COVID-19 Syndrome: Nexus with Herbivory and Exposure Dynamics for Monitoring Livestock Welfare and Agro-Environment
Abstract
:1. Introduction
1.1. Herbivory and Intricacies of Exposure Dynamics
1.2. Justification for the Review
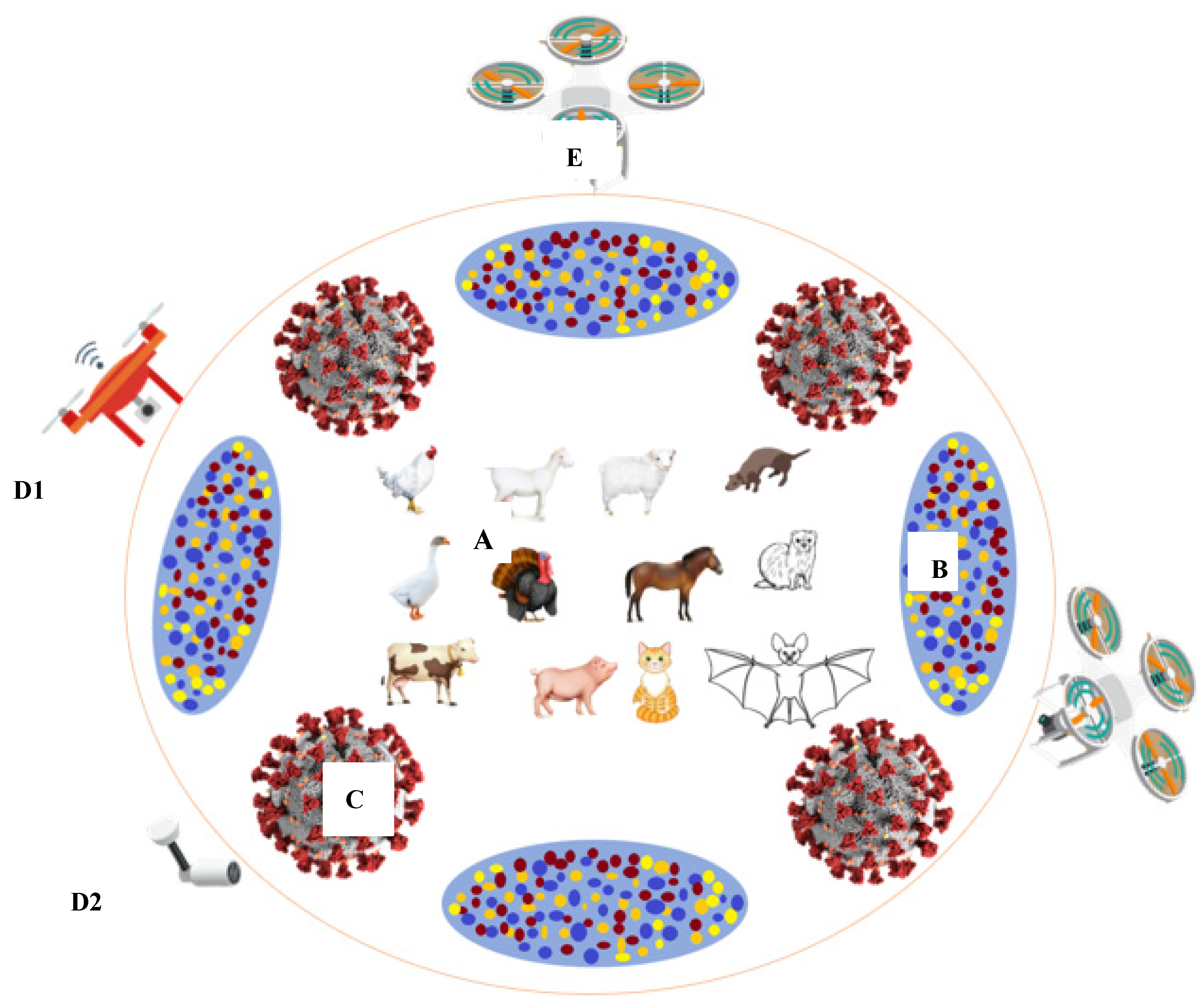
2. Comorbidity of Coronavirus Infections across Species
2.1. Pyrogenicity and Vital Signs of COVID-19 Infection
2.2. Endocytosis of Coronaviruses
3. Viral and Host Receptor Interaction
3.1. Viral Tropism and Immunogenicity of Coronaviruses
3.2. Evolution and Mutability of SARS-CoV-2
4. Exposure Dynamics and Organ Functionality
4.1. Blood Types and Susceptibility to Coronavirus Variants
4.2. Herd Immunity Versus Eco-Exposure to Viral Infections
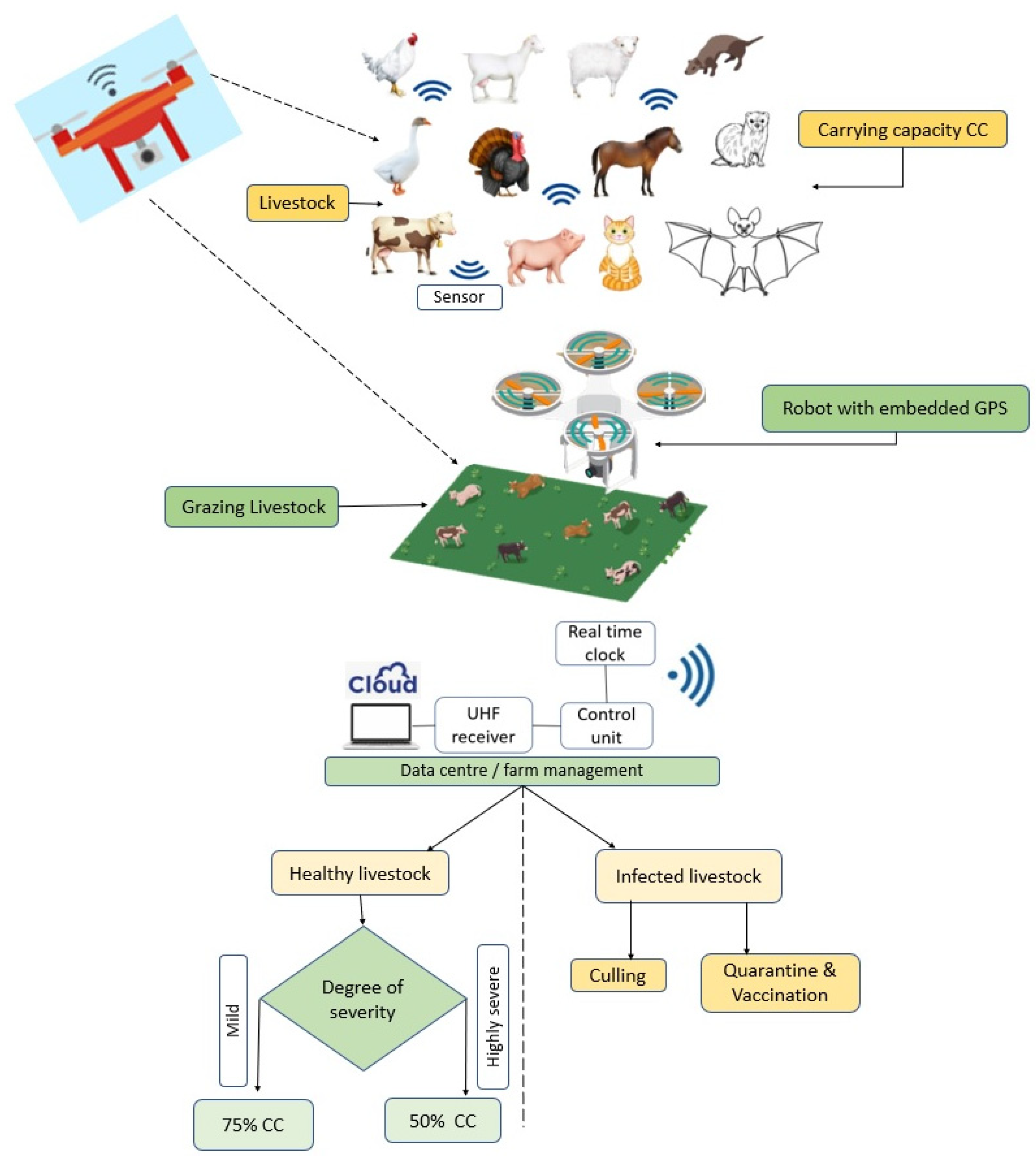
4.3. Biosensing and Smart Precision Farming Models in COVID-19 Era
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hui, D.S.; IAzhar, E.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntosh, A.; Bachmann, M.; Siedner, M.J.; Gareta, D.; Seeley, J.; Herbst, K. Effect of COVID-19 lockdown on hospital admissions and mortality in rural KwaZulu-Natal, South Africa: Interrupted time series analysis. BMJ Open 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Pizzol, D.; Marotta, C.; Antunes, M.; Racalbuto, V.; Veronese, N.; Smith, L. Coronavirus Diseases (COVID-19) Current Status and Future Perspectives: A Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 1-11. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med Res. 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019-novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Worldometers, 2021. COVID-19 Coronavirus Pandemic. Available online: worldometers.info/coronavirus/?zarsrc=130 (accessed on 21 August 2021).
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2021, 12, 1-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, A.R.; Erdogan, A.; Agaoglu, P.M.; Dineri, Y.; Cakirci, A.Y.; Senel, M.E.; Okyay, R.A.; Tasdogan, A.M. Novel Coronavirus (COVID-19) Outbreak: A review of the current literature. EJMO 2020, 4, 1–7. [Google Scholar]
- Peiris, J.S.M.; Chu, C.M.; Cheng, V.C.C.; Chan, K.S.; Hung, I.F.N.; Poon, L.M.; Law, K.I.; Tang, B.S.F.; Hon TY, W.; Chan, C.S.; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- World Health Organization. WHO Statement Regarding Cluster of Pneumonia Cases in Wuhan, China Geneva (updated 9–14 January 2020). Available online: https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding-cluster-of-pneumoniacases-in-wuhan-china (accessed on 21 August 2021).
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Coronaviruses. In Fenner and White’s Medical Virology; Elsevier: Philadelphia, PA, USA, 2017; pp. 437–446. [Google Scholar]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Beijerinck, M.W. Concerning a contagium vivum fluidum as cause of the spot disease of tobacco leaves. American Phytopathological Society, Johnson, J., Ed. Classics 1898, 7, 33–52. [Google Scholar]
- Takahashi, H.; Fukuhara, T.; Kitazawa, H.; Kormelink, R. Virus latency and the impact on plants. Front. Microbiol. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Shi, M.; Holmes, E.C. Using metagenomics to characterize an expanding virosphere. Cell 2018, 172, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.A.A. (Ed.) Defining Principles; Sustainability Science and Engineerings Series; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 513–518. [Google Scholar]
- Schowalter, T.; Pandeya, M.; Presley, S.J.; Willig, M.R.; Zimmerman, J.K. Arthropods are not declining but are responsive to disturbance in the Luquillo Experimental Forest, Puerto Rico. Proc. Nat. Acad. Sci. USA 2021, 118, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hefner, J.T.; Linde, K.C. Nasal Aperture shape: Functional Morphology. In Atlas of Human Cranial Macromorphoscopic Traits, 1st ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 73–88. [Google Scholar]
- Woolhouse, M.E.J.; Adair, K.; Brierley, L. RNA viruses: A case study of the biology of emerging infectious diseases. Microbiol. Spectr. 2013, 1, 1–16. [Google Scholar] [CrossRef]
- Ashour, H.M.; Elkhatib, W.F.; Rahman, M.M.; Elshabrawy, H.A. Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens 2020, 9, 1-15. [Google Scholar] [CrossRef] [Green Version]
- Al-Ahmadi, A.; Alahmadi, M.; Al-Zahrani, A. Spatial association between primary Middle East respiratory syndrome coronavirus infection and exposure to dromedary camels in Saudi Arabia Zoo. Public Health 2020, 67, 382–390. [Google Scholar]
- Cossart, P.; Helenius, A. Endocytosis of viruses and bacteria. Cold Spring Habor Perspect. Biol. 2014, 4, 1–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maclachlan, N.J.; Edward, J.; Dubovi, E.J. Coronaviridae. In Fenner’s Veterinary Virology, 5h ed.; Elsevier: London, UK, 2017; pp. 435–461. [Google Scholar]
- Almendros, A. Can companion animals become infected with Covid-19? Vet. Record 2020, 186, 388–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.I.; Kim, S.G.; Kim, S.M.; Kim, E.H.; Park, S.J.; Yu, K.M.; Chang, J.H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Principi, N. School Closure during the Coronavirus Disease 2019 (COVID-19) Pandemic: An Effective Intervention at the Global Level? JAMA Pediatr. 2021, 174, 921–922. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wei, M.; Yuan, J.; Liu, Y.; Fu, T.; Yu, X.; Zhang, Z.J. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA 2020, 323, 1313–1314. [Google Scholar] [CrossRef]
- Desforges, M.; Coupanec, A.L.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dube, M.; Talbot, P.J. Human. Coronaviruses opportunistic pathogens of the central nervous system? Viruses 2019, 12, 1-28. [Google Scholar] [CrossRef] [Green Version]
- Chow, E.J.; Schwartz, N.G.; Tobolowsky, F.A.; Zacks, R.L.; Huntington-Frazier, M.; Reddy, S.C.; Rao, A.K. Symptom screening at illness onset of health care personnel with SARS-CoV-2 Infection in King County, Washington. JAMA 2020, 323, 2087–2089. [Google Scholar] [CrossRef]
- Ogoina, D. Fever, fever patterns and diseases called ‘fever’—A review. J. Infec. Public Health 2011, 4, 108–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartung, T. Pyrogen testing revisited on occasion of the 25th anniversary of the whole blood monocyte activation test. ALTEX 2021, 38, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Pranitha, N.; Sthira, S.S.; Mohanan, P.V. Pyrogens, a polypeptide produces fever by metabolic changes in hypothalamus: Mechanisms and detections. Immunol. Lett. 2018, 204, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Helenius, A. Virus entry at a glance. J. Cell Sci. 2013, 126, 1289–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; She, G.; Wu, T.; Xue, C.; Cao, Y. PEDV enters cells through clathrin, caveolae and lipid raft-mediated endocytosis and trafcs via the endo-lysosome pathway. Vet. Res. 2020, 51, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Carrasco-Hernandez, R.; Jácome, R.; LópezVidal, Y.; Ponce de León, S. Are RNA viruses candidate agents for the Next Global Pandemic? A Review. ILAR J. 2017, 58, 343–358. [Google Scholar] [CrossRef] [Green Version]
- Beachboard, D.C.; Horner, S.M. Innate immune evasion strategies of DNA and RNA viruses. Curr. Opin. Microbiol. 2016, 32, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Han, S.; Wang, L.F.; Shi, Z. Immunogenicity difference between the SARS coronavirus and the bat SARS-like coronavirus spike(S) proteins. Biochem. Biophy. Res. Com. 2009, 387, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE-2 protein, the functional receptor for SARS coronavirus: A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade long structural studies of SARS. J. Virol. 2020, 94, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE-2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 1–5. [Google Scholar] [CrossRef]
- Nomaguchi, M.; Fujita, M.; Miyazaki YAdachi, A. Viral tropism. Front. Microbiol. 2012, 3, 281. [Google Scholar] [CrossRef] [Green Version]
- Lescure, F.-X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.-H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Centre for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Abdul Amir, A.S.; Hafidh, R.R. The possible immunological pathways for the variable immunopathogenesis of COVID-19 Infections among healthy adults, elderly and children. Electron. J. Gen. Med. 2020, 17, 1–4. [Google Scholar]
- Katze, M.G.; He, Y.; Gale, M. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Chen, J.; Xiang, R.; Song, H.; Shu, S.; Chen, L.; Liang, L.; Zhou, J.; You, L.; et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N. Engl. J. Med. 2020, 382, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J. Animal coronaviruses: What can they teach us about the severe acute respiratory syndrome? Rev. Sci. Technol. 2004, 23, 643–660. [Google Scholar] [CrossRef]
- Goubau, D.; Deddouche, S.; Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 2013, 38, 855–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and altered glycan theory of autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, I.; Tsumoto, K. Antigen-Antibody binding. Encycl. Life Sci. 2016, 1–8. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Walsh, K.B.; Rice, S.; Rosen, H.; Oldstone, M.B.A. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 3799–3804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.J.; Su, I.J.; Theron, M.; Wu, Y.C.; Lai, S.K.; Liu, C.C.; Lei, H.Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005, 75, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Powell, M.A.; Staedtke, V.; Bai, R.-Y.; Thomas, D.L.; Fischer, N.M.; Huq, S.; Khalafallah, A.M.; Koenecke, A.; Xiong, R.; et al. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. J. Clin. Investig. 2020, 130, 3345–3347. [Google Scholar] [CrossRef]
- Effros, R.B. Telomerase induction in T cells: A cure for aging and disease? Exp. Geront. 2007, 42, 416–420. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.L.; Lin, C.Y. Open reading frame phylogenetic analysis on the cloud. Int. J. Genom. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Rodgers, K.; McVey, M. Error-prone repair of DNA-double-strand breaks. J. Cell. Physiol. 2016, 231, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjuan, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral mutation rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef] [Green Version]
- Stern, A.; Andino, R. Viral evolution: It is all about mutations. In Viral Pathogenesis: From Basics to System Biology, 3rd ed; Elsevier: Amsterdam, The Netherlands, 2016; pp. 233–240. [Google Scholar]
- Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018, 16, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrault, D.; Moineau, S.; Duchaine, C. Methods for sampling of airborne viruses. Microbiol. Mol. Biol. Rev. 2008, 72, 413–444. [Google Scholar]
- Aliabadi, A.A.; Rogak, S.N.; Bartlett, K.H.; Green, S.I. Preventing airborne disease transmission: Review of methods for ventilation design in health care facilities. Adv. Prev. Med. 2011, 2011, 124064. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.; Chu, D.K.; Shiu, E.Y.; Chan, K.H.; McDevitt, J.J.; Hau, B.J.; Yen, H.L.; Li, Y.; Ip, D.K.; Peiris, J.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaman, J.; Kohn, M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. USA 2009, 106, 3243–3248. [Google Scholar] [CrossRef] [Green Version]
- Forni, D.; Cagliani, R.; Clerici, M.; Sironi, M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017, 25, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.; Lau, E.; Wong, J.Y.; et al. Early Transmission dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, W.; Li, B.; Li, D.J.; Zhang, J.; Zhao, F. Association Between ABO Blood Group System and COVID-19 Susceptibility in Wuhan. Front. Cell. Inf. Microbiol. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Rowe, E.; Dawkins, M.S.; Gebhardt-Henrich, S.G. A Systematic Review of Precision Livestock Farming in the Poultry Sector: Is Technology Focused on Improving Bird Welfare? Animals 2019, 9, 1-18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Del Valle, D.M.; Schulze, S.K.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Kikkert, M. Innate Immune Evasion by Human Respiratory RNA Viruses. J. Innate Immun. 2020, 12, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yu, H.; Yang, H.; Xue, F.; Wu, Z.; Shen, W.; Li, J.; Zhou, Z.; Ding, Y.; Zhao, Q.; et al. Structures of two coronavirus main proteases: Implications for substrate binding and antiviral drug design. J. Virol. 2008, 82, 2515–2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, K.V. SARS coronavirus: A new challenge for prevention and therapy. J. Clin. Investig. 2009, 111, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Di Maria, E.; Latini, A.; Borgiani, P.; Novelli, G. Genetic variants of the human host influencing the coronavirus-associated phenotypes (SARS, MERS and COVID-19): Rapid systematic review and field synopsis. Hum. Genom. 2020, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Volz, E.; Hill, V.; McCrone, J.T.; Price, A.; Jorgensen, D.; O’Toole, Á.; Southgate, J.; Johnson, R.; Jackson, B.; Nascimento, F.F.; et al. Evaluating the Effects of SARS-CoV-2 Spike Mutation D614G on Transmissibility and Pathogenicity. Cell 2020, 184, 64–75. [Google Scholar] [CrossRef]
- Tasneem, A.A.; Abdulhamd, M.A. ABO blood groups among Coronavirus disease 2019 patients. Ibero Am. J. Med. 2020, 2, 268–274. [Google Scholar]
- Song, J.; Chen, F.; Campos, M.; Bolgiano, D.; Houck, K.; Chambless, L.E.; Wu, K.K.; Folsom, A.R.; Couper, D.; Boerwinkle, E.; et al. Quantitative Influence of ABO Blood Groups on Factor VIII and Its Ratio to von Willebrand Factor, Novel Observations from an ARIC Study of 11,673 Subjects. PLoS ONE 2015, 10, e0132626. [Google Scholar] [CrossRef] [Green Version]
- Reid, M.E.; Lomas-Francis, C. The Blood Group Antigen Facts Book, 2nd ed.; Elsevier Academic Press: New York, NY, USA, 2004. [Google Scholar]
- Walls, A.; Park, Y.; Tortorici, M.; Wall, A.; McGuire, A.; Veesler, D.A. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Paul, E.M.; Fine, K.M.; Scott, J.A.; Edmunds, W.J. Community Protection. Plotkin’s Vaccines: USA, 7th ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 1512–1531. [Google Scholar]
- Alobid, M.; Derardja, B.; Szucs, I. Food Gap Optimization for Sustainability Concerns, the Case of Egypt. Sustainability 2021, 13, 1-17. [Google Scholar] [CrossRef]
- Van Boheemen, S.; de Graaf, M.; Lauber, C.; Bestebroer, T.M.; Raj, V.S.; Zaki, A.M. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 2012, 3, 473–512. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Holmes, E.C.; O’Toole, Á.; Hill, V.; McCrone, J.T.; Ruis, C.; du Plessis, L.; Pybus, O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020, 5, 1403–1407. [Google Scholar] [CrossRef]
- Lin, J.; Wang, R.; Jiao, P.; Li, Y.; Liao, M. An impedance immunosensor based on low-cost microelectrodes and specific monoclonal antibodies for rapid detection of avian influenza virus H5N1 in chicken swabs. Biosens. Bioelectron. 2015, 67, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Callaway, Z.; Wang, Y.; Zhang, B.; Zhang, T.; Costello, T.A.; Slavik, M.F.; Li, Y. A portable impedance biosensing system for rapid detection of avian influenza virus. Sensors 2016, 17, 1–15. [Google Scholar]
- Fu, Y.; Callaway, Z.; Lum, J.; Wang, R.; Lin, J.; Li, Y. Exploiting enzyme catalysis in ultra-low ion strength media for impedance biosensing of avian influenza virus using a bare interdigitated electrode. Anal. Chem. 2014, 86, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Das, A.; Dhawane, A.N.; Sweeney, J.; Zhang, X.; Chivukula, V.; Iyer, S.S. Highly specific and rapid glycan-based amperometric detection of influenza viruses. Chem. Sci. 2017, 8, 3628–3634. [Google Scholar] [CrossRef] [Green Version]
- Aydinlik, S.; Ozkan-Ariksoysal, D.; Kara, P.; Ayiner, A.A.; Ozsoz, M.S. A nucleic acid-based electrochemical biosensor for the detection of influenza B virus from PCR samples using gold nanoparticle-adsorbed disposable graphite electrode and Meldola’s blue as an intercalator. Anal. Methods 2011, 3, 1607–1614. [Google Scholar] [CrossRef]
- Tian, J.; Wang, D.; Zheng, Y.T.; Jing, A. High Sensitive Electrochemical Avian Influenza Virus H7 Biosensor Based on CNTs/MoSx Aerogel. Int. J. Electrochem. Sci. 2017, 12, 2658–2668. [Google Scholar] [CrossRef]
- Lam-Dai, T.; Binh Hai, N.; Nguyen Van, H.; Hoang Vinh, T.; Huy Le, N.; Phuc Xuan, N. Electrochemical detection of short HIV sequences on chitosan/Fe3O4 nanoparticle based screen printed electrodes. Mater. Sci. Eng. C 2011, 31, 477–485. [Google Scholar]
- Qureshi, A.; Kang, W.P.; Davidson, J.L.; Gurbuz, Y. Review on carbon- derived, solid-state, micro and nano sensors for electrochemical sensing applications. Diam. Rel. Mat. 2009, 18, 1401–1420. [Google Scholar] [CrossRef] [Green Version]
- Braustein, H.E.; Braustein, I.E. Real time diagnostic point of care by amperometric immuno-biosensor kit by flow technology. ECS Trans. 2014, 58, 1–17. [Google Scholar] [CrossRef]
- Mashhadizadeh, H.M.; Talemi, R.P. A highly sensitive and selective hepatitis B DNA biosensor using gold nanoparticle electrodeposition on an Au electrode and mercaptobenzaldehyde. J. Anal. Methods 2014, 6, 8956–8964. [Google Scholar] [CrossRef]
- Malecka, K.; Grabowska, I.; Radecki, J.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Radecka, H. Electrochemical detection of avian influenza virus genotype using amino-ssDNA probe modified gold electrodes. Electroanalysis 2013, 25, 1871–1878. [Google Scholar] [CrossRef]
- Kiilerich-Pedersen, K.; Dapra, J.; Cherre SRozlosnik, N. High sensitivity point-of-care device for direct virus diagnostics. Biosens. Bioelectron. 2003, 49, 374–379. [Google Scholar] [CrossRef]
- Jarocka, U.; Sawicka, R.; Gora-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecki, J.; Radecka, H. An immunosensor based on antibody binding fragments attached to gold nanoparticles for the detection of peptides derived from avian influenza hemagglutinin H5. Sensors 2014, 14, 15714–15728. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for highly accurate Severe Acute Respiratory Syndrome Coronavirus-2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dincer, C.; Kling, A.; Urban, G.A.; Bruch, R. Single-Channel Multianalyte Biosensor. Patent WO 2019/134741 A1, 2019. [Google Scholar]
- Kaur, H.; Shorie, M. Nanomaterial based aptasensors for clinical and environmental diagnostic applications. Nanoscale Adv. 2019, 1, 2123–2138. [Google Scholar] [CrossRef] [Green Version]
- Bora, U.; Sett, A.; Singh, D. Nucleic Acid Based Biosensors for Clinical Applications. Biosens. J. 2013, 2, 1–8. [Google Scholar] [CrossRef]
- Slütter, B.; Jiskoot, W. Sizing the optimal dimensions of a vaccine delivery system: A particulate matter. Expert Opin. Drug Deliv. 2016, 13, 167–170. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Fayemi, O.E.; Adekunle, S.A.; Ebenso, E.E. A Sensor for the Determination of Lindane Using PANI/Zn, Fe(III) Oxides and Nylon 6,6/MWCNT/Zn, Fe(III) Oxides Nanofibers Modified Glassy Carbon Electrode. J. Nanomater. 2016, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Stringer, R.C.; Schommer, S.; Hoehn, D.; Grant, S.A. Development of an optical biosensor using gold nanoparticles and quantum dots for the detection of Porcine Reproductive and Respiratory Syndrome Virus. Sens. Actuators B Chem. 2008, 134, 427–431. [Google Scholar] [CrossRef]



| Host Species | Phylogenetic Genera of Coronaviridae Family | Designation of Viral Isolate/Prototype | Implication of Infection |
|---|---|---|---|
| Domestic fowl (Gallus gallus domesticus) Family/Order: Phasianidae/Pangalliformes | Chicken coronavirus (γ-coronavirus) | Infectious bronchitis virus (IBV) strain; chicken-dominant coronavirus (CdCoV). | Replication of virions in the epithelial layers weaken the immune response, causes nutrient malabsorption, enterotropism and poor welfare. |
| Duck (Anas platyrhynchos) Family/Order: Anatidae/Anseriformes | Duck coronavirus (γ-coronavirus) | Infectious bronchitis virus (IBV), DdCoV/GD/2014 | Fatal, rapidly spreading viral infection of young ducklings. |
| Domestic geese (Anser anser) Family/Order: Anatidae/ Anseriformes | Goose coronavirus (unclassified γ-coronavirus) | Goose coronavirus (GCoV); infectious bronchitis virus (IBV) | Precociously infected geese exhibit respirotropism, retarded growth, abnormal growth of feathers. |
| Pheasant (Phasianus colchicus) Family/Order: Phasianidae/ Galliformes | Pheasant coronavirus (γ-coronavirus) | Ph/UK/27/B287-4/99; Ph/UK/24/B114-4/99; Ph/UK/24/B307-12/98; Ph/UK/24/B88-4/99; γCoV/ph/China/I0623/17 (I0623/17), γCoV/ph/China/I0710/17 (I0710/17) | Distortion of respiratory tract and renal blot, nephritis, visceral gout, air sacculitis, conjunctivitis, sinusitis, splenomegaly, poor hatchability, excess mortality. |
| Domestic Pigeon (Columba livia domestica) Family/Order: Columbidae/ Columbiformes | Pigeon coronavirus | N/A | Ruffled feathers, dyspnoea and excessive mucus from the beak, high susceptability to secondary infections. |
| Guinea fowl (Numida meleagris) Family/Order: Numididae/ Galliformes | Guinea fowl coronavirus (GfCoV) | GfCoV/FR/2011; GfCoV/2014 | Neonatal respiratory distress syndrome, enteritis, low feed intake, poor flock performance, excess mortality. |
| Turkey (Meleagris gallopavo) Family/Order: | Turkey coronavirus (TCoV) γ-coronavirus | N/A | Bluecomb (enteric) disease and diarrhoea, poult enteritis complex or intestinal disorders at starter phase, anorexia, emaciation, morbidity/mortality (5–100%), poor egg quality (shell deformation, albumen thinning). |
| Host Species | Phylogenetic Genera of Coronaviridae | Designation of Viral Isolate/Prototype | Implication of Infection |
|---|---|---|---|
| Bats (Miniopterus spp.) Family/Order: Microchiroptera/Therapsid | Bat coronavirus (α-coronavirus) | Bat-CoV/China/A515/2005; Bat-CoV/P, Bat-CoV/133/2005, BM48-31/BGR/2008, HKU4, HKU5, Bat-CoV-273/2005, RsSHC014; Bat-CoV/HKU9–1/China/2007 | Diminishing bat genetic conservation. Reducing annual crop pollination, seed dispersal and pest control. |
| Cattle Family/Order: Bovidae/ Artiodactyla | Bovine coronavirus (BCoV) | Isolate Alpaca, AH187, E-AH187, E-AH187-TC, E-AH65, E-AH65-TC, R-AH187, R-AH65, R-AH65-TC | Severe diarrhoea in neonate calves, winter dysentery in cattle, respiratory infections in calves. Silvopastoral grazing/ranching restriction, tacit weight loss, morbidity, emergency culling, low meat and milk yield. |
| Dromedary Camel | Camel coronavirus (α-coronavirus) | DcCoV UAE-HKU23; MERS-like CoV | Source of zoonotic Middle East respiratory syndrome (MERS-CoV) infecting unciliated bronchial epithelial cells, type II pneumocytes. |
| Feline Family/Order: Felidae/ Carnivora | Feline enteric coronavirus (FeCoV) | Feline infectious peritonitis (FIP), UU4-54; feline APN, feline infectious peritonitis (FIP), virulent | Asymptomatic carriers experience seroconversion among cats. Biotypes replicate in macrophages, causing severe and lethal disease. |
| Giraffe (Giraffa camelopardalis) | Giraffe coronavirus (GiCoV) | CoV (GiCoV-OH3) US/OH3/2003, US/OH3-TC/2006 | Weight loss, malabsorption of nutrients and water due to diarrhoea, decline in tourism and economic outcomes for hospitality industry. |
| Human Family/Order: Hominidae/Primates | Human coronavirus (β-coronavirus) | Human CoV-OC43, HCoV-229E, HKU1, HCoV-NL63 | Induces acute respiratory distress syndrome, cytokine storm and multiple complications in immunocompetent adults and infants. |
| Mink (Neovison vison or Mustela lutreola) Family/Order: Mustelidae/Carnivora | Mink coronavirus | WD1127, WD1133, MV1-Lu, NB3 SARS-CoV-2, NB7 SARS-CoV-2 | Raises secondary viral host, respiratory disease, emergency culling, high mortality. |
| Murine Family/Order: Muridae/ Rodentia | Murine coronavirus | Murine hepatitis virus: MHV-1, MHV-3, MHV-JHM.IA, RA59/R13, RA59/SJHM, RJHM/A, SA59/RJHM | Receptor (CEACAM1) binds MHV S-protein to activate virus–cell membrane fusion A59 strain. It infects mice liver and brain, demyelinating disease peaking at about 1 month postinfection. |
| Pig | Porcine coronavirus (Δ-coronavirus, unsegmented) | Porcine transmissible gastroenteritis virus (TGEV), porcine respiratory coronavirus (PRCoV); porcine haemagglutinating encephalomyelitis coronavirus (PHEV) | Infects ciliated bronchial epithelial cells and type II pneumocytes causing swine acute diarrhoea syndrome (SADS-CoV) enteritis among piglets or neonates, mortality. |
| Rabbit (Oryctolagus cuniculus/ Family/Order: Lagomorpha | Rabbit coronavirus (β-coronavirus) | RbCoV-HKU14 | Infects upper respiratory tract, sparing the lungs. Shortage of wool, meat, gourmet products. |
| Coronavirus Variants | Brief Description of the Variants |
|---|---|
| COVID-19 | Earliest viral lineage sharing nucleotide positions (8782 in ORF1ab and 28,144 in ORF8) with the closest species, i.e., bat (BetaCov/Wuhan/WH01/2019) viruses (RaTG13 and RmYN02). |
| Genome sequence of early lineage (GenBank accession No. MN908947) similar to the phylogeny of SARS-CoV-2. | |
| B.1.1.7 | B.1.1.7, first called VUI 202012/01. It has 14 nonsynonymous amino acid altering mutations, 6 synonymous (non-AA-altering) mutations, and 3 deletions (69/70 deletion, P681H, ORF8 stop codon (Q27stop)). |
| Mutates in the receptor-binding domain (RBD) of spike protein at position 501, where amino acid asparagine (N) is replaced with tyrosine (Y). | |
| N501Y | Mutation denotes a change from asparagine (N) to tyrosine (Y) in amino acid position 501. It binds to ACE2 receptors in humans and spreads faster between people. Detected in the United Kingdom. |
| Variant 501.V2 has 10–20 mutations and spreads faster than the original SARS-CoV-2. 501.V2 was first detected in South Africa and increases binding of the virus to receptors in human cells. | |
| D614G | Emerges from an ancestral D residue found in the glycosylated region of the viral spike protein. |
| G (glycine) replaces D (aspartic acid) in the RBD of spike glycoprotein to boost transmission rate of SARS-CoV-2 in China and Italy. Loss of smell (anosmia) is linked with this variant. | |
| B.1.1.7 deletions identified in spike protein; harbours a truncated ORF8 gene. | |
| Y839 variant | The spike Y839 variant is a descendant of G614 variant, the strongest trigger of the first wave of SARS-CoV-2 transmission in Italy and Portugal. |
| Nextstrain clade 20B | Common ancestors are located in the S-gene, viral genome of 13% and nonsynonymous substitutions. |
| Delta variant and “delta plus” variant | It is a “variant of concern”, first identified in India, found to be 40–60% more transmissible than the α-variant of SARS-CoV-2. |
| Multiple spike protein mutations | |
| Having protein mutations (deletion 69–70, deletion 144, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H) in various genomic regions and mutations (N501Y) located within the receptor-binding domain. | |
| Biosensor | Target Genome | Influenza Serotype | Bioreceptor |
|---|---|---|---|
| Impedimetric | Single-stranded, negative-sense RNA virus | H5N1 panzootic in poultry (bird flu) | Antibody |
| Impedimetric | Virus | H5N1 | Aptamer |
| Impedimetric | Avian virus, roughly spherical (120 nM) and genome consisting of 8 RNA fragments | H5N1: has haemagglutinin type 5 combined with neuraminidase type 1, with glycoprotein spikes on the surface encoding 10 proteins | Aptamer |
| Amperometric | NA | H1N1 (swine flu) H3N2 (human influenza-A) | 4,7-Di-Ome N-acetylneuraminic acid |
| Voltammetric | Virus | Influenza B | DNA probe |
| Voltammetric | Virus | HPAIV H7N1 (Muscovy duckling flu) | Antibody |
| Voltammetric | HIV-1 | Methylene blue | Chitosan/Fe3O4 |
| DNA | Diamond nanowire | ||
| Voltammetric | Linear, double-stranded DNA lymphotropic virus with major CpG island methylator phenotype | Epstein–Barr virus (EBV) (human γ-herpesvirus 4) activates retrovirus HERV-W/MSRV causing infectious mononucleosis or kissing disease | Oxirane-derivatised beads |
| Impedance | Partly double-stranded circular DNA virus with ~3.2 kbp that replicates by a reverse transcriptase via an RNA intermediate | Hepatitis B virus (HBV) encodes 4 overlapping open reading frames (ORFs) (S, C, P and X) and infects humans with liver cancer | AuNPs, |
| Impedance | Negative-sense RNA viruses from Orthomyxoviridae | AIV H5N1 | NH2-ssDNA probe |
| Impedance | Virus | Antibody | Conductive polymer |
| Impedance | Virus from Orthomyxoviridae | H5N2 | Magnetic nanobeads/antibody |
| PPT, LSPR | Virus from Coronavidae | SARS-CoV and SARS-CoV-2 | Gold nanoislands (AuNIs) |
| Biosensing RNA Viruses for Organ Functionality in Animal and Domestic Avian Species | ||
|---|---|---|
| Target Organ | Common Symptoms | Biosensor |
| Lungs | Acute respiratory syndrome due to dyspnea or tachypnea that provokes other symptoms found in respiratory infections, e.g., fever and cough. | Gold nanoparticles (AuNPs) and quantum dots for detecting viral respiratory syndrome virus |
| Severe infection can lead to pneumonia as the lung tissue fills with fluid and pus, reducing the air sacs’ ability to transfer oxygen into the blood | ||
| Heart | Acute coronary syndrome, congestive heart failure, arrhythmias causing inflammation of heart muscle or myocarditis, abnormal rhythms, progressive heart failure, sudden cardiac death | Wearable viral trackers |
| Brain/nervous vessels | Blood clotting, nerve damage or burst vessels in the brain, e.g., dementia-like syndrome, delirium, stroke and seizures | Real-time lab-on-chip (LOC), point-of-care (POC) pathogen detecting sensors |
| Disruption of brain signalling, temporary loss of smell (anosmia)/taste, depression | ||
| Gastrointestinal system | General malaise, bowel abnormalities, acute pancreatitis, sudden inflammation | Enzymatic biosensors (enzyme–target analyte interaction), whole-cell biosensor |
| Body temperature | Pyrexia/pyrogen | Wearable fitness trackers |
| Blood vessels | Destruction of endothelial cells or cytokine storm; constriction of blood vessels, leaky vessel walls, pulmonary embolism, hypercoagulability and low oxygen levels in blood | Immunosensors (antibody–antigen interaction) |
| Kidney | Cytokine storm can trigger kidney failure or systemic abnormalities in the renal system | Biosensor (AuNPs) for detecting creatinine levels in kidney healthy states and disease progression |
| Abnormally high levels of liver enzymes, indicating at least temporary damage | ||
| Biosensing RNA viruses in aerosols and animal by-products and agri-environment | ||
| Livestock feeds, water, saliva, urine, sweat, tears | Noninvasive, miniaturised POC sensor for real-time monitoring, wearable microfluidic multisensory biosensors, electrochemical sweat biosensors | |
| Meat/meat products | Lab-on-a-chip (LOC) wearable biosensor analysis and intracellular analysis in a miniaturised multifunctional chip with real-time, noninvasive, and nonirritating sensing capacities for cell sorting, single-cell capture and captured-cell transport; lipid-based nanosystem; liposome containing siRNA nanoparticles | |
| Milk/dairy products | ||
| Aerosols/air quality | Telemetry wearable microfluidic for sensing air quality; portable mouth-guard biosensor, micro total analysis systems (µTAS), optical RNA sensor for sampling aerosolised pathogens, aerodynamic particle spectrometer | |
| Fur, hide and skin | Epidermal potentiometric sensor, wearable skin-interfaced analytics body sensor | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayemi, P.O.; Fayemi, O.E.; Joel, L.O.; Ogungbuyi, M.G. COVID-19 Syndrome: Nexus with Herbivory and Exposure Dynamics for Monitoring Livestock Welfare and Agro-Environment. Sustainability 2021, 13, 12381. https://doi.org/10.3390/su132212381
Fayemi PO, Fayemi OE, Joel LO, Ogungbuyi MG. COVID-19 Syndrome: Nexus with Herbivory and Exposure Dynamics for Monitoring Livestock Welfare and Agro-Environment. Sustainability. 2021; 13(22):12381. https://doi.org/10.3390/su132212381
Chicago/Turabian StyleFayemi, Peter Olutope, Omolola Esther Fayemi, Luke Oluwaseye Joel, and Michael Gbenga Ogungbuyi. 2021. "COVID-19 Syndrome: Nexus with Herbivory and Exposure Dynamics for Monitoring Livestock Welfare and Agro-Environment" Sustainability 13, no. 22: 12381. https://doi.org/10.3390/su132212381