.; Szpyrokwicz, L. Chromium recovery from chrome tan wastewater. Int. J. Environ. Stud. 1996, 51, 125–145. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Elbisser, B. 2nd European Meeting on Environmental Chemistry. In Proceedings of the 2nd European Meeting on Environmental Chemistry, Dijon, France, 12–15 December 2001; p. 273. [Google Scholar]
- Vegliò, F.; Quaresima, R.; Fornari, P.; Ubaldini, S. Recovery of valuable metals from electronic and galvanic industrial wastes by leaching and electrowinning. Waste Manag. 2003, 23, 245–252. [Google Scholar] [CrossRef]
- Dahbi, S.; Azzi, M.; Saib, N.; De la Guardia, M.; Faure, R.; Durand, R. Removal of trivalent chromium from tannery waste waters using bone charcoal. Anal. Bioanal. Chem. 2002, 374, 540–546. [Google Scholar] [CrossRef]
- Park, S.-J.; Jung, W.-Y. Removal of chromium by activated carbon fibers plated with copper metal. Carbon Lett. 2001, 2, 15–21. [Google Scholar]
- Bai, R.; Abraham, T. Studies on enhancement of Cr(VI) biosorption by chemically modified biomass of Rhizopus nigricans. Water Res. 2002, 36, 1224–1236. [Google Scholar] [CrossRef]
- Chong, K.H.; Volesky, B. Metal biosorption equilibria in a ternary system. Biotechnol. Bioeng. 1996, 49, 629–638. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, J.H.; Ahn, Y.; Chung, J.W. Comparison of Heavy Metal Adsorption by Peat Moss and Peat Moss-Derived Biochar Produced Under Different Carbonization Conditions. Water Air Soil Pollut. 2015, 226, 9. [Google Scholar] [CrossRef]
- Ofomaja, A.; Ho, Y.-S. Effect of pH on cadmium biosorption by coconut copra meal. J. Hazard. Mater. 2007, 139, 356–362. [Google Scholar] [CrossRef]
- Meunier, N.; Laroulandie, J.; Blais, J.; Tyagi, R. Cocoa shells for heavy metal removal from acidic solutions. Bioresour. Technol. 2003, 90, 255–263. [Google Scholar] [CrossRef]
- Tan, L.C.; Choa, V.; Tay, J.H. The Influence of pH on Mobility of Heavy Metals from Municipal Solid Waste Incinerator Fly Ash. Environ. Monit. Assess. 1997, 44, 275–284. [Google Scholar] [CrossRef]
- Foday Jr, E.H.; Ramli, N.A.S.; Ismail, H.N.; Malik, N.A.; Basri, H.F.; Aziz, F.S.A.; Nor, N.S.M.; Jumhat, F. Municipal solid waste characteristics in Taman Universiti, Skudai, Johore, Malaysia. J. Adv. Res. Des. 2017, 38, 13–20. [Google Scholar]
- Abia, A.; Asuquo, E. Lead (II) and nickel (II) adsorption kinetics from aqueous metal solutions using chemically modified and unmodified agricultural adsorbents. Afr. J. Biotechnol. 2006, 5, 1475–1482. [Google Scholar]
- Al-Asheh, S.; Banat, F.; Al-Rousan, D. Adsorption of Copper, Zinc and Nickel Ions from Single and Binary Metal Ion Mixtures on to Chicken Feathers. Adsorpt. Sci. Technol. 2002, 20, 849–864. [Google Scholar] [CrossRef]
- Abu Al-Rub, F.A.; Kandah, M.; Al-Dabaybeh, N. Competitive Adsorption of Nickel and Cadmium on Sheep Manure Wastes: Experimental and Prediction Studies. Sep. Sci. Technol. 2003, 38, 483–497. [Google Scholar] [CrossRef]
- Özdemir, N.; Horn, R.; Friedt, W. Construction and characterization of a BAC library for sunflower (Helianthus annuus L.). Euphytica 2004, 138, 177–183. [Google Scholar] [CrossRef]
- Ajmal, M.; Rao, R.A.K.; Anwar, S.; Ahmad, J.; Ahmad, R. Adsorption studies on rice husk: Removal and recovery of Cd(II) from wastewater. Bioresour. Technol. 2003, 86, 147–149. [Google Scholar] [CrossRef]
- Ajmal, M.; Rao, R.A.K.; Ahmad, R.; Ahmad, J. Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni(II) from electroplating wastewater. J. Hazard. Mater. 2000, 79, 117–131. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I.; Martínez, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb (II), Ni (II), Cu (II) and Cd (II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 2006, 50, 132–140. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Heavy metal biosorption sites in Aspergillus niger. Bioresour. Technol. 1997, 61, 221–227. [Google Scholar] [CrossRef]
- Vaishya, R.; Prasad, S. Adsorption of copper (II) on sawdust. Indian J. Environ. Prot. 1991, 11, 284–289. [Google Scholar]
- Vázquez, G.; Antorrena, G.; González-Álvarez, J.; Doval, M. Adsorption of heavy metal ions by chemically modified Pinus pinaster bark. Bioresour. Technol. 1994, 48, 251–255. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Gupta, V.K.; Mohan, D. Removal of Lead and Chromium by Activated Slag—A Blast-Furnace Waste. J. Environ. Eng. 1997, 123, 461–468. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.; Adrian, D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Keskinkan, O.; Goksu, M.; Basibuyuk, M.; Forster, C. Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresour. Technol. 2004, 92, 197–200. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.; Peralta-Videa, J.; Montes, M.; de la Rosa, G.; Corral-Diaz, B. Bioaccumulation of cadmium, chromium and copper by Convolvulus arvensis L.: Impact on plant growth and uptake of nutritional elements. Bioresour. Technol. 2004, 92, 229–235. [Google Scholar] [CrossRef]
- Dumitriu, S. Polysaccharides as Biomaterials. In Polymeric Biomaterials, Revised and Expanded; CRC Press: New York, NY, USA, 2001; pp. 15–76. [Google Scholar]
- Brown, P.; Gill, S.; Allen, S.J. Determination of optimal peat type to potentially capture copper and cadmium from solu-tion. Water Environ. Res. 2001, 73, 351–362. [Google Scholar] [CrossRef]
- Celis, R.; Hermosín, M.C.; Cornejo, J. Heavy Metal Adsorption by Functionalized Clays. Environ. Sci. Technol. 2000, 34, 4593–4599. [Google Scholar] [CrossRef]
- Cameron, H.; Mata, M.T.; Riquelme, C. The effect of heavy metals on the viability of Tetraselmis marina AC16-MESO and an evaluation of the potential use of this microalga in bioremediation. PeerJ 2018, 6, e5295. [Google Scholar] [CrossRef]
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. [Google Scholar] [CrossRef] [PubMed]
- Priatni, S.; Kosasih, W.; A Budiwati, T.; Ratnaningrum, D. Production of peptone from boso fish (Oxyeleotris marmorata) for bacterial growth medium. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Tangerang, Indonesia, 2017; p. 012009. [Google Scholar]
- Upadhyay, A.; Singh, N.; Singh, R.; Rai, U. Amelioration of arsenic toxicity in rice: Comparative effect of inoculation of Chlorella vulgaris and Nannochloropsis sp. on growth, biochemical changes and arsenic uptake. Ecotoxicol. Environ. Saf. 2016, 124, 68–73. [Google Scholar] [CrossRef]
- Gómez-Jacinto, V.; García-Barrera, T.; Gómez-Ariza, J.L.; Garbayo-Nores, I.; Vílchez-Lobato, C. Elucidation of the defence mechanism in microalgae Chlorella sorokiniana under mercury exposure. Identification of Hg–phytochelatins. Chemi-Co-Biol. Interact. 2015, 238, 82–90. [Google Scholar] [CrossRef]
- Balaji, S.; Kalaivani, T.; Sushma, B.; Pillai, C.V.; Shalini, M.; Rajasekaran, C. Characterization of sorption sites and differ-ential stress response of microalgae isolates against tannery effluents from Ranipet industrial area—An application towards phycoremediation. Int. J. Phytoremediat. 2016, 18, 747–753. [Google Scholar] [CrossRef]
- Devars, S.; Avilés, C.; Cervantes, C.; Moreno-Sánchez, R. Mercury uptake and removal by Euglena gracilis. Arch. Microbiol. 2000, 174, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Costa, L.; Rybski, D.; Lucht, W.; Kropp, J.P. A systematic study of sustainable development goal (SDG) in-teractions. Earth’s Future 2017, 5, 1169–1179. [Google Scholar] [CrossRef]
- Ibuot, A.; Dean, A.P.; McIntosh, O.A.; Pittman, J.K. Metal bioremediation by CrMTP4 over-expressing Chlamydomonas reinhardtii in comparison to natural wastewater-tolerant microalgae strains. Algal Res. 2017, 24, 89–96. [Google Scholar] [CrossRef]


| Drinking Water Acceptable Standards in (mg L−1) | |||||
|---|---|---|---|---|---|
| Metals | WHO [20] | USEPA [21] | EU Standard [22] | MEE-China [23] | DWI-UK [24] |
| Nickel (Ni) | 0.07 | - | 0.020 | 0.000 | 0.02 |
| Lead (Pb) | 0.01 | 0.015 | 0.005 | 0.010 | 0.01 |
| Zinc (Zn) | - | 5.0 | - | 0.05 | - |
| Copper (Cu) | 2.0 | 1.0 | 2.000 | 1.000 | 2.0 |
| Cadmium (Cd) | 0.003 | 0.005 | 0.005 | 0.005 | 0.005 |
| Mercury (Hg) | 0.006 | 0.002 | 0.001 | 0.00005 | 0.001 |
| Arsenic (As) | 0.01 | 0.010 | 0.01 | 0.050 | 0.01 |
| Chromium (Cr) | 0.05 | 0.100 | 0.025 | 0.050 | 0.05 |
| Antimony | 0.02 | - | 0.01 | - | 0.005 |
| Bromate | 0.01 | - | 0.01 | - | 0.01 |
| Uranium | 0.03 | 0.03 | 0.03 | - | - |
| Common Name (Phylum) | Body Form | Size | Pigments | Colour Composition | Cell Walls |
|---|---|---|---|---|---|
| Brown algae (Phaeophyta) | Multicellular | 60 cm–60 m | Chlorophyll, Fucoxanthin, and several other xanthophylls | Golden-brown, Greenish-brown | Cellulose, Alginate, Fucoidan |
| Red algae (Rhodophyta) | Multicellular | 50 cm–2 m | Chlorophyll, Phycocyanin, Phycoerythrin, and several xanthophylls | Brownish red, Purple | Cellulose, Xylans, Galactans |
| Green algae (Chlorophyta) | Unicellular, Colonial, Filamentous, Multicellular | 1–1000 μm | a and b Chlorophyll and several xanthophylls | Green | Cellulose Hydroxyl –proline glucosides β- xylans, β-mannans |
| Species of Algae | Metal Ions | qmax (mmol/g) | pH | References |
|---|---|---|---|---|
| Green Algae | ||||
| Ulva lactuca | Pb(II) | 0.61 | 4.5 | [45] |
| Cladophora glomerata | 0.35 | 4.5 | [45] | |
| Ulva sp. | 1.46 | 5.0 | [33] | |
| Codium vermilara | 0.30 | 5.0 | [46] | |
| Spirogyra insignis | 0.24 | 5.0 | [46] | |
| Spirogyra neglecta | 0.56 | 5.0 | [47] | |
| Caulerpa lentillifera | 0.13 | 5.0 | [48] | |
| Spirogyra sp. | 0.43 | 5.0 | [49] | |
| Cladophora sp. | 0.22 | 5.0 | [49] | |
| Ulva sp. | Cu(II) | 0.75 | 5.0 | [33] |
| Codium vermilara | 0.26 | 5.0 | [46] | |
| Spirogyra insignis | 0.30 | 4.0 | [46] | |
| Spirogyra neglecta | 1.80 | 4.5 | [47] | |
| Ulva fasciata | 1.14 | 5.5 | [50] | |
| Caulerpa lentillifera | 0.08 | 5.0 | [48] | |
| Cladophora sp | 0.23 | 5.0 | [49] | |
| Spirogyra sp | 0.53 | 5.0 | [51] | |
| Ulva sp. | Cd(II) | 0.58 | 5.5 | [33] |
| Chaetomorpha linum | 0.48 | 5.0 | [52] | |
| Codium vermilara | 0.19 | 6.0 | [46] | |
| Spirogyra insignis | 0.20 | 6.0 | [46] | |
| Ulva lactuca | 0.25 | 5.0 | [53] | |
| Oedogonium sp. | 0.79 | 5.0 | [54] | |
| Caulerpa lentillifera | 0.04 | 5.0 | [48] | |
| Spirogyra sp. | 0.006 a | - | [55] | |
| Ulva sp. | Zn(II) | 0.54 | 5.5 | [33] |
| Codium vermilara | 0.36 | 6 | [46] | |
| Spirogyra insignis | 0.32 | 6 | [46] | |
| Caulerpa lentillifera | 0.04 | 5 | [48] | |
| Spirogyra s | 0.02 a | - | [55] | |
| Ulva sp. | Ni(II) | 0.29 | 5.5 | [33] |
| Codium vermilara | 0.22 | 6.0 | [46] | |
| Spirogyra insignis | 0.29 | 6.0 | [46] | |
| Ulva lactuca | 1.14 | 4.5 | [56] | |
| Red Algae | ||||
| Gracilaria corticata | Pb(II) | 0.26 | 4.5 | [45] |
| Gracilaria canaliculata | 0.20 | 4.5 | [45] | |
| Polysiphonia violacea | 0.49 | 4.5 | [45] | |
| Gracillaria sp. | 0.45 | 5.0 | [33] | |
| Asparagopsis armata | 0.30 | 4.0 | [46] | |
| Jania rubens | 0.14 | 5.0 | [57] | |
| Pterocladia capillacea | 0.16 | 5.0 | [57] | |
| Corallina mediterranea | 0.31 | 5.0 | [57] | |
| Galaxaura oblongata | 0.42 | 5.0 | [57] | |
| Asparagopsis armata | 0.33 | 5.0 | [46] | |
| Chondrus crispus | 0.63 | 4.0 | [46] | |
| Gelidium | 0.51 | 5.3 | [58] | |
| Gracilaria changii | 0.23 | 5.0 | [52] | |
| Gracilaria edulis | 0.24 | 5.0 | [52] | |
| Gracilaria Salicornia | 0.16 | 5.0 | [52] | |
| Asparagopsis armata | 0.28 | 6.0 | [46] | |
| Ceramium virgatum | 0.35 | 5.0 | [59] | |
| Mastocarpus stellatus | 0.59 | 6.0 | [60] | |
| Jania rubens | 0.27 | 5.0 | [57] | |
| Corallina mediterranea | 0.57 | 5.0 | [57] | |
| Hypnea valentiae | 0.15 | 6.0 | [61] | |
| Palmaria palmate | Cr | 0.57 (Cr(III)) | 4.5 (Cr(III | [62] |
| 0.65 (Cr(VI)) | 2 (Cr(VI)) | |||
| Polysiphonia lanosa | 0.65 (Cr(III)) | 4.5(Cr(III)) | [62] | |
| 0.88 (Cr(VI)) | 2 (Cr(VI)) | |||
| Jania rubens | 0.54 (Cr(III)) | 5.0 (Cr(III)) | [57] | |
| Pterocladia capillacea | 0.66 (Cr(III)) | 5.0 (Cr(III)) | [57] | |
| Corallina mediterranea | 1.35 (Cr(III)) | 5.0 (Cr(III)) | [57] | |
| Galaxaura oblongata | 2.02 (Cr(III)) | 5.0 (Cr(III)) | [57] | |
| Jania rubens | Co(II) | 0.55 | 5.0 | [57] |
| Pterocladia capillacea | 0.89 | 5.0 | [57] | |
| Corallina mediterranea | 1.29 | 5.0 | [57] | |
| Galaxaura oblongata | 1.25 | 5.0 | [57] | |
| Brown Algae | ||||
| Ascophyllum nodosum | Pb(II) | 1.31 | 3.5 | [63] |
| Fucus vesiculosus | 1.11 | 3.5 | [63] | |
| Sargassum vulgare | 1.10 | 3.5 | [63] | |
| Sargassum hystrix | 1.37 | 4.5 | [45] | |
| Sargassum natans | 1.14 | 4.5 | [45] | |
| Padina pavonia | 1.04 | 4.5 | [45] | |
| Sargassum sp. | 1.16 | 5.0 | [33] | |
| Padina sp. | 1.25 | 5.0 | [33] | |
| Fucus vesiculosus | 1.02 | 5.0 | [38] | |
| Fucus spiralis | 0.98 | 3.0 | [46] | |
| Ascophyllum nodosu | 0.86 | 3.0 | [46] | |
| Padina sp. | Cu(II) | 1.14 | 5.0 | [33] |
| Sargassum vulgarie | 0.93 | 4.5 | [64] | |
| Sargassum fluitans | 0.80 | 4.5 | [64] | |
| Sargassum filipendula | 0.89 | 4.5 | [64] | |
| Fucus vesiculosus | 1.66 | 5.0 | [38] | |
| Fucus spiralis | 1.10 | 4.0 | [46] | |
| Ascophyllum nodosum | 0.91 | 4.0 | [46] | |
| Sargassum filipendula | 1.32 | 4.5 | [65] | |
| Fucus serratus | 1.60 | 5.5 | [66] | |
| Sargassum sp. | 1.13 | 5.5 | [50] | |
| Sargassum sp. | Cd(II) | 0.76 | 5.5 | [33] |
| Padina sp | 0.75 | 5.5 | [33] | |
| Sargassum siliquosum | 0.73 | 5.0 | [52] | |
| Sargassum baccularia | 0.74 | 5.0 | [52] | |
| Padina tetrastomatica | 0.53 | 5.0 | [52] | |
| Sargassum vulgarie | 0.79 | 4.5 | [64] | |
| Sargassum fluitans | 0.71 | 4.5 | [64] | |
| Sargassum muticum | 0.68 | 4.5 | [64] | |
| Fucus vesiculosus | 0.96 | 6.0 | [38] | |
| Fucus spiralis | 1.02 | 6.0 | [46] | |
| Ascophyllum nodosum | 0.78 | 6.0 | [46] | |
| Sargassum filipendula | 1.17 | 5.0 | [67] | |
| Bifurcaria bifurcate | 0.65 | 4.5 | [68] | |
| Saccorhiza polyschides | 0.84 | 4.5 | [68] | |
| Ascophyllum nodosum | 0.70 | 4.5 | [68] | |
| Laminaria ochroleuca | 0.56 | 4.5 | [68] | |
| Pelvetia caniculata | 0.66 | 4.5 | [68] | |
| Macrocystis pyrifera | 0.89 | 3.0 | [69] | |
| Sargassum sp. | Zn(II) | 0.50 | 5.5 | [33] |
| Padina sp. | 0.81 | 5.5 | [33] | |
| Fucus spiralis | 0.81 | 6.0 | [46] | |
| Ascophyllum nodosum | 0.64 | 6.0 | [46] | |
| Sargassum filipendula | 0.71 | 5.0 | [67] | |
| Macrocystis pyrifera | 0.91 | 4.0 | [69] | |
| Sargassum fluitans | Ni(II) | 0.75 | 3.5 | [63] |
| Ascophyllum nodosum | 0.69 | 3.5 | [63] | |
| Sargassum natans | 0.41 | 3.5 | [63] | |
| Fucus vesiculosus | 0.39 | 3.5 | [63] | |
| Sargassum vulgare | 0.09 | 3.5 | [63] | |
| Sargassum sp | 0.61 | 5.5 | [33] | |
| Padina sp. | 0.63 | 5.5 | [33] | |
| Cystoseria indica | 0.85 | 6.0 | [70] | |
| Nizmuddinia zanardini | 0.94 | 6.0 | [70] | |
| Sargassum glaucescensand | 0.94 | 6.0 | [70] | |
| Padina australis | 0.46 | 6.0 | [70] | |
| Fucus spiralis | 0.85 | 6.0 | [46] | |
| Ascophyllum nodosum | 0.73 | 6.0 | [46] | |
| Sargassum filipendula | 1.07 | 4.5 | [65] | |
| Fucus vesiculosus | Cr | 1.21 (Cr(III)) | 4.5 (Cr(III)) | [62] |
| 0.82 (Cr(VI)) | 2 (Cr(VI)) | |||
| Fucus spiralis | 1.17 (Cr(III)) | 4.5 (Cr(III)) | [62] | |
| 0.68 (Cr(VI)) | 2 (Cr(VI)) | |||
| Sargassum sp. | 0.60 (Cr(VI)) | 2 (Cr(VI)) | [71] | |
| Sargassum muticum | 3.77 (Cr(VI)) | 2 (Cr(VI)) | [72] | |
| Materials Used | Heavy Metals | References |
|---|---|---|
| Polymers | Fe and Cr | [74] |
| Sawdust and tree barks | Hg, Pb, and Zn | [75] |
| Electronic waste along with galvanic wastes | Cu, Ni, Mn, Pb, Sn | [76] |
| charcoal: | Cr(III) | [77] |
| Clay | Cr(III) | [78] |
| Fungi | Cr, Fe | [79] |
| Dead biomass | Cr | [80] |
| Peat moss | Cr, Fe | [81] |
| Peanut shells, Rice husk, Straw, and walnut cover | Cr, Cu, Ni | [82] |
| Cocoa shell | Al, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn | [83] |
| Coconut husk | Cr, As | [82] |
| Caol and fly ashes | Cr, Cu, Ni | [84] |
| Banana pith and peels | Ni, Pb | [85] |
| Cassava fiber | Pb, Co | [86] |
| Chicken feathers | Al, As | [87] |
| Sheep manure wastes | Ca, Cd | [88] |
| Sunflower | Co, Cr | [89] |
| Rice byproducts | Cu, Fe | [90] |
| Orange peels | Cu, Fe, Hg | [91] |
| Palm kernel fiber | Fe, Hg | [82] |
| Grape stalks | Cr, Fe, Hg | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foday Jr, E.H.; Bo, B.; Xu, X. Removal of Toxic Heavy Metals from Contaminated Aqueous Solutions Using Seaweeds: A Review. Sustainability 2021, 13, 12311. https://doi.org/10.3390/su132112311
Foday Jr EH, Bo B, Xu X. Removal of Toxic Heavy Metals from Contaminated Aqueous Solutions Using Seaweeds: A Review. Sustainability. 2021; 13(21):12311. https://doi.org/10.3390/su132112311
Chicago/Turabian StyleFoday Jr, Edward Hingha, Bai Bo, and Xiaohui Xu. 2021. "Removal of Toxic Heavy Metals from Contaminated Aqueous Solutions Using Seaweeds: A Review" Sustainability 13, no. 21: 12311. https://doi.org/10.3390/su132112311
APA StyleFoday Jr, E. H., Bo, B., & Xu, X. (2021). Removal of Toxic Heavy Metals from Contaminated Aqueous Solutions Using Seaweeds: A Review. Sustainability, 13(21), 12311. https://doi.org/10.3390/su132112311







