Application of Soil Washing and Thermal Desorption for Sustainable Remediation and Reuse of Remediated Soil
Abstract
:1. Introduction
2. Deterioration of Soil Quality during Remediation and Revitalization of Remediated Soil for Sustainable Soil Management
2.1. Deterioration of Soil Quality during Soil Remediation
2.1.1. Soil Washing (SW)
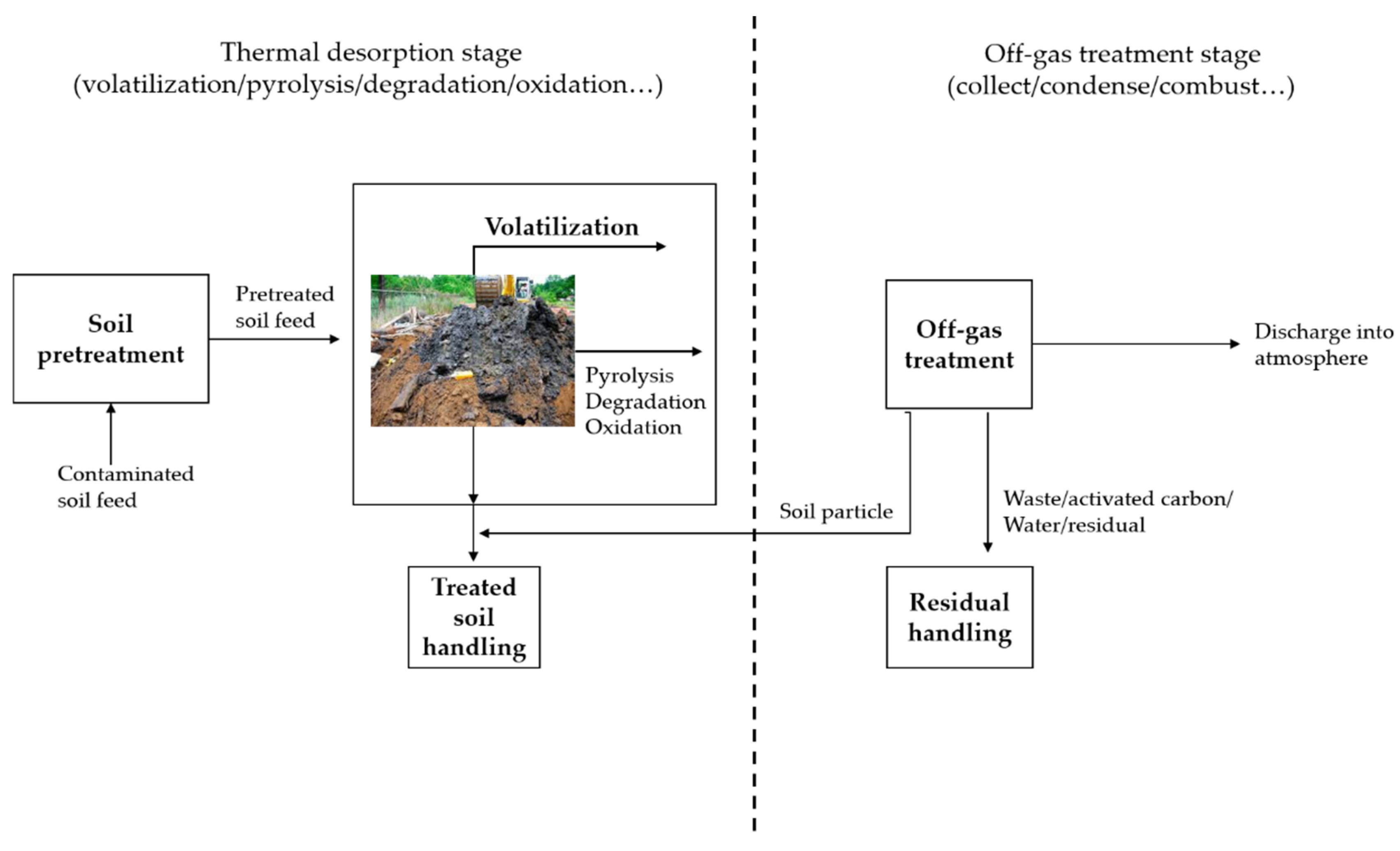
2.1.2. Thermal Desorption (TD)
2.2. Revitalization of Disturbed Soil
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Banwart, S. Save our soils. Nature 2011, 474, 151–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Ma, D.; Qiu, R.; Tang, Y.; Du, C. Non-thermal plasma technology for organic contaminated soil remediation: A review. Chem. Eng. J. 2017, 313, 157–170. [Google Scholar] [CrossRef]
- FRTR. Remediation Technologies Screening Matrix and Reference Guide, 4th ed.; FRTR: Washington, DC, USA, 2002. [Google Scholar]
- Sierra, C.; Martínez-Blanco, D.; Blanco, J.A.; Gallego, J.R. Optimisation of magnetic separation: A case study for soil washing at a heavy metals polluted site. Chemosphere 2014, 107, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Udovic, M.; Lestan, D. Pb, Zn and Cd mobility, availability and fractionation in aged soil remediated by EDTA leaching. Chemosphere 2009, 74, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Villa, R.D.; Trovó, A.G.; Nogueira, R.F.P. Soil remediation using a coupled process: Soil washing with surfactant followed by photo-Fenton oxidation. J. Hazard. Mater. 2010, 174, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Holland, K.S. A framework for sustainable remediation. Environ. Sci. Technol. 2011, 45, 7116–7117. [Google Scholar] [CrossRef] [PubMed]
- Bardos, P.; Bone, B.; Boyle, R.; Ellis, D.; Evans, F.; Harries, N.; Smith, J. Applying sustainable development principles to contaminated land management using the SuRF-UK framework. Remediation 2011, 21, 77–100. [Google Scholar] [CrossRef]
- Favara, P.; Krieger, T.; Boughton, B.; Fisher, A.; Bhargava, M. Guidance for performing footprint analyses and life-cycle assessment for the remediation industry. Remediation 2011, 21, 38–79. [Google Scholar] [CrossRef]
- Fortuna, M.; Simion, I.; Gravrilescu, M. Sustainability in environmental remediation. Environ. Eng. Manag. J. 2011, 10, 1987–1996. [Google Scholar]
- Cundy, A.; Bardos, R.; Church, A.; Puschenreiter, M.; Friesl-Hanl, W.; Müller, I.; Neu, S.; Mench, M.; Witters, N.; Vangronsveld, J. Developing principles of sustainability and stakeholder engagement for ‘gentle’ remediation approaches: The European context. J. Environ. Manag. 2013, 129, 283–291. [Google Scholar] [CrossRef]
- Hou, D.; Al-Tabbaa, A. Sustainability: A new imperative in contaminated land remediation. Environ. Sci. Policy 2014, 39, 25–34. [Google Scholar] [CrossRef]
- Rizzo, E.; Bardos, P.; Pizzol, L.; Critto, A.; Giubilato, E.; Marcomini, A.; Albano, C.; Darmendrail, D.; Doberl, G.; Harcletode, M.; et al. Comparison of international approaches to sustainable remediation. J. Environ. Manag. 2016, 184, 4–17. [Google Scholar] [CrossRef] [PubMed]
- CL: AIRE. A Framework for Assessing the Sustainability of Soil and Groundwater Remediation; SuRF: London, UK, 2010. [Google Scholar]
- Ellis, D.E.; Hadley, P.W. Sustainable remediation white paper—Integrating sustainable principles, practices, and metrics into remediation projects. Remed. J. 2009, 19, 5–114. [Google Scholar]
- Farag, A.M.; Hull, R.N.; Clements, W.H.; Glomb, S.; Larson, D.L.; Stahl, R.; Stauber, J. Restoration of impaired ecosystems: An ounce of prevention or a pound of cure? Integr. Environ. Assess. Manag. 2015, 12, 247–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bone, J.; Head, M.; Barraclough, D.; Archer, M.; Scheib, C.; Flight, D.; Voulvoulis, N. Soil quality assessment under emerging regulatory requirements. Environ. Int. 2010, 36, 609–622. [Google Scholar] [CrossRef] [Green Version]
- Ehrenfield, J. Defining the limits of restoration: The need for realistic goals. Restor. Ecol. 2000, 8, 2–9. [Google Scholar] [CrossRef]
- O’Brien, P.L.; DeSutter, T.M.; Casey, F.X.M.; Khan, E.; Wick, A.F. Thermal remediation alters soil properties—A review. J. Environ. Manag. 2018, 206, 826–835. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.L.; DeSutter, T.M.; Casey, F.X.M.; Wick, A.F.; Khan, E. Wheat growth in soils treated by ex situ thermal desorption. J. Environ. Qual. 2017, 30, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wei, Z.; Wu, Q.; Li, C.; Qian, T.; Zheng, W. Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: Field experiments. Chemosphere 2016, 147, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemicaltechnologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef]
- Zupanc, V.; Kastelec, D.; Lestan, D.; Grcman, H. Soil physical characteristics after EDTA washing and amendment with inorganic and organic additives. Environ. Pollut. 2014, 186, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.M.; Sung, K. Influence of washing treatment on the qualities of heavy metal-contaminated soil. Ecol. Eng. 2015, 81, 89–92. [Google Scholar] [CrossRef]
- Gautam, P.; Bajagain, R.; Jeong, S.W. Combined effects of soil particle size with washing time and soil-to water ratio on removal of total petroleum hydrocarbon from fuel contaminated soil. Chemosphere 2020, 250, 126206. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.; Chang, Y.Y.; Lee, C.H.; Kim, K.W. Assessment of pilot-scale acid washing of soil contaminated with As, Zn and Ni using the BCR three-step sequential extraction. J. Hazard. Mater. 2005, 127, 1–13. [Google Scholar] [CrossRef]
- Jelusic, M.; Grcman, H.; Vodnik, D.; Suhadolc, M.; Lestan, D. Functioning of metal contaminated garden soil after remediation. Environ. Pollut. 2013, 174, 63–70. [Google Scholar] [CrossRef]
- Hu, P.; Yang, B.; Dong, C.; Chen, L.; Gao, X.; Zhao, J.; Wu, L.; Luo, Y.; Christie, P. Assessment of EDTA heap leaching of an agricultural soil highly contaminated with heavy metals. Chemosphere 2014, 117, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, L.; Wang, G.; Chen, Y.; Shen, Z.; Luo, C. Assessment of amendments for the immobilization of Cu in soils containing EDDS leachates. Environ. Sci. Pollut. Res. 2015, 22, 16525–16534. [Google Scholar] [CrossRef] [PubMed]
- Beiyuan, J.; Tsang, D.C.W.; Valix, M.; Zhang, W.; Yang, X.; Ok, Y.S.; Li, X.D. Selective dissolution followed by EDDS washing of an e-waste contaminated soil: Extraction efficiency, fate of residual metals, and impact on soil environment. Chemosphere 2017, 166, 489–496. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Surfactant-enhanced remediation of contaminated soil: A review. Eng. Geol. 2001, 60, 371–380. [Google Scholar] [CrossRef]
- Hong, K.J.; Tokunaga, S.; Kajiuchi, T. Evaluation of remediation process with plant-derived biosurfactant for recovery of heavy metals from contaminated soils. Chemosphere 2002, 49, 379–387. [Google Scholar] [CrossRef]
- Chae, Y.; Cui, R.; Kim, S.W.; An, G.; Jeong, S.W. Exoenzyme activity in contaminated soils, before and after soil washing: Glucosidase activity as a biological indicator of soil health. Ecotoxicol. Environ. Saf. 2017, 135, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Yang, K.; Jho, E.H.; Nam, K. Effect of different soil washing solutions on bioavailability of residual arsenic in soils and soil properties. Chemosphere 2015, 138, 253–258. [Google Scholar] [CrossRef]
- Udovic, M.; Lestan, D. EDTA and HCl leaching of calcareous and acidic soils polluted with potentially toxic metals: Remediation efficiency and soil impact. Chemosphere 2012, 88, 718–724. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, G.; Zhang, G.; He, Q.; Wei, Z.; Zheng, W.; Qian, T.; Wu, Q. Effect of mixed chelators of EDTA, GLDA, and citric acid on bioavailability of residual heavy metals in soils and soil properties. Chemosphere 2018, 209, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Jelusic, M.; Lestan, D. Effect of EDTA washing of metal polluted garden soils. Part I: Toxicity hazards and impact on soil properties. Sci. Total Environ. 2014, 475, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, S.; Farahbakhsh, M.; Heydarpoor, G.; Besalatpour, A.A. Mitigation in availability and toxicity of multi metal contaminated soil by combining soil washing and organic amendments stabilization. Ecotoxicol. Environ. Saf. 2020, 201, 110807. [Google Scholar] [CrossRef] [PubMed]
- Barona, A.; Aranguiz, I.; Elias, A. Metal associations in soils before and after EDTA extractive decontamination: Implications for the effectiveness of further clean up procedures. Environ. Pollut. 2001, 113, 79–85. [Google Scholar] [CrossRef]
- Lei, M.; Liao, B.H.; Zeng, Q.R.; Qin, P.F.; Khan, S. Fraction distributions of lead, cadmium, copper, and zinc in metal-contaminated soil before and after extraction with disodium ethylenediaminetetraacetic acid. Commun. Soil Sci. Plant Anal. 2008, 39, 1963–1978. [Google Scholar] [CrossRef]
- West, C.C.; Harwell, J.H. Surfactants and subsurface remediation. Environ. Sci. Technol. 1992, 26, 2324–2330. [Google Scholar] [CrossRef]
- Laha, S.; Tansel, B.; Ussawarujikulchai, A. Surfactant–soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: A review. J. Environ. Manag. 2009, 90, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Nortemann, B. Biodegradation of EDTA. Appl. Microbiol. Biotechnol. 1999, 51, 751–759. [Google Scholar] [CrossRef]
- Wen, J.; Stacey, S.P.; McLaughlin, M.J.; Kirby, J.K. Biodegradation of rhamnolipid, EDTA and citric acid in cadmium and zinc contaminated soils. Soil Biol. Biochem. 2009, 41, 2214–2221. [Google Scholar] [CrossRef]
- Rahman, I.M.M.; Hossain, M.M.; Begum, Z.A.; Rahman, M.A.; Hasegawa, H. Environmental consequences associated with chelant-assisted phytoremediation of metal-contaminated soil. In Handbook of Phytoremediation; Golubev, I., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 709–722. [Google Scholar]
- Grcman, H.; Velifonja-Bolta, S.; Vodnik, D.; Lestan, D. EDTA enhanced heavy metal phytoextraction: Metal accumulation, leaching and toxicity. Plant Soil 2001, 235, 105–114. [Google Scholar] [CrossRef]
- Mühlbachova, G. Soil microbial activities and heavy metal mobility in long term contaminated soils after addition of EDTA and EDDS. Ecol. Eng. 2011, 37, 1064–1071. [Google Scholar] [CrossRef]
- Epelde, L.; Hernandez, A.J.; Becerril, J.M.; Blanco, F.; Garbisu, C. Effects of chelates on plants and soil microbial community: Comparison of EDTA and EDDS for lead phytoextraction. Sci. Total Environ. 2008, 401, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Renella, G.; Wirth, S.; Islam, R. Secondary salinity effects on soil microbial biomass. Biol. Fertil. Soils 2010, 46, 445–449. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Effect of soil washing on heavy metal removal and soil quality: A two-sided coin. Ecotoxicol. Environ. Saf. 2020, 203, 110981. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Zhang, Q.; Zeng, G. Remediation of multiple heavy metal-contaminated soil through the combination of soil washing and in situ immobilization. Sci. Total Environ. 2018, 635, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoon, S.; Kwon, H.; Choi, Y. Effects of treatment agents during acid washing and pH neutralization on the fertility of heavy metal-impacted dredged marine sediment as plant-growing soil. Environ. Pollut. 2020, 267, 115416. [Google Scholar] [CrossRef] [PubMed]
- Kaurin, A.; Cernilogar, Z.; Lestan, D. Revitalisation of metal-contaminated, EDTA-washed soil by addition of unpolluted soil, compost and biochar: Effects on soil enzyme activity, microbial community composition and abundance. Chemosphere 2018, 193, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wei, Z.; Penn, C.J.; Xu, T.; Wu, Q. Effect of soil washing and liming on bioavailability of heavy metals in acid contaminated soil. Soil Sci. Soc. Am. J. 2013, 77, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wei, H.; Yang, X.H.; Xia, B.; Liu, J.M.; Su, C.Y.; Qiu, R.L. Influence of the selective EDTA derivative phenyl diaminetetraacetic acid on the speciation and extraction of heavy metals from a contaminated soil. Chemosphere 2014, 109, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.I.; Nam, S.H.; Kim, S.W.; Bajagain, R.; Jeong, S.W. Changes in soil properties after remediation influence the performance and survival of soil algae and earthworm. Ecotoxicol. Environ. Saf. 2019, 174, 189–196. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, Y.; Feng, Y.; Li, Y.; Dong, Y. Thermal desorption for remediation of contaminated soil: A review. Chemosphere 2019, 229, 841–855. [Google Scholar] [CrossRef]
- Pape, A.; Switzer, C.; McCosh, N.; Knapp, C.W. Impacts of thermal and smouldering remediation on plant growth and soil ecology. Geoderma 2015, 243–244, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zihms, S.G.; Switzer, C.; Irvine, J.; Karstunen, M. Effects of high temperature processes on physical properties of silica sand. Eng. Geol. 2013, 164, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Sierra, M.J.; Millan, R.; Lopez, F.A.; Alguacil, F.J.; Canadas, I. Sustainable remediation of mercury contaminated soils by thermal desorption. Environ. Sci. Pollut. Res. 2016, 23, 4898–4907. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.L.; DeSutter, T.M.; Casey, F.X.M.; Derby, N.E.; Wick, A.F. Implications of using thermal desorption to remediate contaminated agricultural soil: Physical characteristics and hydraulic processes. J. Environ. Qual. 2016, 45, 1430–1436. [Google Scholar] [CrossRef]
- Bonnard, M.; Devin, S.; Leyval, C.; Morel, J.L.; Vasseur, P. The influence of thermal desorption on genotoxicity of multipolluted soil. Ecotoxicol. Environ. Saf. 2010, 73, 955–960. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Guerrero, C.; García-Orenes, F.; Barcenas-Moreno, G.; Pilar Torres, M. Forest fire effects on soil microbiology. In Fire Effects on Soils and Restoration Strategies; Cerda, A., Robichaud, P.R., Eds.; Science Publishers: London, UK, 2009; pp. 133–175. [Google Scholar]
- Gonzalez-Pérez, J.A.; Gonzalez-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Certinini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.M.; Park, S.; Munster, C.; Kim, G.; Sung, K. Changes in ecological properties of petroleum oil-contaminated soil after low-temperature thermal desorption treatment. Water Air Soil Pollut. 2016, 227, 108. [Google Scholar] [CrossRef]
- Ren, J.; Song, X.; Ding, D. Sustainable remediation of diesel-contaminated soil by low temperature thermal treatment: Improved energy efficiency and soil reusability. Chemosphere 2020, 241, 124952. [Google Scholar] [CrossRef] [PubMed]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 2015, 237–238, 105–116. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Terefe, T.; Mariscal-Sancho, I.; Peregrina, F.; Espejo, R. Influence of heating on various properties of six Mediterranean soils. A laboratory study. Geoderma 2008, 143, 273–280. [Google Scholar] [CrossRef]
- Glass, D.W.; Johnson, D.W.; Blank, R.R.; Miller, W.W. Factors affecting mineral nitrogen transformations by soil heating: A laboratory-simulated fire study. Soil Sci. 2008, 173, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Yusiharni, E.; Gilkes, R.J. Short term effects of heating a lateritic podzolic soil on the availability to plants of native and added phosphate. Geoderma 2012, 191, 132–139. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, Z.; Zhang, J.; Qu, L.; Li, P. Low-thermal remediation of mercury-contaminated soil and cultivation of treated soil. Environ. Sci. Pollut. Res. 2018, 25, 24135–24142. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Hseu, Z.Y.; His, H.C. Influences of thermal decontamination on mercury removal, soil properties, and repartitioning of coexisting heavy metals. Chemosphere 2011, 84, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Barcenas-Moreno, G.; Baath, E. Bacterial and fungal growth in soil heated at different temperatures to simulate a range of fire intensities. Soil Biol. Biochem. 2009, 41, 2517–2526. [Google Scholar] [CrossRef]
- Guerrero, C.; Mataix-Solera, J.; Gomez, I.; Garcia-Orenes, F.; Jordan, M.M. Microbial recolonization and chemical changes in a soil heated at different temperatures. Int. J. Wildland Fire 2005, 14, 385–400. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerda, A.; Arcenegui, V.; Jordan, A.; Zavala, L.M. Fire effects on soil aggregation: A review. Earth Sci. Rev. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- Dixon, J.B. Kaolin and serpentine group minerals. In Minerals in Soil Environments; SSSA Book Series; Dixon, J.B., Weed, S.B., Eds.; SSSA: Madison, WI, USA, 1989; Volume 1, pp. 467–525. [Google Scholar]
- Cébron, A.; Cortet, J.; Criquet, S.; Biaz, A.; Calvert, V.; Caupert, C.; Pernin, C.; Leyval, C. Biological functioning of PAH-polluted and thermal desorption-treated soils assessed by fauna and microbial bioindicators. Res. Microbiol. 2011, 162, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Song, X.; Wei, C.; LaChance, J. A review on the sustainability of thermal treatment for contaminated soils. Environ. Pollut. 2019, 253, 449–463. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y. Field study on the uptake, accumulation, translocation and risk assessment of PAHs in a soil-wheat system with amendments of sewage sludge. Sci. Total Environ. 2016, 560–561, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, Y.; Ji, L.; Zhang, G.; He, Q.; Wei, Z.; Qian, T.; Wu, Q. Revitalization of mixed chelator–washed soil by adding of inorganic and organic amendments. Water Air Soil Pollut. 2019, 230, 112. [Google Scholar] [CrossRef]
- Yoo, J.C.; Beiyuan, J.Z.; Wang, L.; Tsang, D.C.W.; Baek, K.; Bolan, N.S.; Ok, Y.S.; Li, X.D. A combination of ferric nitrate/EDDS-enhanced washing and sludge-derived biochar stabilization of metal-contaminated soils. Sci. Total Environ. 2018, 616–617, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Maček, I.; Šibanc, N.; Kavšček, M.; Lestan, D. Diversity of arbuscular mycorrhizal fungi in metal polluted and EDTA washed garden soils before and after soil revitalization with commercial and indigenous fungal inoculum. Ecol. Eng. 2016, 95, 330–339. [Google Scholar] [CrossRef]

| Soil Quality Indicators | Deterioration | Ref. |
|---|---|---|
| Physical | Decrease in water and nutrient holding capacities | [24,25] |
| Chemical | Loss of soil organic matter, cation exchange capacity, micro- and macronutrients | [26,27,28] |
| Increase in bioavailability and mobility of residual pollutants | [29,30] | |
| Toxicity of residual extractants | [23,31,32] | |
| Biological | Changes in DNA content and microbial population structure Reduced enzyme activities Reduced germination and growth rates of plants (crops) | [24,27,33,34] |
| Soil Quality Indicators | Deterioration | Ref. |
|---|---|---|
| Physical | Clay-sized particles are cemented with Fe- and Al-hydroxides, altering the distribution of soil particle sizes | [58] |
| Chemical | Can decrease clay content, soil pH, WHC, and CEC | [58,59,61] |
| Biological | Genotoxic effects on coelomocytes of Eisenia fetida Microbial activity (including soil enzyme activities) decreases at higher temperature due to thermal denaturation | [58,62,63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Kim, S.-O.; Lee, S.-W.; Kim, M.-S.; Park, H. Application of Soil Washing and Thermal Desorption for Sustainable Remediation and Reuse of Remediated Soil. Sustainability 2021, 13, 12523. https://doi.org/10.3390/su132212523
Lee S-H, Kim S-O, Lee S-W, Kim M-S, Park H. Application of Soil Washing and Thermal Desorption for Sustainable Remediation and Reuse of Remediated Soil. Sustainability. 2021; 13(22):12523. https://doi.org/10.3390/su132212523
Chicago/Turabian StyleLee, Sang-Hwan, Soon-Oh Kim, Sang-Woo Lee, Min-Suk Kim, and Hyun Park. 2021. "Application of Soil Washing and Thermal Desorption for Sustainable Remediation and Reuse of Remediated Soil" Sustainability 13, no. 22: 12523. https://doi.org/10.3390/su132212523
APA StyleLee, S.-H., Kim, S.-O., Lee, S.-W., Kim, M.-S., & Park, H. (2021). Application of Soil Washing and Thermal Desorption for Sustainable Remediation and Reuse of Remediated Soil. Sustainability, 13(22), 12523. https://doi.org/10.3390/su132212523








