Simulation of Slag–Matte/Metal Equilibria for Complex and Low-Grade Raw Materials
Abstract
:1. Introduction
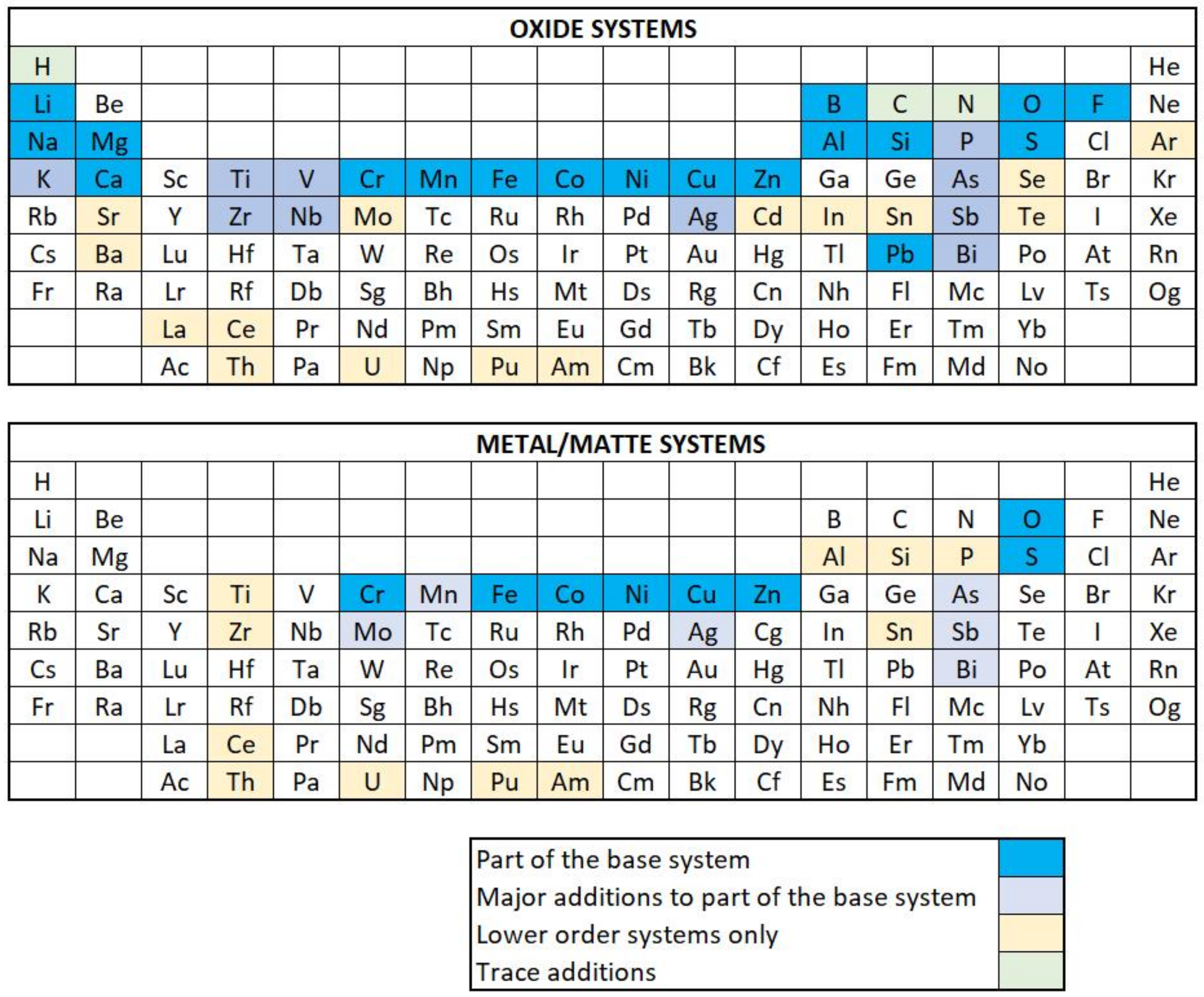
2. Thermodynamic Databases
3. Smelting and Refining Basics
3.1. Refining Equilibria in Secondary Copper Smelting
3.2. Thermodynamics of Trace Elements in Smelting
4. Case Studies
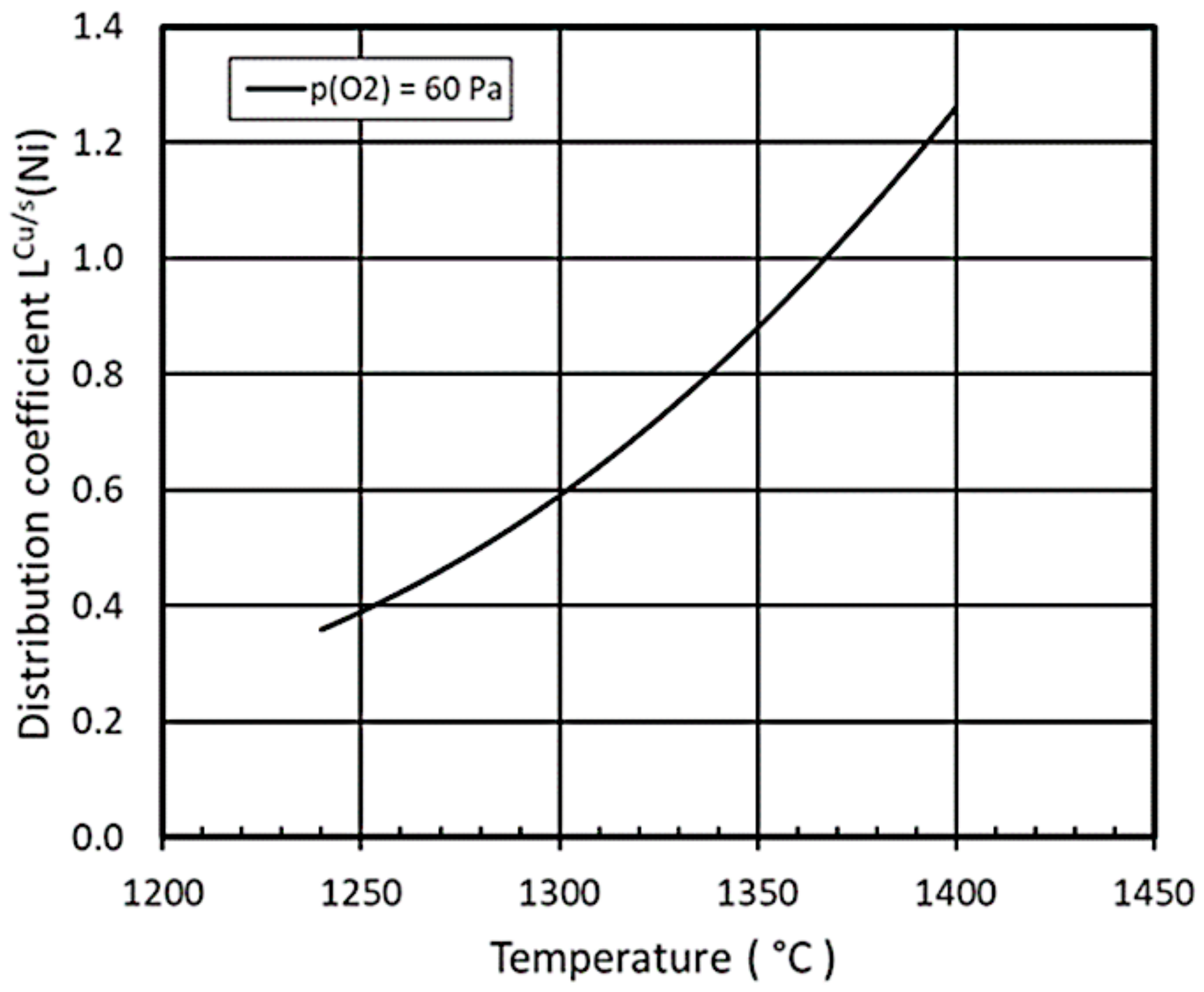
4.1. Case 1: Copper Fire-Refining and Metal Distributions
4.2. Case 2: The Assay of Silica Sand in Matte Smelting—Boundary Conditions in the Copper Smelting
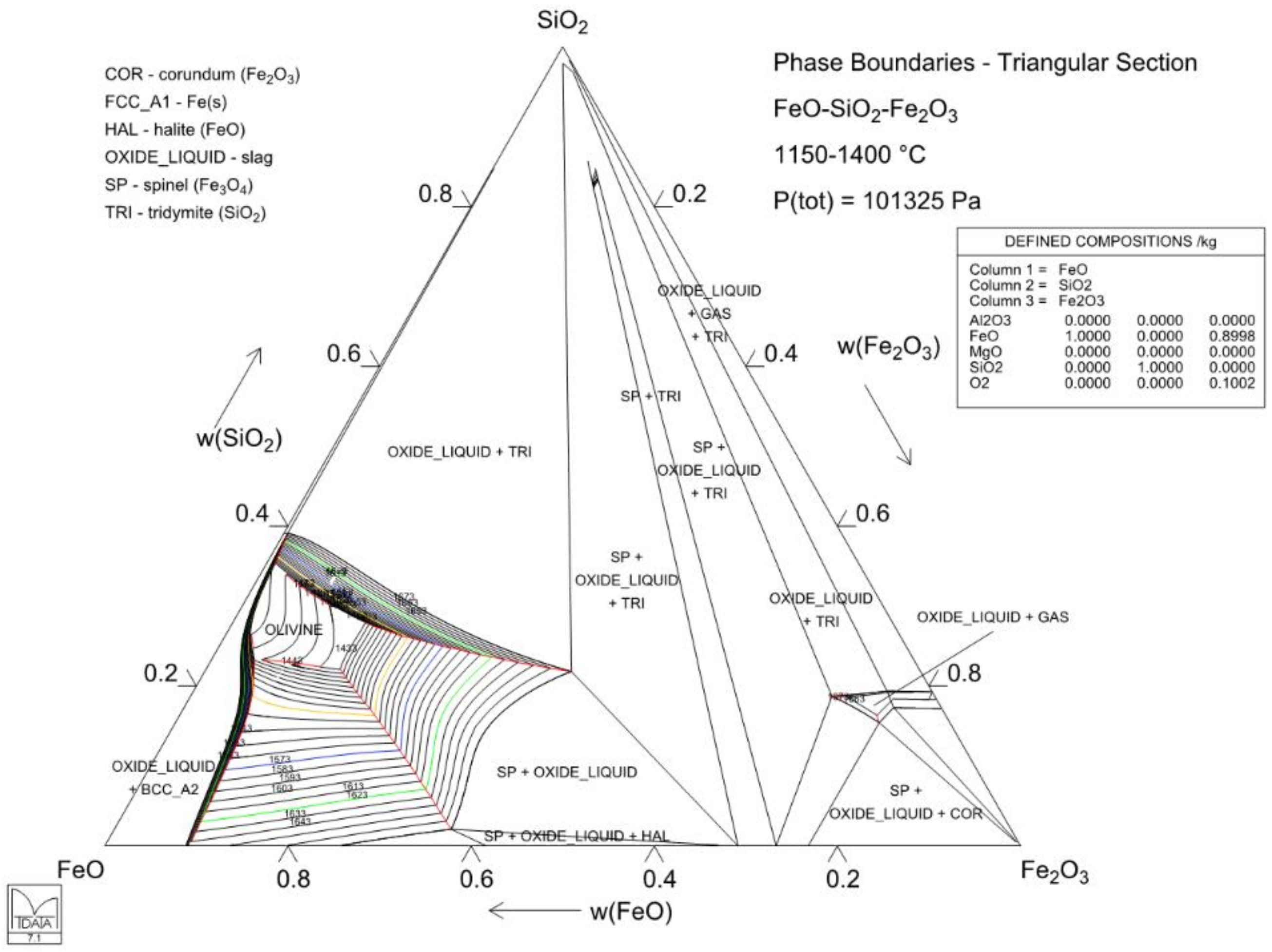
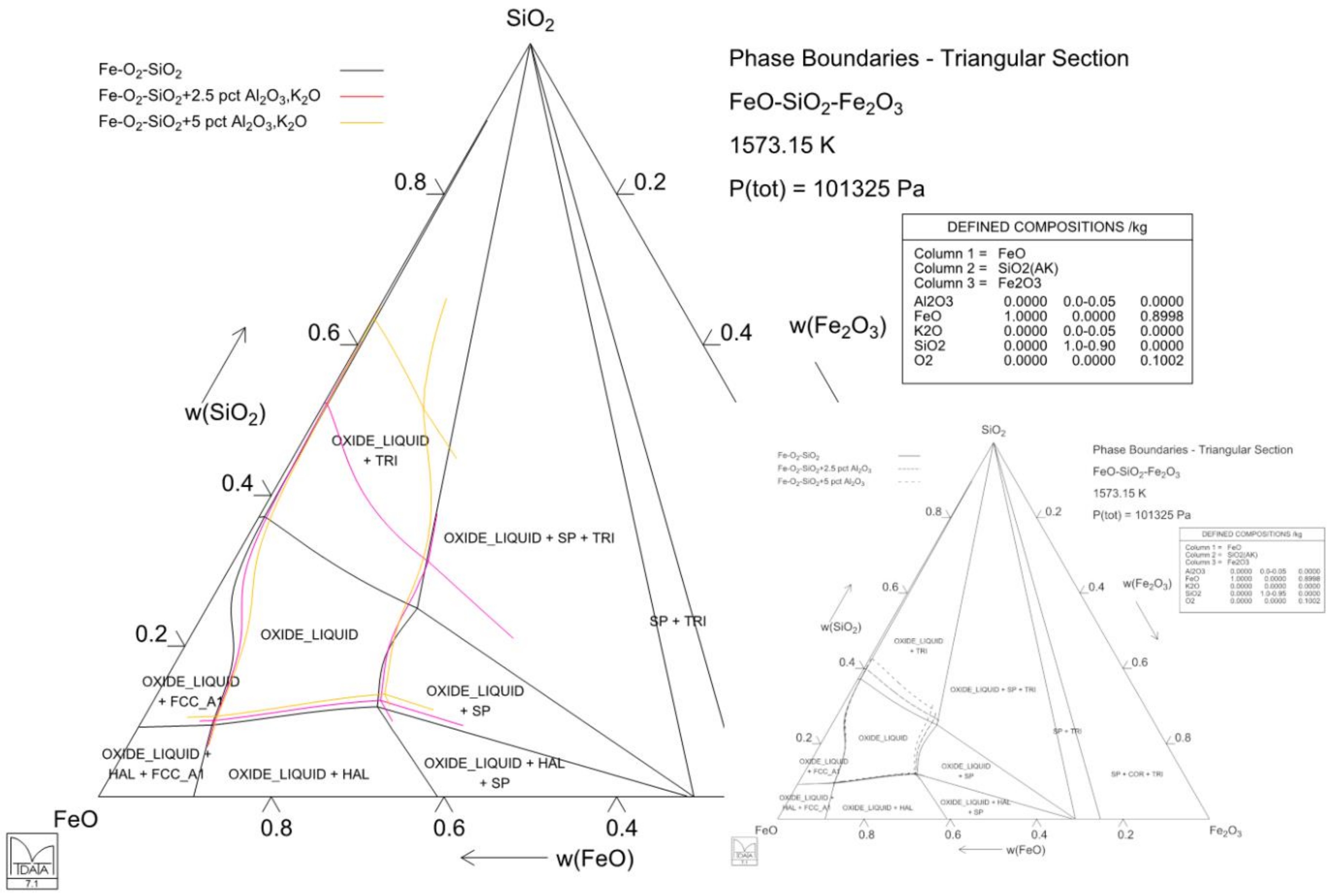
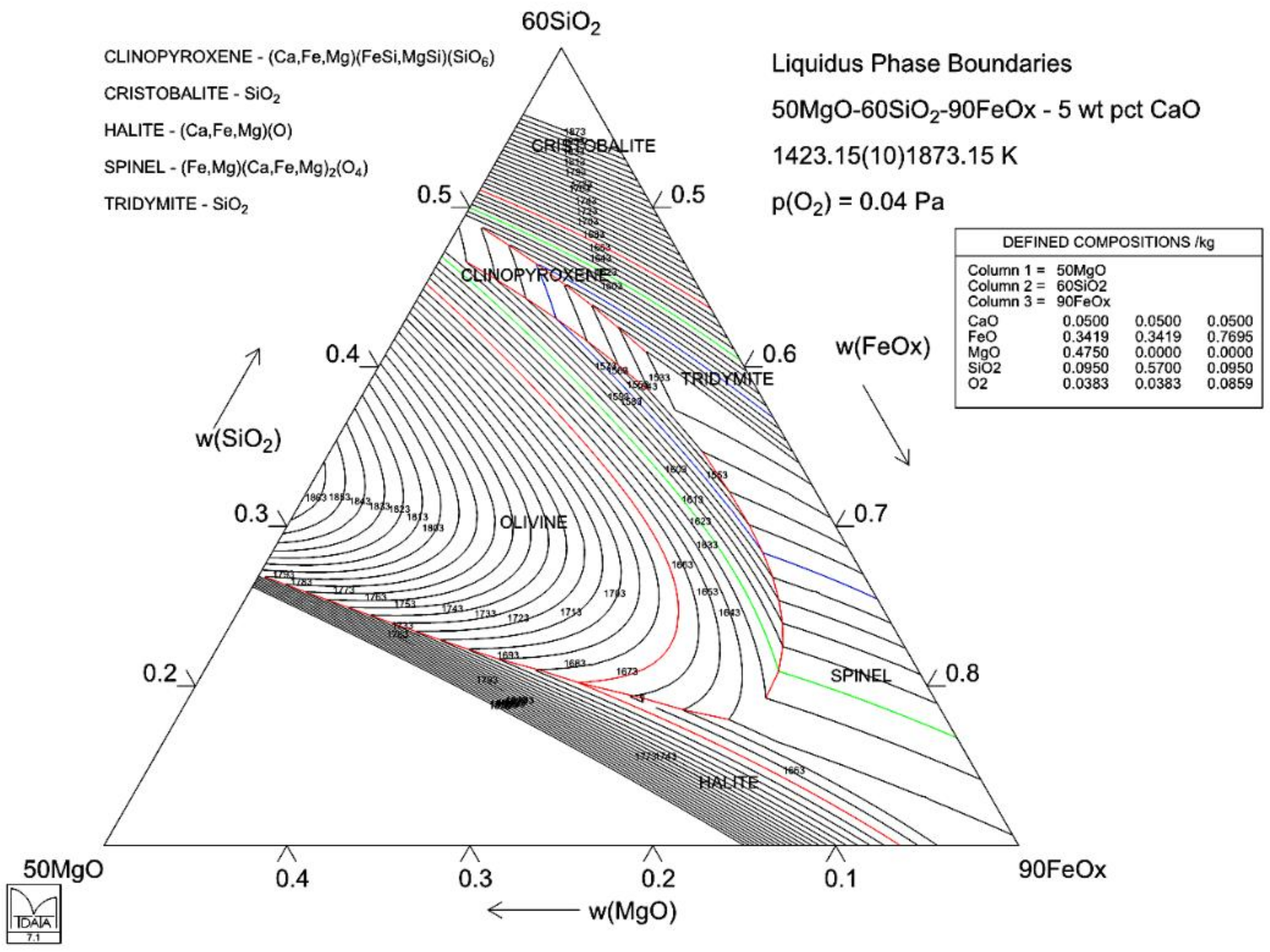
4.3. Case 3: Solubility of Magnesia in Iron Silicate Slags—Slag Modification
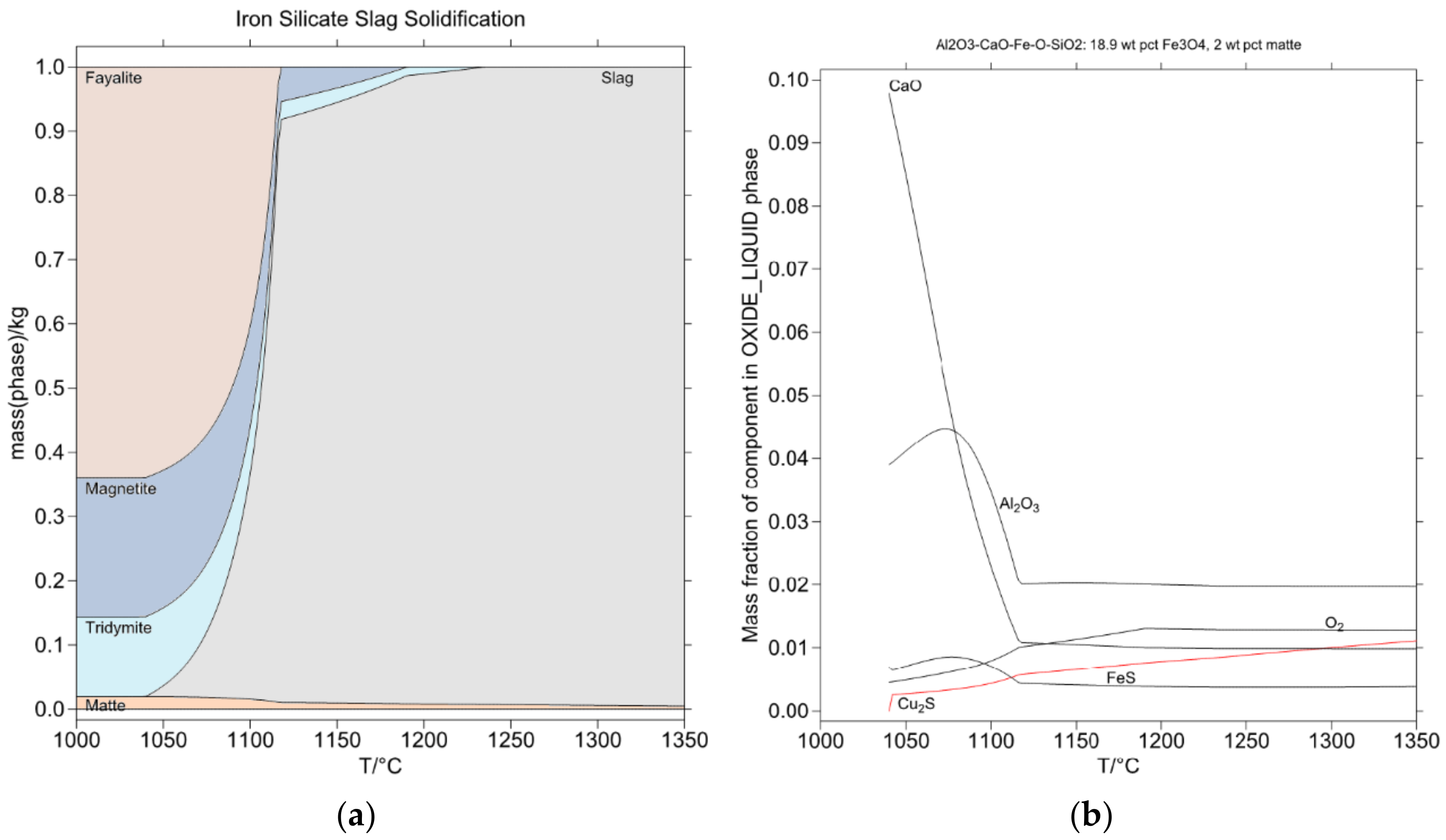
4.4. Case 4: Chemistry of Slag Cooling in Ladles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Moats, M.; Alagha, L.; Awuah-Offei, K. Towards resilient and sustainable supply of critical elements from the copper supply chain: A review. J. Clean. Prod. 2021, 307, 127207. [Google Scholar] [CrossRef]
- Klaffenbach, E.; Mostaghel, S.; Guo, M.; Blanpain, B. Thermodynamic analysis of copper smelting, considering the impact of minor elements behavior on slag application options and Cu recovery. J. Sust. Metall. 2021, 7, 664–683. [Google Scholar] [CrossRef]
- Ayres, R.U.; Talens Peiró, L. Materials efficiency: Rare and critical metals. Phil. Trans. R. Soc. A 2013, 372A, 20110563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhys, C.; Douglas, P.; Dowling, M.; Liversage, G.; Davies, M. Towards Increased Recovery of Critical Metals from WEEE-evaluation of CRMs at a component level and pre-processing methods for interface optimisation with recovery processes. Res. Conserv. Recycl. 2020, 161, 104923. [Google Scholar]
- Alexander, C.; Johto, H.; Lindgren, M.; Pesonen, L.; Roine, A. Comparison of environmental performance of modern copper smelting technologies. Clean. Environm. Syst. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Ordonez, J.I.; Jeldres, R.I.; Serna-Guerrero, R. Toward the Implementation of Circular Economy Strategies: An Overview of the Current Situation in Mineral Processing. Miner. Process. Extr. Met. Rev. 2021, 1–23. [Google Scholar] [CrossRef]
- Alvear Flores, G.; Risopatron, C.; Pease, J. Processing of Complex Materials in the Copper Industry: Challenges and Opportunities Ahead. JOM 2020, 72, 3447–3461. [Google Scholar] [CrossRef]
- Nakamura, S.; Kondo, Y.; Nakajima, K.; Ohno, H.; Pauliuk, S. Quantifying Recycling and Losses of Cr and Ni in Steel Throughout Multiple Life Cycles Using MaTrace-Alloy. Environ. Sci. Technol. 2017, 51, 9469–9476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciacci, L.; Harper, E.M.; Nassar, N.T.; Reck, B.K.; Graedel, T.E. Metal Dissipation and Inefficient Recycling Intensify Climate Forcing. Environ. Sci. Technol. 2016, 50, 11394–11402. [Google Scholar] [CrossRef] [PubMed]
- Copper Development Association Inc.: McLean, VA, USA. 2021. Available online: https://www.copper.org/environment/lifecycle/ukrecyc.html (accessed on 18 November 2021).
- Muchova, L.; Eder, P.; Villanueva, A. End-of-Waste Criteria for Copper and Copper Alloy Scrap; Report EUR 24786 EN; JRC-IPTS: Luxembourg Publications Office of EU: Luxembourg, 2011; p. 118. [Google Scholar]
- Hsu, E.; Barmak, K.; West, A.C.; Park, A.-H. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green. Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Shishin, D.; Shevschenko, M.; Jak, E. Experimental Study and Thermodynamic Calculations in the CaO-Cu2O-FeO-Fe2O3-SiO2 System and Application in Novel Copper-Based Processes. J. Sustain. Metall. 2020, 7, 300–311. [Google Scholar] [CrossRef]
- Rötzer, N.; Schmidt, M. Historical, Current, and Future Energy Demand from Global Copper Production and its Impact on Climate Change. Resources 2020, 9, 44. [Google Scholar] [CrossRef]
- Chen, M.; Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Sukhomlinov, D.; Shi, J.; Taskinen, P.; Jokilaakso, A. Recovery of Precious Metals (Au, Ag, Pt, and Pd) from Urban Mining through Copper Smelting. Metall. Mater. Trans. B 2020, 51B, 1495–1508. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, Y.; Liu, B.; Chang, C. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 63, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Avarmaa, K.; Yliaho, S.; Taskinen, P. Recoveries of rare elements Ga, Ge, In and Sn from Waste Electric and Electronic Equipment through Secondary Copper Smelting. Waste Manag. 2018, 71, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Van Nielen, S.S.; Kleijn, R.; Sprecher, B.; Xicotencatl, B.M.; Tukker, A. Early-stage assessment of minor metal recyclability. Res. Conserv. Recycl. 2022, 176, 105881. [Google Scholar] [CrossRef]
- Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Taskinen, P. Urban mining of precious metals via oxidizing copper smelting. Miner. Eng. 2019, 133, 95–102. [Google Scholar] [CrossRef]
- Shuva, M.A.H.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.; Reuter, M.A.; Firdaus, M. Analysis for Optimum Conditions for Recovery of Valuable Metals from E-waste Through Black Copper Smelting. In Proceedings of the 8th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 26 February–2 March 2017; Springer: Cham, Switzerland, 2017; pp. 419–427. [Google Scholar]
- Reck, B.K.; Graedel, T.E. Challenges in Metal Recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef]
- Hack, K. SGTE Casebook, 2nd ed.; Taylor Francis Inc.: Boca Roca, FL, USA, 2008; p. 300. [Google Scholar]
- Jak, E.; Hidayat, T.; Shishin, D.; Mehrjardi, A.F.; Chen, J.; Hayes, P. Experimental and modelling research in support of energy savings and improved productivity in non-ferrous metal production and recycling. In Proceedings of the European Metallurgical Conference, EMC. Leipzig, Germany, 25–28 June 2017; GDMB Verlag: Clausthal-Zellerfeld, Germany, 2017; pp. 231–250. [Google Scholar]
- Reuter, M.A.; van Schaik, A. Resource Efficient Metal and Material Recycling. In Rewas 2013; Kvithyld, A., Meskers, C., Kirchain, G., Eds.; TMS: Warrendale, PA, USA; pp. 332–340.
- Jung, I.-H. Overview of the applications of thermodynamic databases to steelmaking processes. Calphad 2010, 34, 332–362. [Google Scholar] [CrossRef]
- Gisby, J.; Taskinen, P.; Pihlasalo, J.; Li, Z.; Tyrer, M.; Pearce, J.; Avarmaa, K.; Björklund, P.; Davies, H.; Korpi, M.; et al. MTDATA and the Prediction of Phase Equilibria in Oxide Systems: 30 years of Industrial Collaboration. Metall. Mater. Trans. B 2017, 48B, 91–98. [Google Scholar] [CrossRef]
- Barry, T.; Dinsdale, A.T.; Gisby, J.A. Predictive Thermochemistry and Phase Equilibria of Slags. JOM 1993, 45, 32–38. [Google Scholar] [CrossRef]
- Ham 2020. HTOX Version 9.1—Release Notes; Hampton Thermodynamics: Hampton, UK, 2020.
- Taskinen, P. Thermodynamics of liquid copper-oxygen alloys at 1065–1450 °C. Scand. J. Metall. 1984, 13, 75–82. [Google Scholar]
- Shishin, D.; Decterov, S. Critical assessment and thermodynamic modeling of the Cu-O and Cu-O-S systems. Calphad 2012, 38, 59–70. [Google Scholar] [CrossRef]
- Avarmaa, K.; O’Brien, H.; Klemettinen, L.; Taskinen, P. Precious metal recoveries in secondary copper smelting with high-alumina slags. J. Mater. Cycles Waste Manag. 2020, 22, 642–655. [Google Scholar] [CrossRef] [Green Version]
- Avarmaa, K.; Taskinen, P.; Klemettinen, L.; O’Brien, H.; Lindberg, D. Ni-Fe-Co Alloy—Magnesia-Iron-Silicate Slag Equilibria and the Behavior of Minor Elements Cu and P in Nickel Slag Cleaning. J. Mater. Res. Technol. 2021, 15, 719–730. [Google Scholar] [CrossRef]
- Dańczak, A.; Klemettinen, L.; O’Brien, H.; Taskinen, P.; Lindberg, D.; Jokilaakso, A. Slag Chemistry and Behavior of Nickel and Tin in Black Copper Smelting with Alumina and Magnesia-containing Slags. J. Sust. Metall. 2021, 7, 1–14. [Google Scholar] [CrossRef]
- Shishin, D.; Hayes, P.; Jak, E. Development and Applications of Thermodynamic Database in Copper Smelting. In Proceedings of the Copper 2019 Conference, 2 Pyrometallurgy, Vancouver, VA, Canada, 18–21 August 2019; CIM-MetSoc: Montreal, Canada, 2019; p. 12. [Google Scholar]
- Sineva, S.; Shevchenko, M.; Shishin, D.; Hidayat, T.; Chen, J.; Hayes, P.C.; Jak, E. Phase equilibria and minor element distributions in complex copper/slag/matte systems. JOM 2020, 72, 3401–3409. [Google Scholar] [CrossRef]
- Chen, M.; Avarmaa, K.; Taskinen, P.; Klemettinen, L.; Michallik, R.; O’Brien, H.; Jokilaakso, A. Handling trace elements in WEEE recycling through copper smelting-an experimental and thermodynamic study. Miner. Eng. 2021, 173, 107189. [Google Scholar] [CrossRef]
- Klemettinen, L.; Avarmaa, K.; Taskinen, P. Slag Chemistry of High-alumina Iron Silicate Slags at 1300 °C in WEEE Smelting. J. Sustain. Metall. 2017, 3, 772–781. [Google Scholar] [CrossRef]
- Kapusta, J.; Larouche, F.; Palumbo, E. Adoption of high oxygen bottom blowing in copper matte smelting: Why it is taking so long? In Proceedings of the COM2015 The Conference of Metallurgists, Toronto, ON, Canada, 23–26 August 2015; CIM-MetSoc: Montreal, Canada, 2015; pp. 1–25. [Google Scholar]
- Taskinen, P. Direct-to-blister smelting of copper concentrates: The slag fluxing chemistry. Miner. Process. Extr. Metall. 2011, 120, 240–246. [Google Scholar] [CrossRef]
- Johto, H.; Latostenmaa, P.; Peuraniemi, E.; Osara, K. Review of Boliden Harjavalta Nickel Smelter. In Extraction 2018; Davis, B., Moats, M., Wang, S., Eds.; TMS: Warrendale, PA, USA, 2018; pp. 81–87. [Google Scholar]
- Submaranian, K.N.; Themelis, N.J. Copper recovery by flotation. JOM 1972, 24, 33–38. [Google Scholar] [CrossRef]
- Jalkanen, H.; Vehviläinen, J.; Poijärvi, J. Copper in solidified copper smelter slags. Scand. J. Metall. 2003, 32, 65–70. [Google Scholar] [CrossRef]
- Kim, H.G.; Sohn, H.Y. Minor-Element Behavior and Iron Partition during the Cleaning of Copper Converter Slag under Reducing Conditions. Can. Metall. Q 1997, 36, 31–37. [Google Scholar] [CrossRef]
- Sridhar, R.; Toguri, J.; Simeonov, S. Copper losses and thermodynamic considerations in copper smelting. Metall. Mater. Trans. B 1997, 28B, 191–200. [Google Scholar] [CrossRef]
- Schramm, L.; Behr, G.; Löser, W.; Wetzig, K. Thermodynamic reassessment of the Cu-O phase diagram. J. Phase Equilibria Diffus. 2005, 26, 605–612. [Google Scholar] [CrossRef]
- Hallstedt, B.; Risold, D.; Gauckler, L.J. Thermodynamics Assessment of the Copper-Oxygen System. J. Phase Equil. 1994, 15, 483–499. [Google Scholar] [CrossRef]
- Sineva, S.; Shishin, D.; Starykh, R.; Hayes, P.C.; Jak, E. Equilibrium Distributions of Pb, Bi, and Ag between Fayalite Slag and Copper-Rich Metal, Calcium Ferrite Slag and Copper-Rich Metal. Thermodynamic Assessment and Experimental Study at 1250 °C. J. Sust. Metall. 2021, 7, 569–582. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taskinen, P.; Avarmaa, K. Simulation of Slag–Matte/Metal Equilibria for Complex and Low-Grade Raw Materials. Sustainability 2021, 13, 12826. https://doi.org/10.3390/su132212826
Taskinen P, Avarmaa K. Simulation of Slag–Matte/Metal Equilibria for Complex and Low-Grade Raw Materials. Sustainability. 2021; 13(22):12826. https://doi.org/10.3390/su132212826
Chicago/Turabian StyleTaskinen, Pekka, and Katri Avarmaa. 2021. "Simulation of Slag–Matte/Metal Equilibria for Complex and Low-Grade Raw Materials" Sustainability 13, no. 22: 12826. https://doi.org/10.3390/su132212826
APA StyleTaskinen, P., & Avarmaa, K. (2021). Simulation of Slag–Matte/Metal Equilibria for Complex and Low-Grade Raw Materials. Sustainability, 13(22), 12826. https://doi.org/10.3390/su132212826





