Abstract
The pyrolysis of waste electronically heated tobacco (EHT), consisting of tobacco leaves (TL), a poly-lactic acid (PLA) filter, and a cellulose acetate (CA) filter, was investigated using thermogravimetric (TG) and pyrolyzer–gas chromatography/mass spectrometry (Py-GC/MS) analysis. The pyrolytic properties of waste EHT obtained after smoking were comparable to those of fresh EHT. Although the maximum decomposition temperatures (TmaxS) of waste TL and CA were similar to those of fresh EHT components, the Tmax of waste PLA was slightly higher than that of fresh PLA due to smoldering. The Tmaxs of PLA and CA were lowered when they were co-pyrolyzed with TL due to interactions between pyrolysis intermediates. The apparent activation energies for the non-isothermal pyrolysis of waste EHT components were higher than those of fresh EHT components. Py-GC/MS analysis results indicated that considerable amounts of chemical feedstocks, such as nicotine and limonene from TL, caprolactone and lactide from PLA, and acetic acid and triacetin from CA, can be recovered by simple pyrolysis of EHT. Co-pyrolysis of TL, PLA, and CA revealed that the experimental amount of lactide was much larger than the calculated value, suggesting its synergistic formation.
1. Introduction
Approximately 5.7 trillion conventional cigarettes (CCs) are consumed annually, generating two million tonnes of waste [1]. During smoking, CCs generate smoke and are converted into ash through the complete combustion of tobacco [2]. Electrically heated tobacco (EHT) is tobacco heated at a lower temperature than CCs. Owing to the introduction and growing popularity of EHT, the consumption of CCs is declining, particularly in Europe and the US [3]. Unlike CCs containing cut tobacco leaves (TLs), EHTs are composed of tobacco film grounded and reconstituted as cast-leaf sheets by adding water, glycerine, and cellulose fibers [4]. Most EHTs also contain biopolymers such as poly-lactic acid (PLA) and cellulose acetate (CA). The function of PLA is to cool down the aerosols generated by tobacco films and lower the irritating effects of smoking in the throat. In contrast, CA functions as a mouthpiece filter that imitates the sensory characteristics of cigarettes [5,6]. All EHT products use a controlled heating system to maintain a specific temperature for smoldering the EHT instead of allowing complete burning [7]. Smoking generates considerable amounts of waste EHT; most of which are dumped into the environment without appropriate treatment. The waste generated from EHT is significantly larger than that produced from CCs because EHT remains in most of the tobacco leaves (TL) even after smoking due to the thermal extraction of nicotine without decomposition of hemicellulose, cellulose, and lignin. In addition, PLA and CA filters are rarely treated and properly disposed of, posing another environmental threat [8].
Thermal treatment through pyrolysis and gasification can be effective waste-treatment technologies to reduce EHT waste volume. Numerous studies have been conducted on the pyrolysis of EHT to produce chemical feedstock or fuels. Some researchers have investigated the products released by EHT and their toxicity, while others have examined overall product distribution [9]. However, few have explored the pyrolysis kinetics related to the evolved products from EHT [10]. A literature survey revealed no reports on how to mitigate the environmental impact of EHT waste and recover fuels and chemical feedstocks.
In this study, we investigated the pyrolysis of EHT using thermogravimetric (TG) and pyrolyzer-gas chromatography/mass spectrometry (Py-GC/MS) analysis. An EHT sample was mechanically separated into its components, and their artificial mixture (AM) was also prepared based on the weight ratios of TL, CA, and PLA in EHT. The study examined the individual components of fresh and waste EHT collected after smoking to determine the effect of smoking on pyrolysis kinetics and product distribution.
2. Materials and Methods
2.1. Materials
Fresh EHT (HEETS, Philip Morris Korea Inc., Yangsan, Korea) was purchased from a convenience store, and waste EHTs were prepared by smoking the fresh EHT. The weight ratios of TL, PLA, and CA in both fresh and waste EHT were approximately 40%, 40%, and 20%, respectively. Both fresh and waste EHT were mechanically separated into fresh TL, PLA and CA, and their waste components. All EHT components were milled into small particles, sieved to obtain particles less than 500 μm, and dried at 80 °C for 48 h before the experiment.
2.2. Kinetic Analysis
Kinetic analysis was performed using a TG analyzer (TGA55 Discovery Series, TA Instruments) and 5 mg of TL, PLA, CA, and their artificial mixture (AM, TL/PLA/CA: 2/2/1) were heated at different rates (4, 8, and 16 °C/min) under 100 mL/min of nitrogen flow. A model-free kinetic analysis method, Revised Ozawa [11], was applied to determine the apparent activation energy (Ea) values at each conversion. The final kinetic equation of the Revised Ozawa method is expressed as below and Ea value can be calculated by the slope on the plots of lnβ versus 1/T at each conversion (X).
n: Reaction order; A: Pre-exponential factor (min−1); R: Gas constant (8.314 J/mol·K); : Conversion factor at peak temperature, : Temperature(K).
2.3. Py-GC/MS Analysis
A pyrolyzer (Py) (3030D, Frontier Laboratories Ltd., Fukushima, Japan) combined with gas chromatography (GC)/mass spectroscopy (MS) (7890A/5975C inert, Agilent Technology, Santa Clara, CA, USA) was used to investigate the chemical distribution of products obtained from the pyrolysis of fresh and waste TL, PLA, CA, and AM at 450 °C. 1 mg sample in a deactivated metal cup was introduced to the preheated furnace of the pyrolyzer. The pyrolysis product emitted by the pyrolyzer was transferred to a GC capillary column (UA-5, 30 m × 0.25 mm i.d. × 0.25 μm f.t.) via a split/splitless GC inlet (300 °C, split ratio: 100/1) and cryo-focused with liquid nitrogen at the front point of the column. After cryo-focusing, the pyrolysis products were separated in the column under non-isothermal GC oven heat condition, from 40 °C (3 min hold) to 320 °C (3 min hold) at 20 °C/min and detected by MS in scan mode (m/z, 35–550). All peaks monitored on total ion chromatogram were identified using an MS library (NIST 08) and integrated to compare their amounts indirectly using absolute peak areas. The average MS peak areas for all pyrolysis products, which were obtained through repeated Py-GC/MS analysis, were used as the amounts of pyrolysis products.
3. Results and Discussion
3.1. Physoco-Chemical Propertis of EHT Components
Table 1 provides the results of the proximate and ultimate analyses of all components of fresh EHT. The proximate analysis found that the volatile content was greater than 80% in all EHT components, suggesting a high potential for the production of oil and gas by pyrolysis [12]. All EHT components had high carbon and oxygen contents, suggesting that the recovery of chemical feedstocks would be a more effective target than fuel production.

Table 1.
Physicochemical properties of main components of fresh EHT.
3.2. TG Analysis
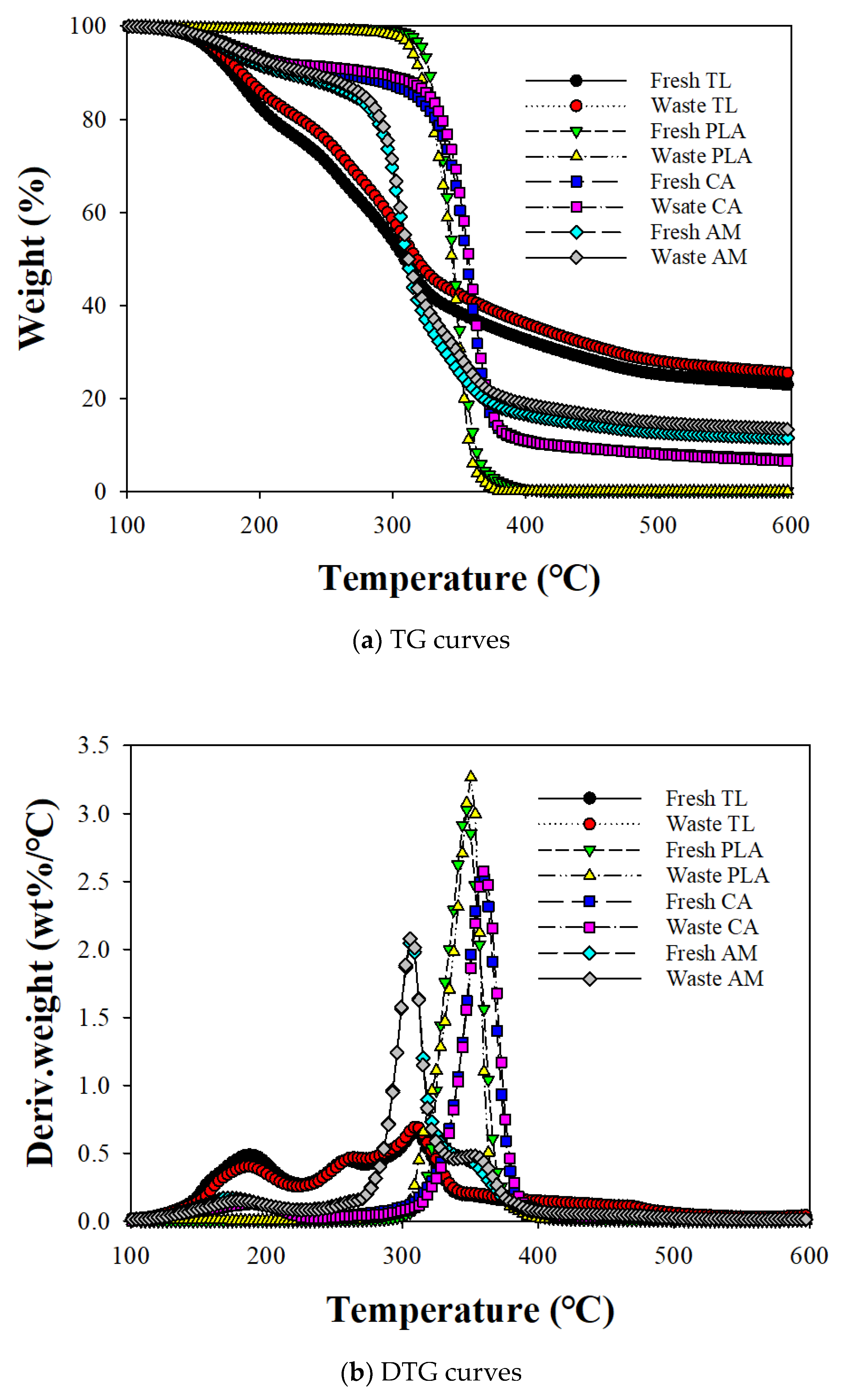
Figure 1 shows the TG and derivative TG (DTG) curves for the non-isothermal decomposition of fresh and waste EHT components and their AMs at 16 °C/min. Thermal decomposition of fresh and waste TL revealed two discrete weight-loss regions. The first decomposition region from 100 to 220 °C can be explained by the concurrent reaction of the vaporization of glycerol, nicotine, and pectin degradation [13]. The peak height for the first decomposition region of waste TL was smaller than that of fresh TL, indicating that large amounts of glycerol and nicotine were eliminated with pectin decomposition during smoking [14]. The second and third peaks between 230 to 350 °C corresponded to the decomposition of hemicellulose [15,16] and cellulose [17,18], respectively. The peak tailing at a temperature greater than 350 °C on the DTG curve for TL can be assigned to the additional decomposition of lignin and char stabilization [19].
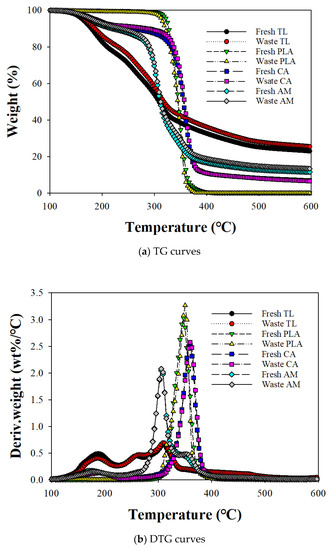
Figure 1.
(a) TG and (b) DTG curves for the individual components of fresh and waste EHT components and their AMs.
The TG and DTG curves for fresh and waste PLA indicated a decomposition reaction from 290 °C to 410 °C. Waste PLA had a higher maximum decomposition temperature (Tmax; 352 °C) compared with fresh PLA (347 °C), suggesting the smoldering effect of smoking. Smoldering is a slow combustion process at low temperatures when EHT is lit during smoking. Some researchers have reported that the thermal stability of PLA is easily disturbed, even by a low temperature smoldering reaction, due to its low heat resistance [20]. The transfer of glycerol and nicotine from TL to PLA during smoking can also lead to higher PLA decomposition temperatures.
The TG and DTG curves for fresh and waste CA indicated a small loss of weight between 100 and 220 °C due to the vaporization of a flavoring agent [8,9]. The Tmax (360 °C) on the DTG curve for CA was in agreement with the findings of previous studies [21,22] and the DTG analysis produced no apparent difference in Tmax for the fresh and waste CAs.
The TG and DTG curves for the AMs of EHT components revealed three decomposition regions. For fresh AM, the first region, up to 220 °C, coincided with the first DTG peak of TL vaporizing glycol and nicotine [7,23]. The second decomposition peak for AM can be assigned to the merged decomposition peak of cellulose and PLA. Although the co-pyrolysis of TL did not change the decomposition temperatures of hemicellulose and cellulose in TL with PLA, that of PLA shifted to a lower temperature than its TG analysis and overlapped with the cellulose decomposition temperature. This suggests that the intermediates of the pyrolysis reaction of TL accelerated the decomposition of PLA [24]. Wang et al. [25] also found that PLA decomposition temperature is lowered when it co-pyrolyzed with biomass due to the effective intermolecular reaction among volatiles/condensates produced from biomass and PLA. The decomposition temperatures of CA on the DTG curves of AMs were also shifted to the lower temperatures, from 359 °C to 351 °C for fresh CA and from 360 to 352 °C for waste CA, due to the interaction of CA pyrolysis intermediates with those of other EHT components [22].
3.3. Kinetic Analysis
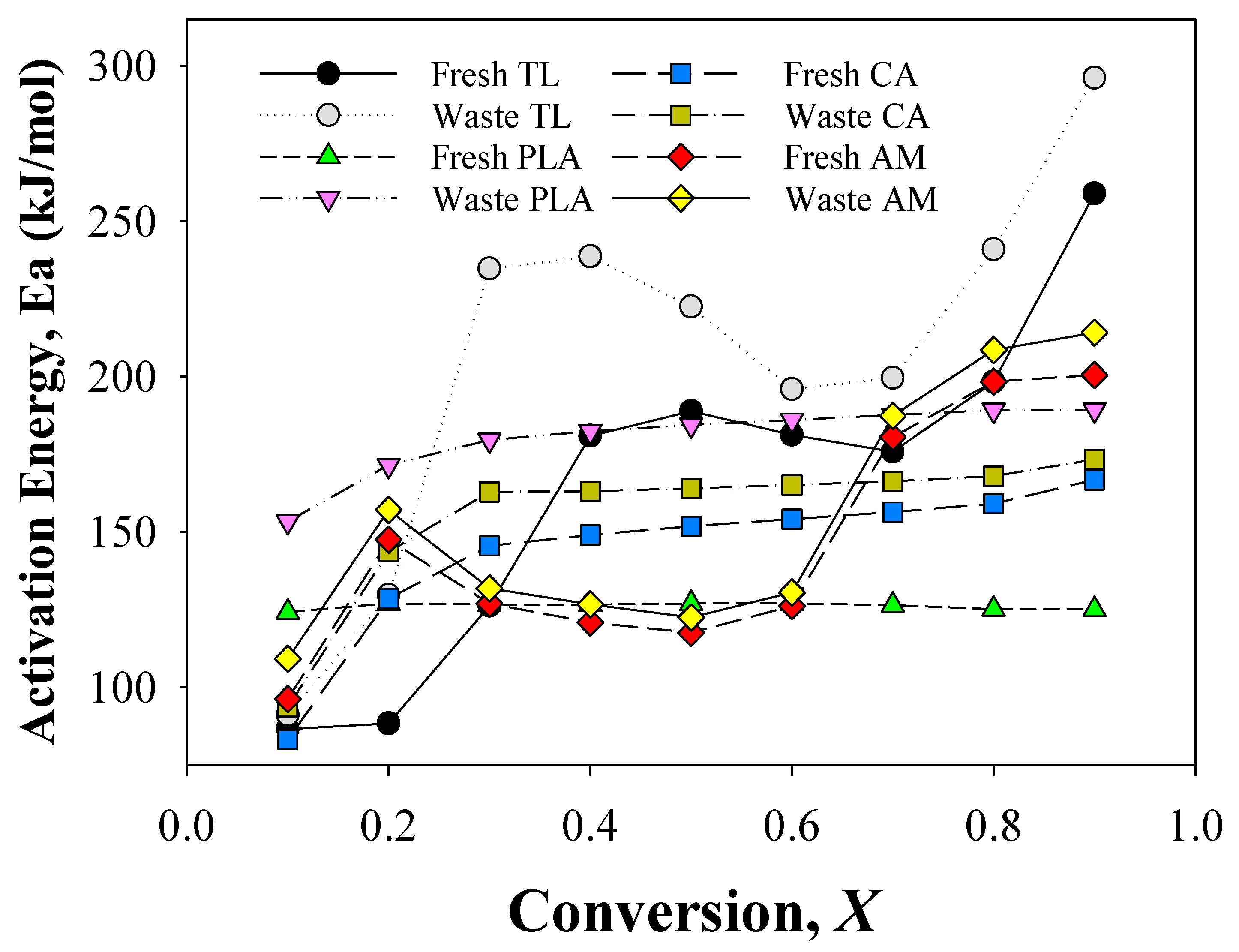
Figure 2 presents the Ea values obtained from the TG analysis of individual components of EHT and their AMs. These values were calculated from the slopes of the plots of lnβ versus 1/T at each conversion (X). All plots had linear correlation coefficients (R2) greater than 0.99, suggesting effective kinetic analysis.
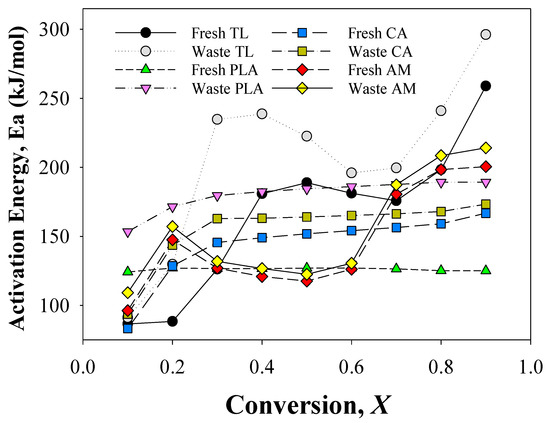
Figure 2.
Activation energies obtained from the kinetic analysis of the individual components of fresh and waste EHT and their mixtures.
The Ea of fresh TL increased from 87 kJ/mol (X: 0.1) to 180 kJ/mol (X: 0.4) and decreased gradually to 176 kJ/mol (X: 0.7), and then re-increased again to 259 kJ/mol (X: 0.9), confirming that TL were decomposed via a multi-step pyrolysis reaction involving nicotine vaporization and pectin decomposition [26], cellulose decomposition, and lignin decomposition and char stabilization [25]. Although the Ea values at each conversion differed, waste TL also revealed a similar tendency in the change of Ea values with fresh TL. This suggests that the overall pyrolysis patterns of fresh and waste TLs were similar. Although waste TL had a similar Ea change tendency with fresh TL, the Ea values at all conversions during the pyrolysis of waste TL were much higher than those of fresh TL. This suggests that smoking EHT leads to the smoldering of TL [27]. The smoldered TL is associated with higher Ea values because of the partial elimination/vaporization of nicotine and glycerin and the partial decomposition of pectin and hemicellulose. Other researchers have also reported that smoldered/torrefied biomass exhibits higher Ea values than their original samples [28].
The change tendency in Ea for fresh PLA was not markedly different from that of waste PLA, confirming the single-step thermal decomposition process of PLA [29,30]. However, waste PLA exhibited a much higher Ea value compared with fresh PLA. The large difference in the Ea of fresh and waste PLAs can be attributed to the smoldering effect on waste samples, which disturbed the thermal stability of PLA [8]. Another possible explanation for the high Ea of waste PLA is the penetration of heat and volatile compounds, such as heterocycles and glycerols, to PLA during the smoking process. Nicolae et al. [20] reported that the thermal stability of PLA is low and can be easily changed even by low-temperature thermal treatment. The Ea of waste CA was also higher than that of fresh CA, suggesting that smoking influences the thermal stability of CA. The Ea values at a conversion rate of 0.1 for fresh and waste CAs were approximately 83 kJ/mol and 94 kJ/mol, respectively. Subsequent increases in the Ea of fresh and waste CAs at each conversion reached their maximums of 167 kJ/mol and 173 kJ/mol, respectively; this is consistent with values reported previously [31,32].
The Ea obtained from the pyrolysis of AM (prepared by mixing TL, PLA, and CA), exhibited the expected trend for each conversion. The Ea for fresh AM started from 96 kJ/mol at X 0.1, while the initial value for the waste AM was 109 kJ/mol at X 0.1. From X 0.2 to X 0.6, the fresh and waste AM values dropped from 147 kJ/mol and 157 kJ/mol to 126 kJ/mol and 130 kJ/mol, respectively. The DTG curves of AMs reveal an overlapped decomposition of cellulose from TL alongside PLA as a result of their intermolecular interaction. The enhanced reactivity caused by the co-pyrolysis between PLA and TL may be the primary reason for the decrease in Ea from X 0.2 to X 0.6 [24]. The increase from 0.7 to 0.9 conversion rates may be the result of char stabilization from TL [25].
3.4. Py-GC/MS Analysis
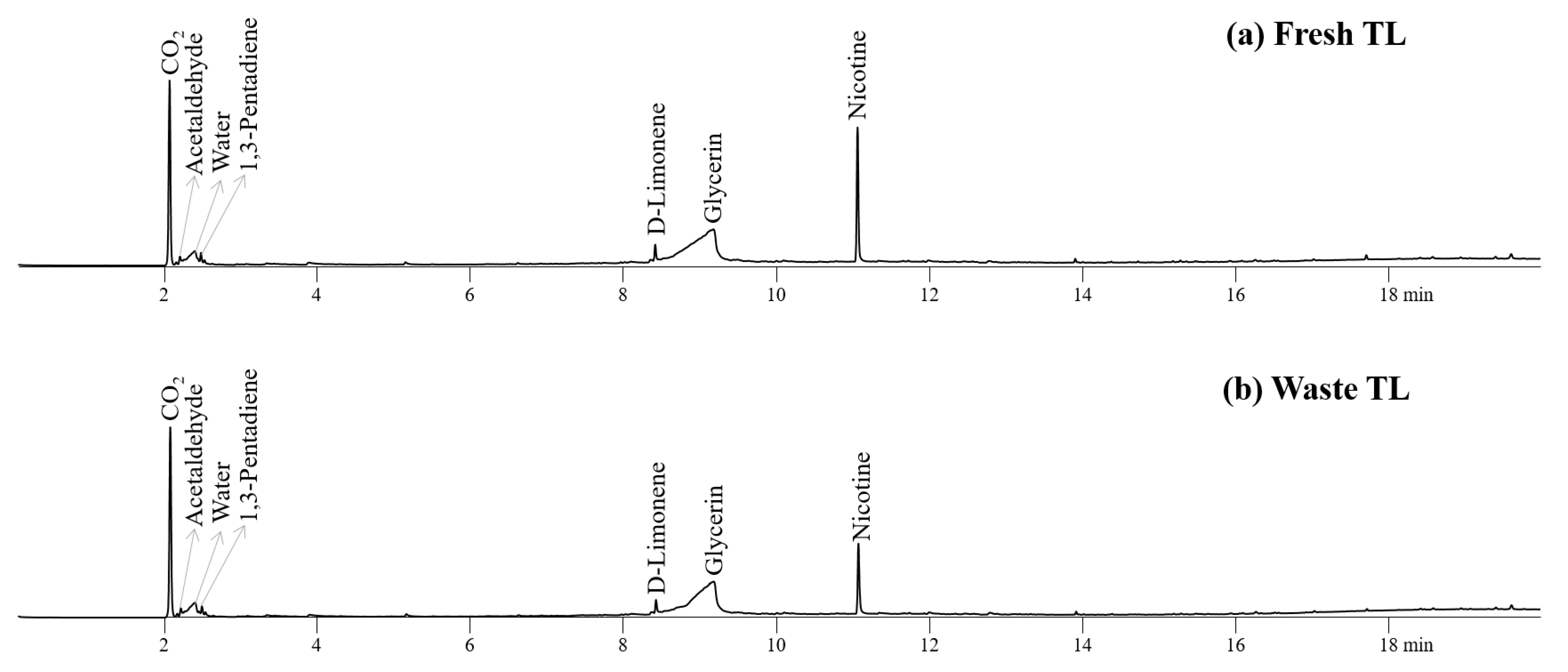
Figure 3 depicts the pyrograms obtained from the Py-GC/MS analysis of fresh and waste TL, PLA, CA, and their AMs at 450 °C. The pyrolyzates of fresh and waste TL consisted of a variety of oxygenates and nitrogen-containing compounds, as reported previously [28,33]. Nitrogen-containing heterocyclic compound (nicotine) is the main pyrolyzate of TL [9,28]. Limonene was produced by the decomposition of pectin, confirming previous reports [26,34]. Glycerol, a humectant in EHT, was also found in the pyrogram of TLs [9]. Other chemicals, such as carbon dioxide, acetaldehyde, water, and 1,3-pentadiene, were produced from the decomposition of the hemicellulose and cellulose parts of TL [35]. Both fresh and waste TLs produced similar amounts of compounds except glycerin and nicotine, suggesting a negligible smoldering effect, which is also in agreement with previous studies [36,37]. The peak intensities for nicotine and glycerin on the pyrogram of waste TL were much lower than those of fresh TL due to their elimination during smoking.
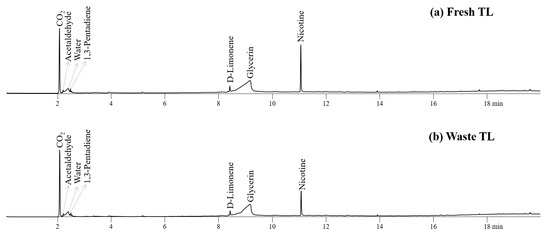
Figure 3.
Pyrograms obtained from Py-GC/MS analysis of (a) fresh TL and (b) waste TL at 450 °C.
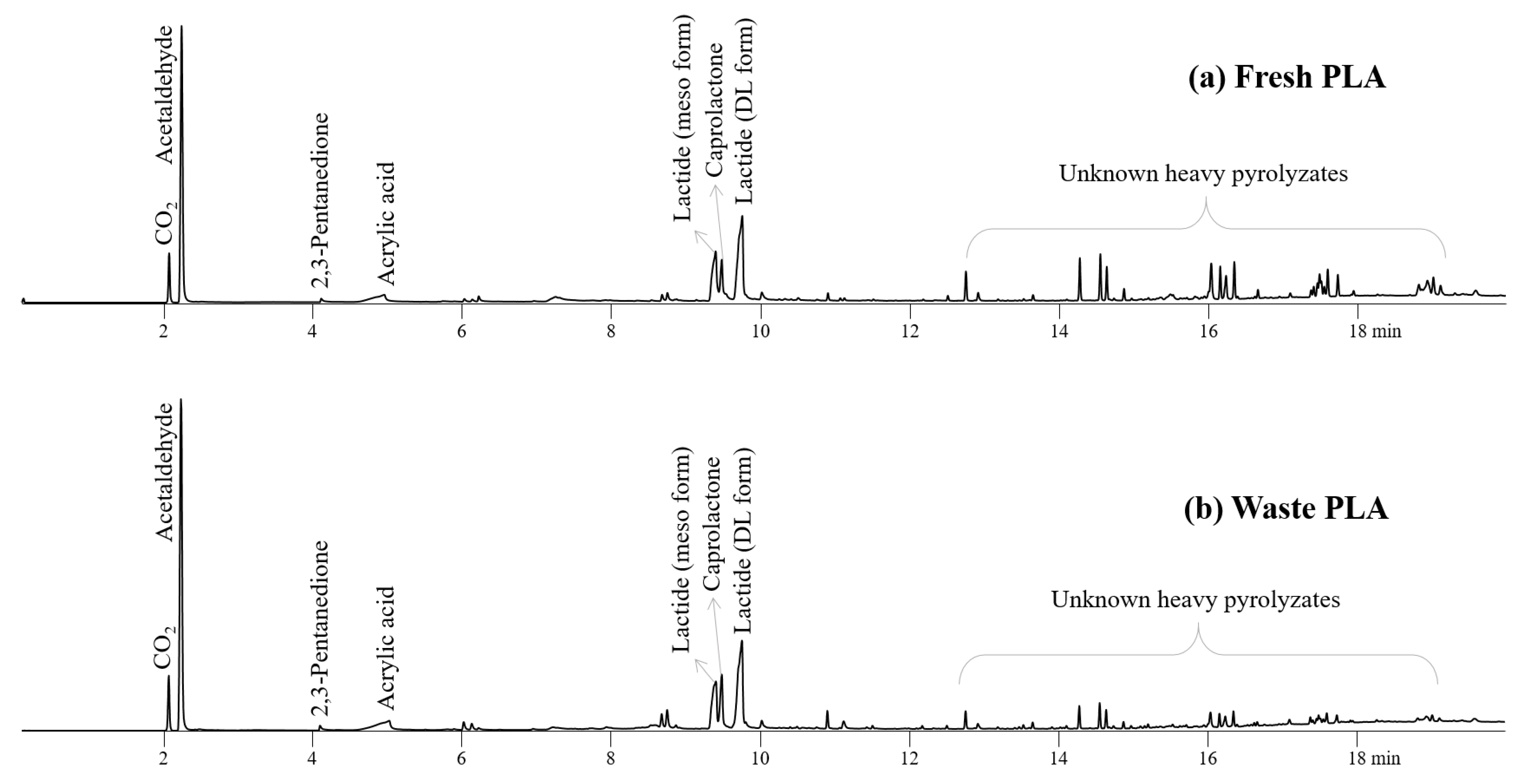
As reported previously, fresh and waste PLA produced a variety of acids, esters, and ketones (Figure 4) [38,39,40]. The pyrolysis of PLA in particular produced a large amount of cyclic esters, caprolactone and lactide, which are important chemical feedstocks in the pharmaceutical and biomedical industries [41,42]. Lactide is also an important feedstock for synthesizing biopolymers in various applications [43,44]. Compared to fresh PLA, waste PLA produced the smaller amount of heavy prolyzates, unknown peaks having the longer retention time than 12 min on Figure 4, with the increased peak intensities for acetaldehyde, lactide, and caprolactone, suggesting that the efficient cracking of heavy pyrolysis intermediates can be achieved during the pyrolysis of waste PLA.
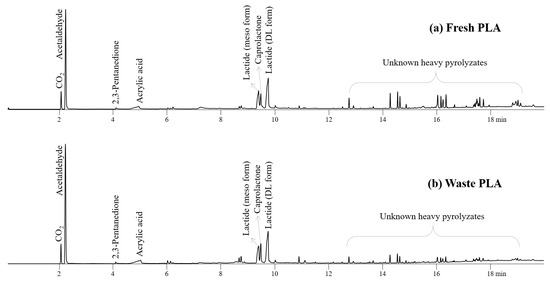
Figure 4.
Pyrograms obtained from Py-GC/MS analysis of (a) fresh PLA and (b) waste PLA at 450 °C.
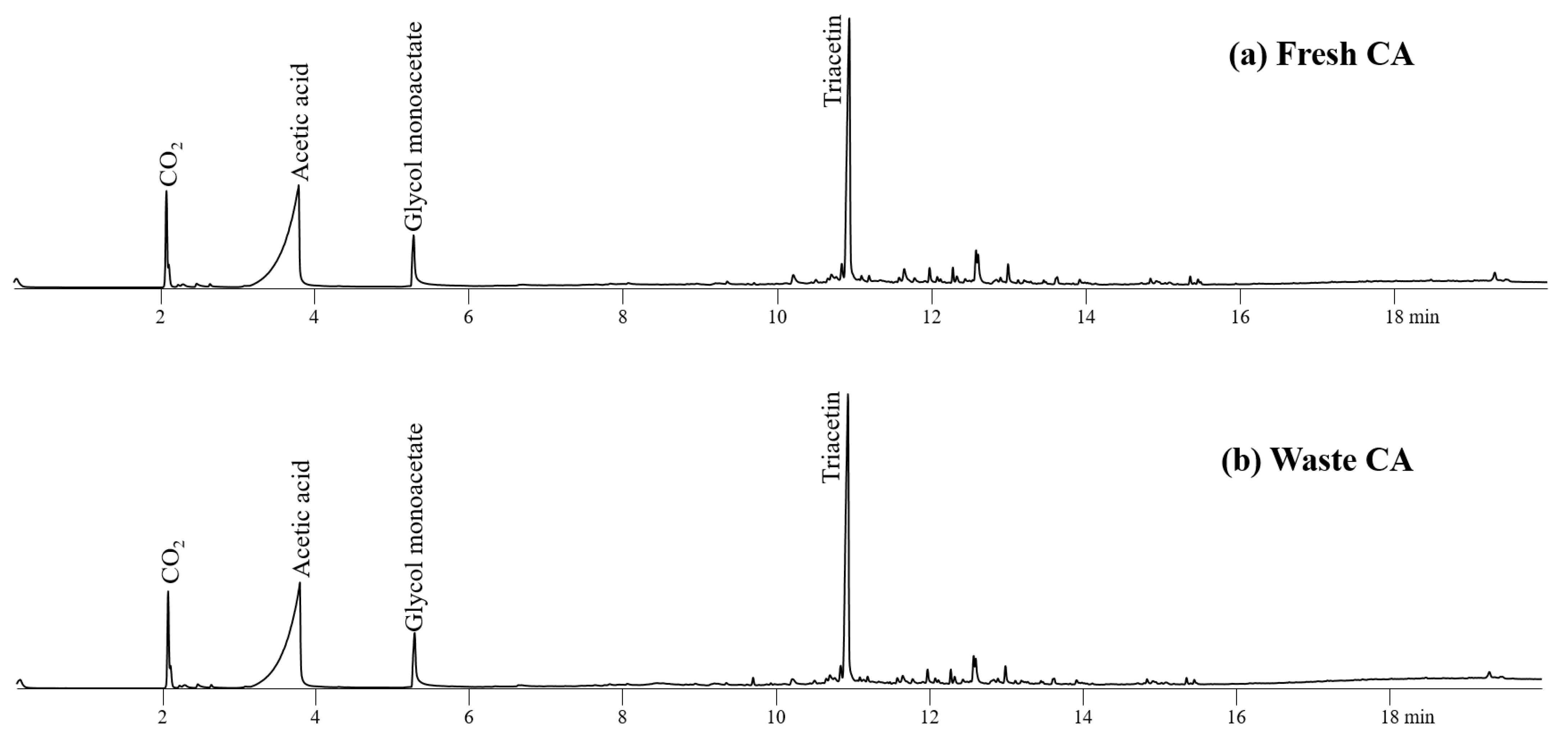
Figure 5 shows the product distribution obtained from fresh and waste CAs. Triacetin, a valuable solvent used as a decaffeinating agent [8] and as plasticizer of tobacco CA filters, was associated with the highest intensity peak on the CA pyrogram. Acetic acid and glycol monoacetate were also observed on the pyrograms of CA.
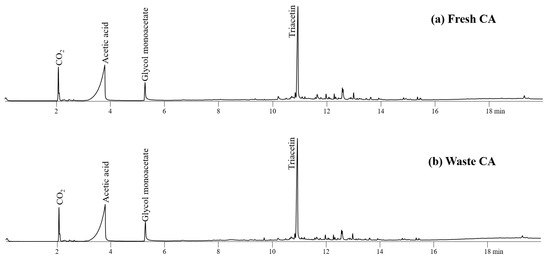
Figure 5.
Pyrograms obtained from Py-GC/MS analysis of (a) fresh CA and (b) waste CA at 450 °C.
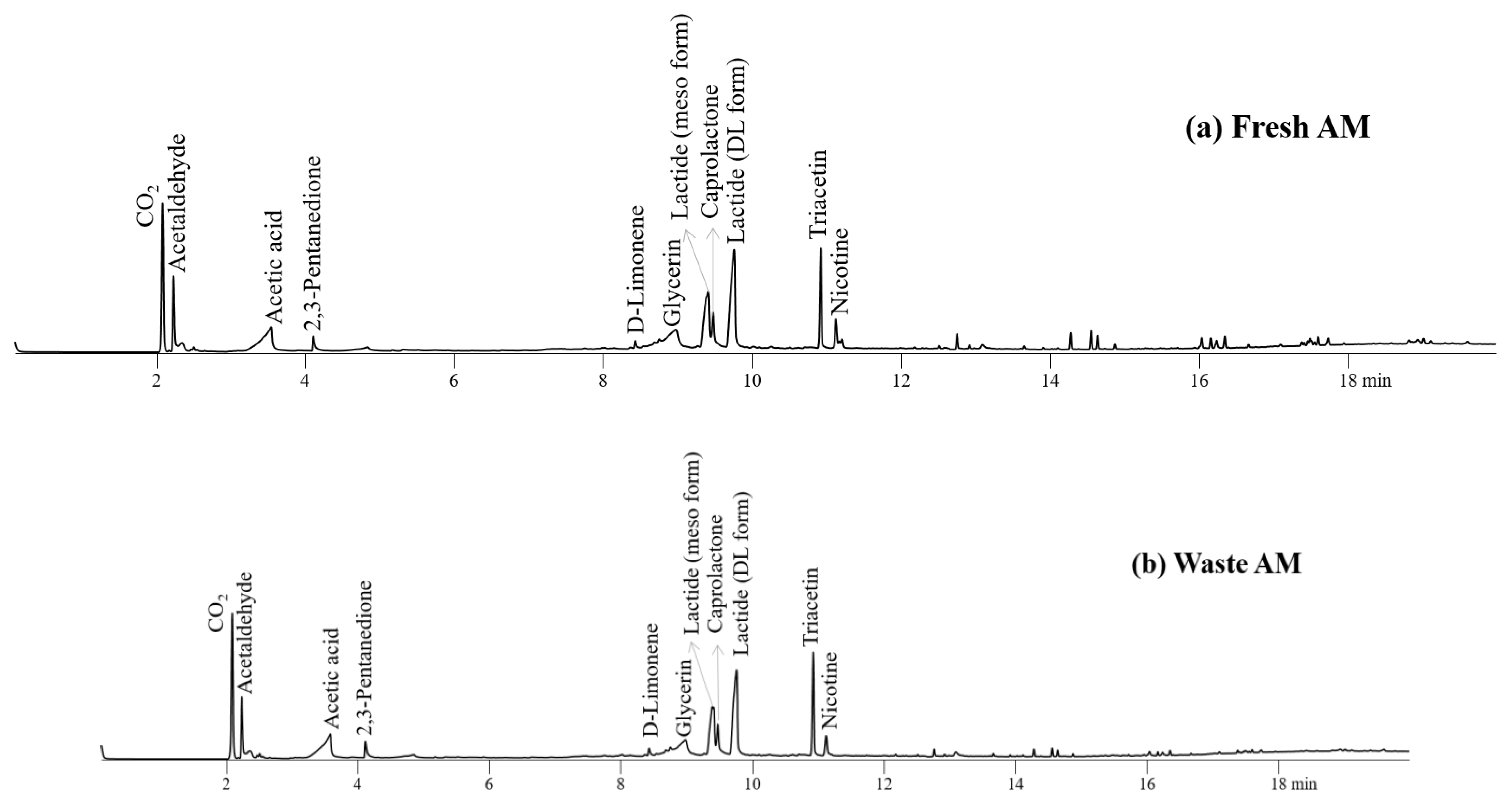
Figure 6 depicts the pyrograms for the AMs of fresh and waste EHT components. The chemicals produced by the pyrolysis of TL, PLA, and CA were also monitored on the pyrograms of their AMs. As expected with the Py-GC/MS results of individual components of EHT, the peak intensities for the main pyrolysis products of fresh and waste AMs were not different largely with those of fresh AMs except nicotine and glycerine.
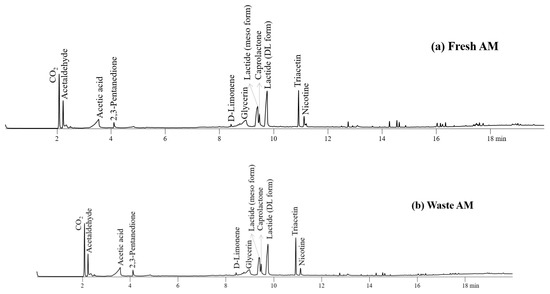
Figure 6.
Pyrograms obtained from Py-GC/MS analysis of (a) fresh AM and (b) waste AM at 450 °C.
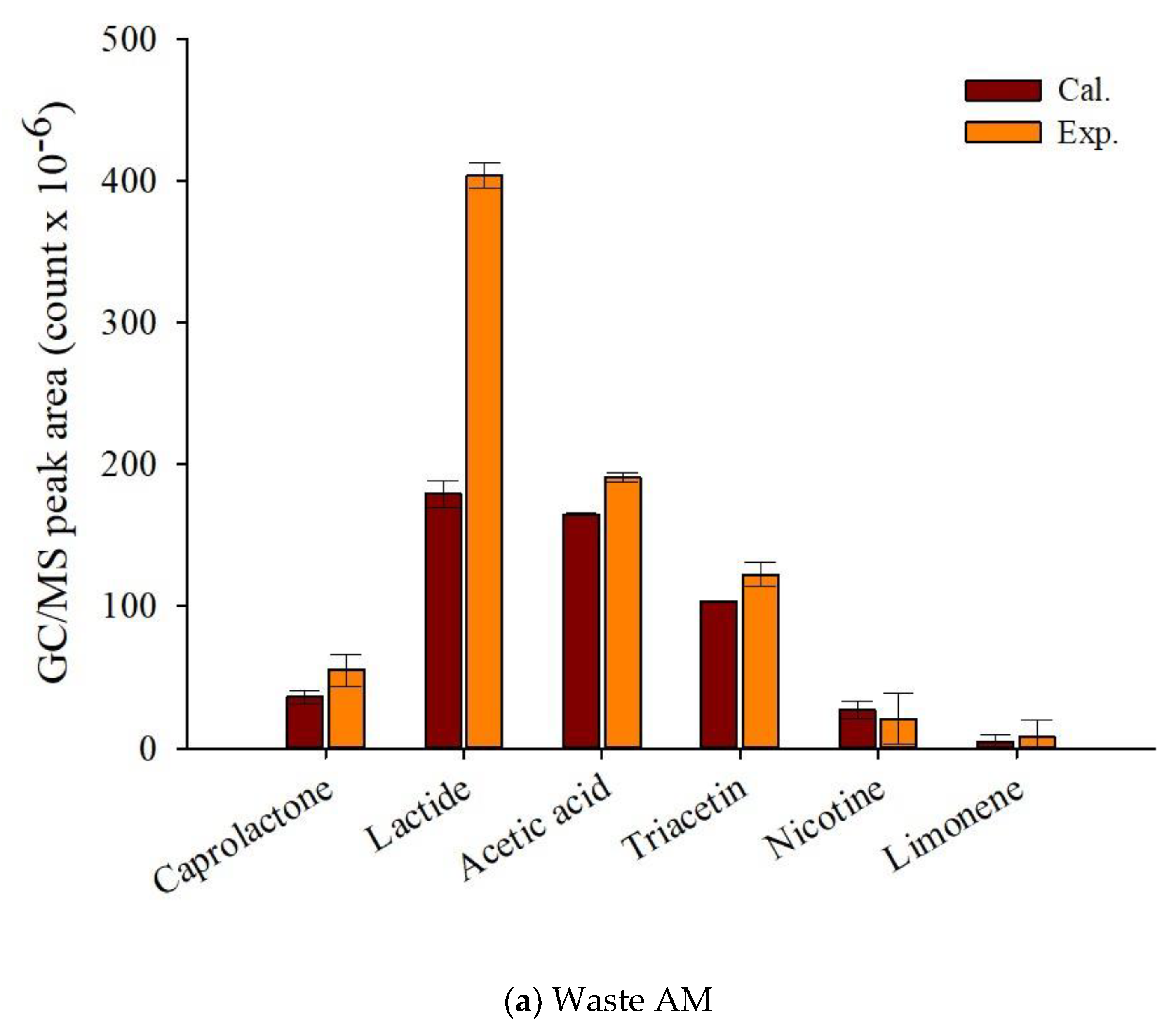
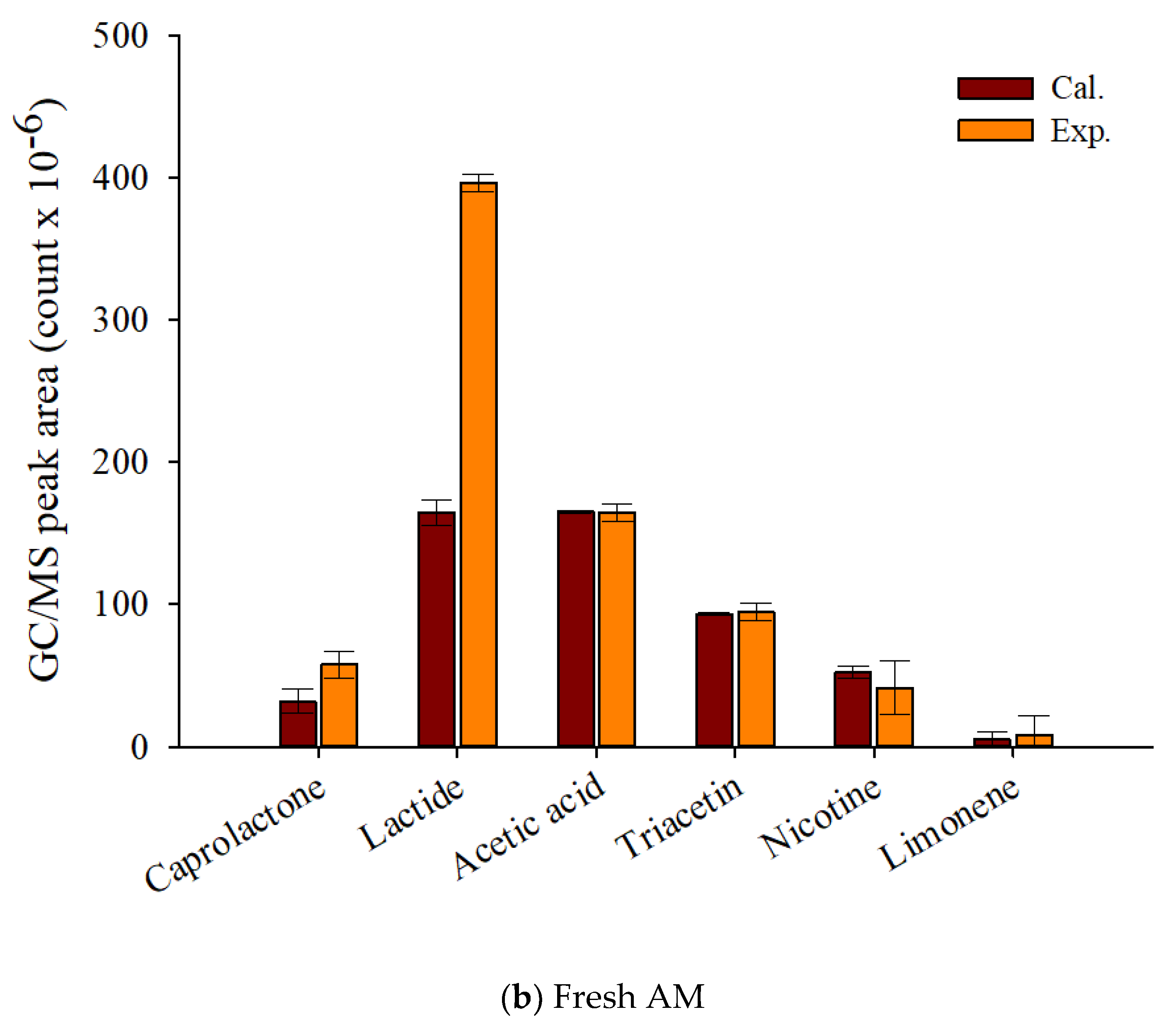
Figure 7 displays that theoretical and experimental MS peak areas for the main pyrolyzates of fresh and waste AM. Although it was difficult to know the synergistic formation of the pyrolyzates of TL and CA, the experimental peak area of lactide, the main pyrolyzate of PLA, was clearly larger than the calculated peak area, suggesting its synergistic formation by the radical interaction with the pyrolysis intermediates of other components of EHT. Sun et al. [45], who investigated the pyrolysis reaction mechanism of PLA, reported that the main pyrolyzates of PLA were obtained by transesterification and free radical reactions. When biomass co-pyrolyzed with PLA, the free radicals promoted the production of caprolactone and lactide. Cornelissen et al. [46] also reported higher experimental values for esteric compounds alongside gaseous yields and oxygenates compared with their theoretically calculated values from the co-pyrolysis of PLA and biomass. The increased production amounts of chemical feedstock on the co-pyrolysis of waste components of EHT suggests that the simple pyrolysis of waste EHT can provide not only the minimization of the pre-treatment to separate the EHT components, but also the maximization of the value on their commercialization.
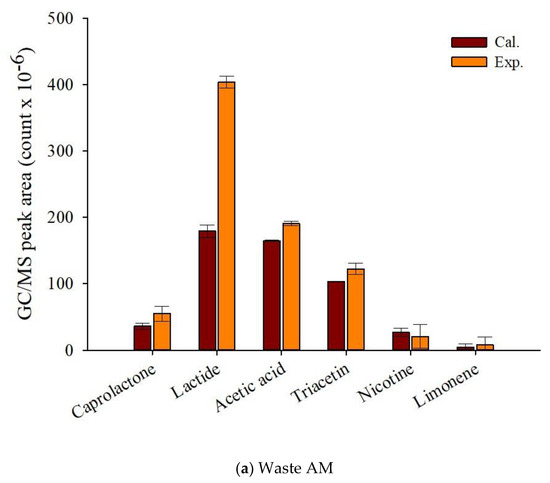
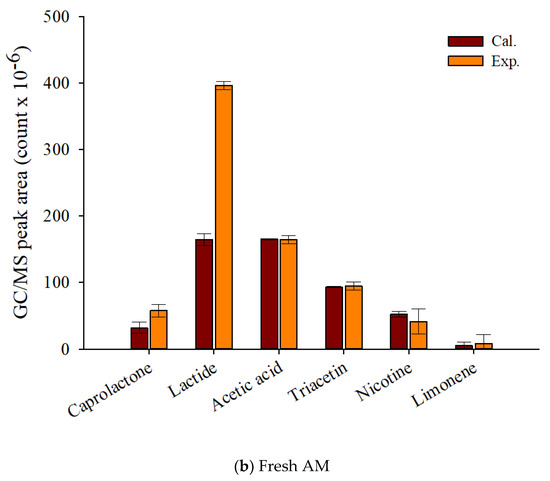
Figure 7.
Py-GC/MS peak area (count × 10−6) of the theoretically calculated and experimental values for the main pyrolyzates of EHT components (a) waste and (b) fresh artificial mixtures at 450 °C.
4. Conclusions
Thermal decomposition of fresh and waste TL and CA was similar; however, waste PLA exhibited a shift to the higher decomposition region (i.e., 352 °C) from its fresh counterpart (i.e., 347 °C) due to the smoldering effect. The AMs of fresh and waste EHT produced dramatic shifts in DTG peaks for the second region consisting of TL and PLA compared with their separated components, due to the effective intermolecular interactions of volatiles and condensates produced by the simultaneous decomposition of TL and PLA. In addition, PLA interacted with CA, resulting in a DTG shift to a lower-temperature region. The Ea values of waste EHT were higher than that of the fresh EHT, highlighting the smoldering effect on waste EHT. The results of Py-GC/MS analysis suggest that important chemical feedstocks, including caprolactone, lactide, acetic acid, triacetin, nicotine, and limonene, can be obtained from the pyrolysis of waste EHT. These chemical feedstocks can be used in other chemical processes along with the re-manufacturing of EHT. Compared with the theoretical values, the higher experimental values for the formation of lactide indicate that co-pyrolysis can be a more appropriate method to produce these valuable compounds, and waste EHT can be pyrolyzed without any pre-treatment requirement.
Author Contributions
Conceptualization, Y.-M.K.; formal analysis, H.K., S.-J.L., G.-J.W.; investigation, Y.C., M.Z.S.; data curation, H.K., S.-J.L., G.-J.W.; writing—original draft preparation, Y.-K.P., Y.C., S.J.; writing—review and editing, S.P., Y.-M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2020R1A5A1019631), by Korea Environment Industry & Technology Institute through Post Plastic, a specialized program of the Graduate School funded by Korea Ministry of Environment (MOE), and by the Soonchunhyang University Research Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baran, W.; Madej-Knysak, D.; Sobczak, A.; Adamek, E. The influence of waste from electronic cigarettes, conventional cigarettes and heat-not-burn tobacco products on microorganisms. J. Hazard. Mater. 2020, 385, 121591. [Google Scholar] [CrossRef] [PubMed]
- Barontini, F.; Tugnoli, A.; Cozzani, V.; Tetteh, J.; Jarriault, M.; Zinovik, I. Volatile products formed in the thermal decomposition of a tobacco substrate. Ind. Eng. Chem. Res. 2013, 52, 14984–14997. [Google Scholar] [CrossRef]
- Abrams, D.B. Can we use them to make combusting of tobacco obsolete-end the “cigarette century” and its preventable deaths? In Consequences Smoking—50 Years Progress; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2014. [Google Scholar]
- Smith, M.R.; Clark, B.; Lüdicke, F.; Schaller, J.P.; Vanscheeuwijck, P.; Hoeng, J.; Peitsch, M.C. Evaluation of the Tobacco Heating System 2.2. Part 1: Description of the system and the scientific assessment program. Regul. Toxicol. Pharmacol. 2016, 81, S17–S26. [Google Scholar] [CrossRef] [PubMed]
- Schorp, M.K.; Tricker, A.R.; Dempsey, R. Reduced exposure evaluation of an Electrically Heated Cigarette Smoking System. Part 1: Non-clinical and clinical insights. Regul. Toxicol. Pharmacol. 2012, 64, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Zenzen, V.; Diekmann, J.; Gerstenberg, B.; Weber, S.; Wittke, S.; Schorp, M.K. Reduced exposure evaluation of an Electrically Heated Cigarette Smoking System. Part 2: Smoke chemistry and in vitro toxicological evaluation using smoking regimens reflecting human puffing behavior. Regul. Toxicol. Pharmacol. 2012, 64, S11–S34. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.; Jakaj, B.; Forster, M.; Nicol, J.; Mavropoulou, E.; Scott, K.; Liu, C.; McAdam, K.; Murphy, J.; Proctor, C.J. Assessment of tobacco heating product THP1.0. Part 2: Product design, operation and thermophysical characterisation. Regul. Toxicol. Pharmacol. 2018, 93, 4–13. [Google Scholar] [CrossRef]
- St. Helen, G.; Goniewicz, M.L.; Dempsey, D.; Wilson, M.; Jacob, P.; Benowitz, N.L. Exposure and kinetics of polycyclic aromatic hydrocarbons (PAHs) in cigarette smokers. Chem. Res. Toxicol. 2012, 25, 952–964. [Google Scholar] [CrossRef]
- Ilies, B.D.; Moosakutty, S.; Kharbatia, N.; Sarathy, M. Identification of volatile constituents released from IQOS heat-not-burn tobacco HeatSticks using a direct sampling method. Tob. Control. 2020. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Liu, Q.; Gu, Y.; Luo, Z.; Cen, K.; Fransson, T. Comparison of the pyrolysis behavior of lignins from different tree species. Biotechnol. Adv. 2009, 27, 562–567. [Google Scholar] [CrossRef]
- Schaller, J.P.; Pijnenburg, J.P.M.; Ajithkumar, A.; Tricker, A.R. Evaluation of the Tobacco Heating System 2.2. Part 3: Influence of the tobacco blend on the formation of harmful and potentially harmful constituents of the Tobacco Heating System 2.2 aerosol. Regul. Toxicol. Pharmacol. 2016, 81, S48–S58. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.K. Characterization of thermal reaction by peak temperature and height of DTG curves. Thermochim. Acta 1995, 264, 137–156. [Google Scholar] [CrossRef]
- Herrington, J.S.; Myers, C. Electronic cigarette solutions and resultant aerosol profiles. J. Chromatogr. A 2015, 1418, 192–199. [Google Scholar] [CrossRef]
- Oja, V.; Hajaligol, M.R.; Waymack, B.E. The vaporization of semi-volatile compounds during tobacco pyrolysis. J. Anal. Appl. Pyrolysis 2006, 76, 117–123. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Cai, J.; Wu, W.; Liu, R.; Huber, G.W. A distributed activation energy model for the pyrolysis of lignocellulosic biomass. Green Chem. 2013, 15, 1331–1340. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Generalized master plots as a straightforward approach for determining the kinetic model: The case of cellulose pyrolysis. Thermochim. Acta 2013, 552, 54–59. [Google Scholar] [CrossRef]
- Park, Y.-K.; Jung, J.; Ryu, S.; Lee, H.W.; Siddiqui, M.Z.; Jae, J.; Watanabe, A.; Kim, Y.-M. Catalytic co-pyrolysis of yellow poplar wood and polyethylene terephthalate over two stage calcium oxide-ZSM-5. Appl. Energy 2019, 250, 1706–1718. [Google Scholar] [CrossRef]
- Senneca, O.; Chirone, R.; Salatino, P.; Nappi, L. Patterns and kinetics of pyrolysis of tobacco under inert and oxidative conditions. J. Anal. Appl. Pyrolysis 2007, 79, 227–233. [Google Scholar] [CrossRef]
- Nicolae, C.; Grigorescu, M.; Gabor, R. An Investigation of Thermal Degradation of Poly (Lactic Acid). Eng. Lett. 2008, 16, 568–571. [Google Scholar]
- Maria da Conceicao, C.L.; de Alencar, A.E.V.; Mazzeto, S.E.; de ASoares, S. The effect of additives on the thermal degradation of cellulose acetate. Polym. Degrad. Stab. 2003, 80, 149–155. [Google Scholar] [CrossRef]
- Rosdi, N.H.; Mohd Kanafi, N.; Abdul Rahman, N. Preparation and thermal properties of cellulose acetate/polystyrene blend nanofibers via electrospinning technique. Pertanika J. Sci. Technol. 2018, 26, 979–990. [Google Scholar]
- Wu, W.; Mei, Y.; Zhang, L.; Liu, R.; Cai, J. Kinetics and reaction chemistry of pyrolysis and combustion of tobacco waste. Fuel 2015, 156, 71–80. [Google Scholar] [CrossRef]
- Cornelissen, T.; Jans, M.; Stals, M.; Kuppens, T.; Thewys, T.; Janssens, G.K.; Pastijn, H.; Yperman, J.; Reggers, G.; Schreurs, S.; et al. Flash co-pyrolysis of biomass: The influence of biopolymers. J. Anal. Appl. Pyrolysis 2009, 85, 87–97. [Google Scholar] [CrossRef][Green Version]
- Wang, G.; Li, A. Thermal Decomposition and Kinetics of Mixtures of Polylactic Acid and Biomass during Copyrolysis. Chin. J. Chem. Eng. 2008, 16, 929–933. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.W.; Lee, S.H.; Kim, S.S.; Park, S.H.; Jeon, J.K.; Kim, S.; Park, Y.K. Pyrolysis properties and kinetics of mandarin peel. Korean J. Chem. Eng. 2011, 28, 2012–2016. [Google Scholar] [CrossRef]
- He, Q.; Ding, L.; Gong, Y.; Li, W.; Wei, J.; Yu, G. Effect of torrefaction on pinewood pyrolysis kinetics and thermal behavior using thermogravimetric analysis. Bioresour. Technol. 2019, 280, 104–111. [Google Scholar] [CrossRef]
- Ru, B.; Wang, S.; Dai, G.; Zhang, L. Effect of Torrefaction on Biomass Physicochemical Characteristics and the Resulting Pyrolysis Behavior. Energy Fuels 2015, 29, 5865–5874. [Google Scholar] [CrossRef]
- Gupta, M.C.; Deshmukh, V.G. Thermal oxidative degradation of poly-lactic acid. Colloid Polym. Sci. 1982, 260, 514–517. [Google Scholar] [CrossRef]
- Huang, Z.; Ye, Q.Q.; Teng, L.J. A comparison study on thermal decomposition behavior of poly(l-lactide) with different kinetic models. J. Therm. Anal. Calorim. 2015, 119, 2015–2027. [Google Scholar] [CrossRef]
- Shamsipur, M.; Pourmortazavi, S.M.; Hajimirsadeghi, S.S.; Atifeh, S.M. Effect of functional group on thermal stability of cellulose derivative energetic polymers. Fuel 2012, 95, 394–399. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, S.; Han, T.U.; Park, Y.K.; Watanabe, C. Pyrolysis reaction characteristics of Korean pine (Pinus Koraiensis) nut shell. J. Anal. Appl. Pyrolysis 2014, 110, 435–441. [Google Scholar] [CrossRef]
- Akalina, M.K.; Karagöz, S. Pyrolysis of tobacco residue. Part 2: Catalytic. BioResources 2011, 6, 1773–1805. [Google Scholar]
- Park, Y.K.; Lee, B.; Watanabe, A.; Lee, H.W.; Lee, J.Y.; Kim, S.; Han, T.U.; Kim, Y.M. Catalytic copyrolysis of cork oak and waste plastic films over HBeta. Catalysts 2018, 8, 318. [Google Scholar] [CrossRef]
- Burton, H.R.; Childs, G. The Thermal Degradation of Tobacco: VI. Influence of Extraction on the Formation of Some Major Gas Phase Constituents. Beiträge Tab. Int./Contrib. Tob. Res. 1975, 8, 174–180. [Google Scholar] [CrossRef][Green Version]
- Yan, B.; Zhang, S.; Chen, W.; Cai, Q. Pyrolysis of tobacco wastes for bio-oil with aroma compounds. J. Anal. Appl. Pyrolysis 2018, 136, 248–254. [Google Scholar] [CrossRef]
- Konsomboon, S.; Commandre, J.M.; Fukuda, S. Torrefaction of Various Biomass Feedstocks and Its Impact on the Reduction of Tar Produced during Pyrolysis. Energy Fuels 2019, 33, 3257–3266. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Parres, F.; López, J.; Jiménez, A. Development of a novel pyrolysis-gas chromatography/mass spectrometry method for the analysis of poly(lactic acid) thermal degradation products. J. Anal. Appl. Pyrolysis 2013, 101, 150–155. [Google Scholar] [CrossRef]
- Miskolczi, N. Co-pyrolysis of petroleum based waste HDPE, poly-lactic-acid biopolymer and organic waste. J. Ind. Eng. Chem. 2013, 19, 1549–1559. [Google Scholar] [CrossRef]
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Conversion of poly(lactic acid) to lactide via microwave assisted pyrolysis. J. Anal. Appl. Pyrolysis 2014, 110, 55–65. [Google Scholar] [CrossRef]
- Domenek, S.; Courgneau, C.; Ducruet, V. Characteristics and Applications of Poly(lactide). In Biopolymers; Kalia, S., Avérous, L., Eds.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Chung, Y.L.; Olsson, J.V.; Li, R.J.; Frank, C.W.; Waymouth, R.M.; Billington, S.L.; Sattely, E.S. A renewable lignin-lactide copolymer and application in biobased composites. ACS Sustain. Chem. Eng. 2013, 1, 1231–1238. [Google Scholar] [CrossRef]
- Riaz, S.; Fatima, N.; Rasheed, A.; Riaz, M.; Anwar, F.; Khatoon, Y. Metabolic engineered biocatalyst: A solution for PLA based problems. Int. J. Biomater. 2018, 2018, 1963024. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, C.; Tan, H.; Zhang, Y. Synergistic effects of wood fiber and polylactic acid during co-pyrolysis using TG-FTIR-MS and Py-GC/MS. Energy Convers. Manag. 2019, 202, 112212. [Google Scholar] [CrossRef]
- Cornelissen, T.; Yperman, J.; Reggers, G.; Schreurs, S.; Carleer, R. Flash co-pyrolysis of biomass with polylactic acid. Part 1: Influence on bio-oil yield and heating value. Fuel 2008, 87, 1031–1041. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).