Grazing Sheep in Organic Vineyards: An On-Farm Study about Risk of Chronic Copper Poisoning
Abstract
:1. Introduction
2. Materials and Methods
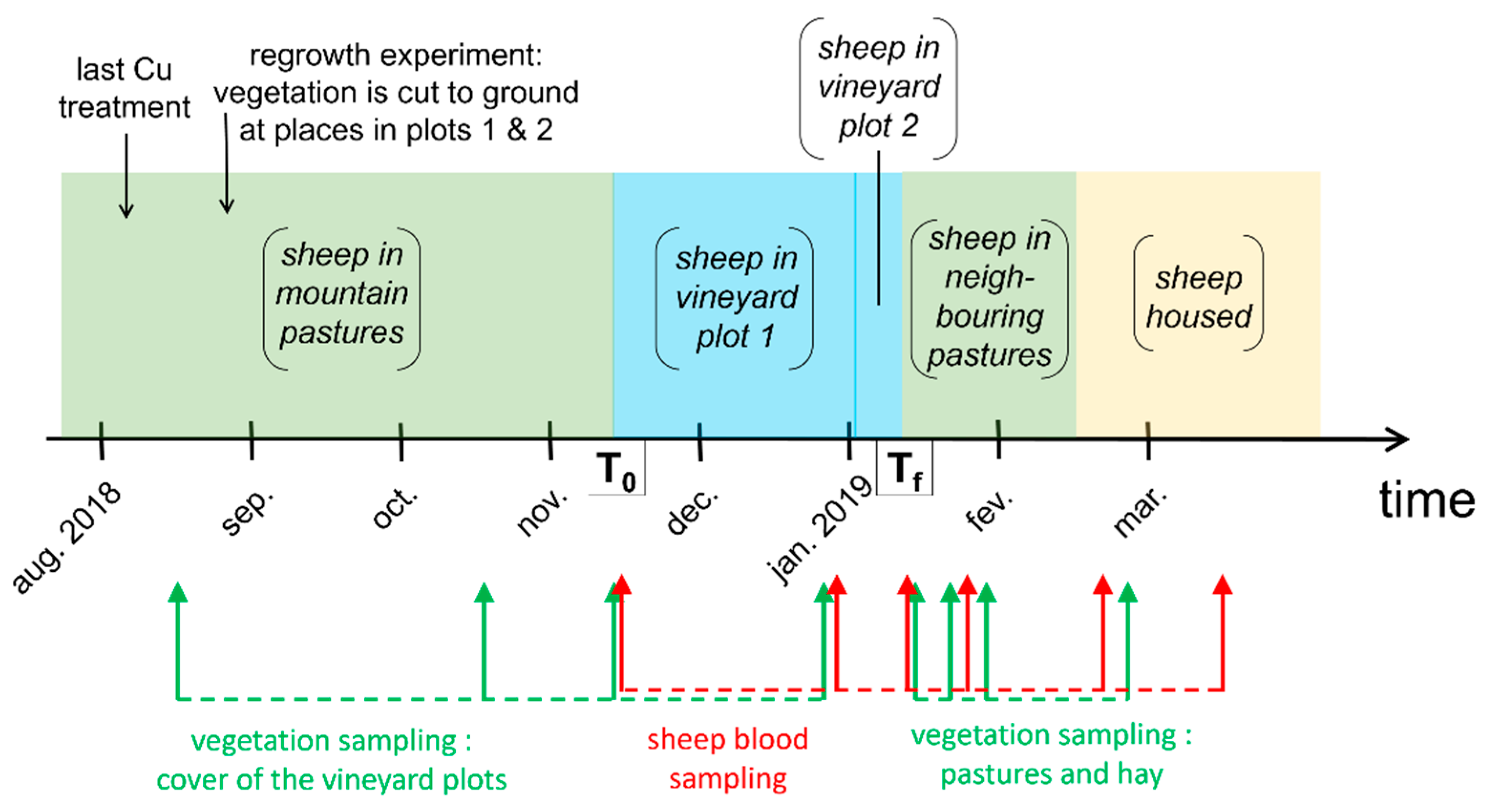
2.1. Experimental Setup
2.2. Sheep and Plot Description
2.3. Sampling and Analysis
2.4. Data Treatment and Calculations
3. Results and Discussion
3.1. Cu in the Vegetation Cover of Vineyard Plots
3.1.1. Dynamics of Cu in the Plants and Soil
3.1.2. Antagonism and Risk of CCP
3.2. Monitoring the CCP Status of Sheep by Blood Analysis
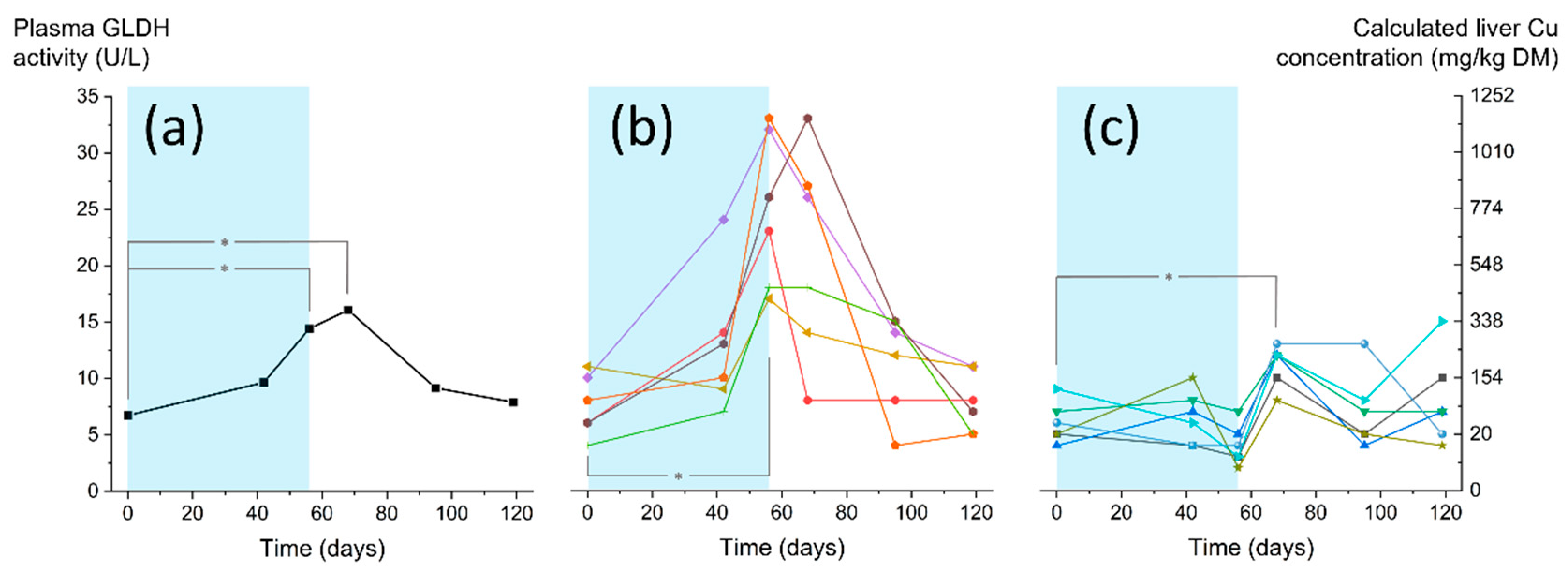
3.2.1. Hepatic Enzymes
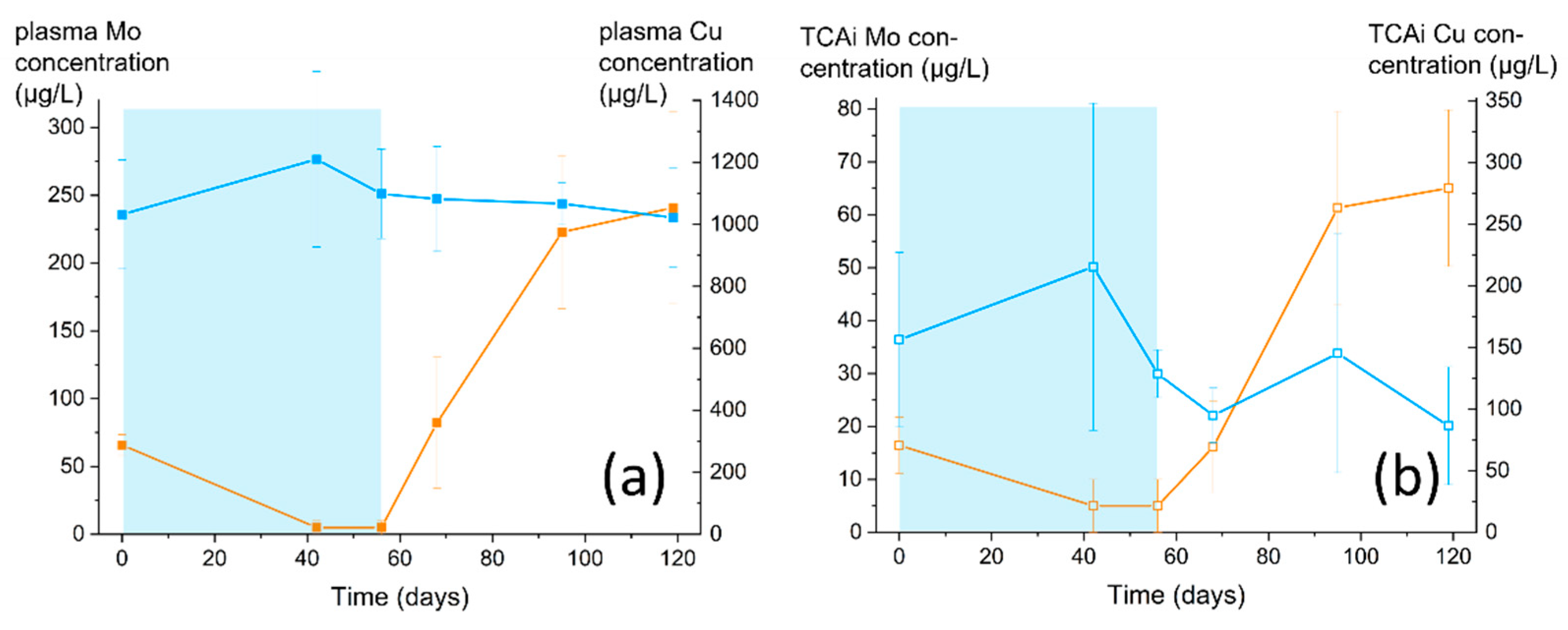
3.2.2. Concentration of Mineral Elements in the Plasma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval
Abbreviations
References
- Smith, J. The History of Temperate Agroforestry. Available online: https://core.ac.uk/download/pdf/10930443.pdf (accessed on 9 November 2021).
- Eichhorn, M.; Paris, P.; Herzog, F.; Incoll, L.; Liagre, F.; Mantzanas, K.; Mayus, M.; Moreno, G.; Papanastasis, V.; Pilbeam, D. Silvoarable systems in Europe–Past, present and future prospects. Agrofor. Syst. 2006, 67, 29–50. [Google Scholar] [CrossRef]
- Niles, M.T.; Garrett, R.D.; Walsh, D. Ecological and economic benefits of integrating sheep into viticulture production. Agron. Sustain. Dev. 2017, 38, 1. [Google Scholar] [CrossRef] [Green Version]
- Brewer, K.M.; Gaudin, A.C. Potential of crop-livestock integration to enhance carbon sequestration and agroecosystem functioning in semi-arid croplands. Soil Biol. Biochem. 2020, 149, 107936. [Google Scholar] [CrossRef]
- Burgess, P.J.; Chinery, F.; Eriksson, G.; Pershagen, E.; Pérez-Casenave, C.; Upson, M.; Garcia de Jalon, S.; Giannitsopoulos, M.; Graves, A. Lessons Learnt—Grazed Orchards in England and Wales. Available online: https://www.agforward.eu/documents/LessonsLearnt/WP3_UK_grazed_orchards_lessons%20learnt.pdf (accessed on 9 November 2021).
- Paut, R.; Dufils, A.; Derbez, F.; Dossin, A.-L.; Penvern, S. Orchard Grazing in France: Multiple Forms of Fruit Tree–Livestock Integration in Line with Farmers’ Objectives and Constraints. Forests 2021, 12, 1339. [Google Scholar] [CrossRef]
- Hammond, J.; Stephenson, A. Copper Poisoning in Sheep Grazing in a Vineyard. (Winter). Available online: http://www.flockandherd.net.au/sheep/ireader/copper-poisoning-vineyard.html (accessed on 9 November 2021).
- Oruc, H.H.; Cengiz, M.; Beskaya, A. Chronic copper toxicosis in sheep following the use of copper sulfate as a fungicide on fruit trees. J. Vet. Diagn. Investig. 2009, 21, 540–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagostin, S.; Schärer, H.-J.; Pertot, I.; Tamm, L. Are there alternatives to copper for controlling grapevine downy mildew in organic viticulture? Crop. Prot. 2011, 30, 776–788. [Google Scholar] [CrossRef]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.-C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K.A.; Muller, T.; Kandeler, E. Remediation of copper in vineyards—A mini review. Environ. Pollut. 2012, 167, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Brun, L.; Maillet, J.; Richarte, J.; Herrmann, P.; Remy, J. Relationships between extractable copper, soil properties and copper uptake by wild plants in vineyard soils. Environ. Pollut. 1998, 102, 151–161. [Google Scholar] [CrossRef]
- National Research Council. Mineral Tolerance of Animals; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Villar, D.; Carson, T.L.; Janke, B.H.; Pallarés, F.J.; Fernández, G.; Kinker, J.A. Retrospective study of chronic copper poissoning in sheep. An. Vet. Murcia 2002, 18, 53–60. [Google Scholar]
- Humann-Ziehank, E.; Coenen, M.; Ganter, M.; Bickhardt, K. Long-term observation of subclinical chronic copper poisoning in two sheep breeds. J. Vet. Med. Ser. Physiol. Pathol. Clin. Med. 2001, 48, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Suttle, N.F. Copper. In Mineral Nutrition of Livestock, 4th ed.; CABI: Wallingford, UK, 2010; pp. 255–305. [Google Scholar] [CrossRef]
- Zervas, G.; Nikolaou, E.; Mantzios, A. Comparative study of chronic copper poisoning in lambs and young goats. Anim. Sci. 1990, 50, 497–506. [Google Scholar] [CrossRef]
- Bull, L.; Dick, A.; Keast, J.; Edgar, G. An experimental investigation of the hepatotoxic and other effects on sheep of consumption of Heliotropium europaeum L.: Heliotrope poisoning of sheep. Aust. J. Agric. Res. 1956, 7, 281–332. [Google Scholar] [CrossRef]
- White, R.; Swick, R.; Cheeke, P. Effects of dietary copper and molybdenum on tansy ragwort (Senecio jacobaea) toxicity in sheep. Am. J. Vet. Res. 1984, 45, 159–161. [Google Scholar] [PubMed]
- Ortolani, E.; Machado, C.H.; Sucupira, M. Assessment of some clinical and laboratory variables for early diagnosis of cumulative copper poisoning in sheep. Vet. Hum. Toxicol. 2003, 45, 289–293. [Google Scholar]
- MacPherson, A.; Hemingway, R. The relative merit of various blood analyses and liver function tests in giving an early diagnosis of chronic copper poisoning in sheep. Br. Vet. J. 1969, 125, 213–221. [Google Scholar] [CrossRef]
- Suttle, N. Control of hepatic copper retention in Texel ram lambs by dietary supplementation with copper antagonists followed by a copper depletion regimen. Anim. Feed. Sci. Technol. 2012, 173, 194–200. [Google Scholar] [CrossRef]
- Kumaratilake, J.; Howell, J.M. Intravenously administered tetra-thiomolybdate and the removal of copper from the liver of copper-loaded sheep. J. Comp. Pathol. 1989, 101, 177–199. [Google Scholar] [CrossRef]
- Hidiroglou, M.; Heaney, D.; Hartin, K. Copper poisoning in a flock of sheep. Copper excretion patterns after treatment with molybdenum and sulfur or penicillamine. Can. Vet. J. 1984, 25, 377. [Google Scholar]
- Kincaid, R. Assessment of trace mineral status of ruminants: A review. J. Anim. Sci. 2000, 77, 1–10. [Google Scholar] [CrossRef]
- Schumann, G.; Bonora, R.; Ceriotti, F.; Férard, G.; Ferrero, C.A.; Franck, P.F.; Gella, F.-J.; Hoelzel, W.; Jørgensen, P.J.; Kanno, T. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin. Chem. Lab. Med. 2002, 40, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koontz, L. Chapter One–TCA Precipitation. In Methods in Enzymology; Lorsch, J., Ed.; Academic Press: San Diego, CA, USA, 2014; Volume 541, pp. 3–10. [Google Scholar] [CrossRef]
- Van Ryssen, J. Estimation of liver mass in sheep. J. S. Afr. Vet. Assoc. 1980, 51, 37–39. [Google Scholar]
- Molot, B.; Gaimon, C. Réduction des Apports Cupriques en Viticulture Biologique: Étude du Lessivage Foliaire Sous Simulateur de Pluie. Available online: https://orgprints.org/6947/ (accessed on 9 November 2021).
- Perez-Rodriguez, P.; Soto-Gomez, D.; De La Calle, I.; Lopez-Periago, J.E.; Paradelo, M. Rainfall-induced removal of copper-based spray residues from vines. Ecotoxicol. Environ. Saf. 2016, 132, 304–310. [Google Scholar] [CrossRef]
- Hunsche, M.; Alexeenko, A.; Damerow, L.; Noga, G. Rain-induced removal of copper from apple leaves: Influence of rain properties and tank-mix adjuvants on deposit characteristics at the micro scale. Crop. Prot. 2011, 30, 495–501. [Google Scholar] [CrossRef]
- Pepper, I.L.; Gerba, C.P.; Gentry, T.J. Environmental Microbiology, 3rd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar] [CrossRef]
- MacPherson, A. Trace-mineral Status of Forages. In Forage Evaluation in Ruminant Nutrition; Givens, D.I., Owen, E., Axford, R.F.E., Omed, H.M., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 345–371. [Google Scholar] [CrossRef]
- Ivan, M.; Proulx, J.G.; Morales, R.; Codagnone, H.C.V.; Dayrell, M.D.S. Copper accumulation in the liver of sheep and cattle fed diets supplemented with copper sulfate or copper chloride. Can. J. Anim. Sci. 1990, 70, 727–730. [Google Scholar] [CrossRef]
- Suttle, N. The role of organic sulphur in the copper-molybdenum-S interrelationship in ruminant nutrition. Br. J. Nutr. 1975, 34, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pott, E.; Henry, P.; Zanetti, M.; Rao, P.; Hinderberger, E., Jr.; Ammerman, C. Effects of high dietary molybdenum concentration and duration of feeding time on molybdenum and copper metabolism in sheep. Anim. Feed. Sci. Technol. 1999, 79, 93–105. [Google Scholar] [CrossRef]
- Ivan, M.; Veira, D. Effects of copper sulfate supplement on growth, tissue concentration, and ruminal solubilities of molybdenum and copper in sheep fed low and high molybdenum diets. J. Dairy Sci. 1985, 68, 891–896. [Google Scholar] [CrossRef]
- Hidiroglou, M.; Morris, G.; Ivan, M. Chemical composition of sheep bones as influenced by molybdenum supplementation. J. Dairy Sci. 1982, 65, 619–624. [Google Scholar] [CrossRef]
- Mason, J.; Lamand, M.; Tressol, J. The influence of dietary sulphur, molybdate and copper on the absorption, excretion and plasma fraction levels of 99Mo in sheep. Ann. De Rech. Vétérinaires 1978, 9, 577–586. [Google Scholar]
- Mason, J.; Lamand, M.; Tressol, J.; Mulryan, G. Studies of the changes in systemic copper metabolism and excretion produced by the intravenous administration of trithiomolybdate in sheep. Br. J. Nutr. 1988, 59, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.; Cardin, C. The competition of molybdate and sulphate ions for a transport system in the ovine small intestine. Res. Vet. Sci. 1977, 22, 313–315. [Google Scholar] [CrossRef]
- Tejada-Jiménez, M.; Galván, A.; Fernández, E. Algae and humans share a molybdate transporter. Proc. Natl. Acad. Sci. USA 2011, 108, 6420–6425. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Cu | +/− | Mo | +/− | S | +/− | Cu/Mo | Absorbable Cu | Calculated Pasture Time | |
|---|---|---|---|---|---|---|---|---|---|
| mg/kg DM | mg/kg DM | mg/kg DM | % | Days | |||||
| Plot 1 (rows) | 98.0 | 17.5 | 0.46 | 0.02 | 1560 | 245 | 214.5 | 6.0 | 16 |
| Plot 1 (inter-rows) | 27.9 | 0.8 | 0.58 | 0.01 | 2600 | 184 | 48.5 | 4.2 | 82 |
| Plot 2 (inter-rows) | 36.8 | 15.9 | 0.46 | 0.08 | 2775 | 318 | 80.8 | 4.6 | 57 |
| Pastures | 5.1 | 0.4 | 2.44 | 1.49 | 2430 | 834 | 2.1 | 1.4 | 1335 |
| Underwood pastures | 4.5 | 1.3 | 0.82 | 0.38 | 1130 | 77 | 5.5 | 5.0 | 430 |
| Hay | 1.0 | 0.3 | 5.91 | 2.38 | 1123 | 375 | 0.2 | 7.4 | 1259 |
| pH | OM | CEC | Cu | +/− | Cu EDTA | +/− | |
|---|---|---|---|---|---|---|---|
| g/kg | méq/kg | mg/kg | mg/kg | ||||
| Plot 1 | 7.94 | 29.1 | 161 | 32.0 | 5.8 | 16.6 | 4.1 |
| Plot 2 | 7.83 | 31.4 | 117 | 13.1 | 6.1 | 6.4 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trouillard, M.; Lèbre, A.; Heckendorn, F. Grazing Sheep in Organic Vineyards: An On-Farm Study about Risk of Chronic Copper Poisoning. Sustainability 2021, 13, 12860. https://doi.org/10.3390/su132212860
Trouillard M, Lèbre A, Heckendorn F. Grazing Sheep in Organic Vineyards: An On-Farm Study about Risk of Chronic Copper Poisoning. Sustainability. 2021; 13(22):12860. https://doi.org/10.3390/su132212860
Chicago/Turabian StyleTrouillard, Martin, Amélie Lèbre, and Felix Heckendorn. 2021. "Grazing Sheep in Organic Vineyards: An On-Farm Study about Risk of Chronic Copper Poisoning" Sustainability 13, no. 22: 12860. https://doi.org/10.3390/su132212860
APA StyleTrouillard, M., Lèbre, A., & Heckendorn, F. (2021). Grazing Sheep in Organic Vineyards: An On-Farm Study about Risk of Chronic Copper Poisoning. Sustainability, 13(22), 12860. https://doi.org/10.3390/su132212860







