Harnessing the Wild Relatives and Landraces for Fe and Zn Biofortification in Wheat through Genetic Interventions—A Review
Abstract
:1. Introduction
2. Green Revolution and Its Effect on Quality Traits
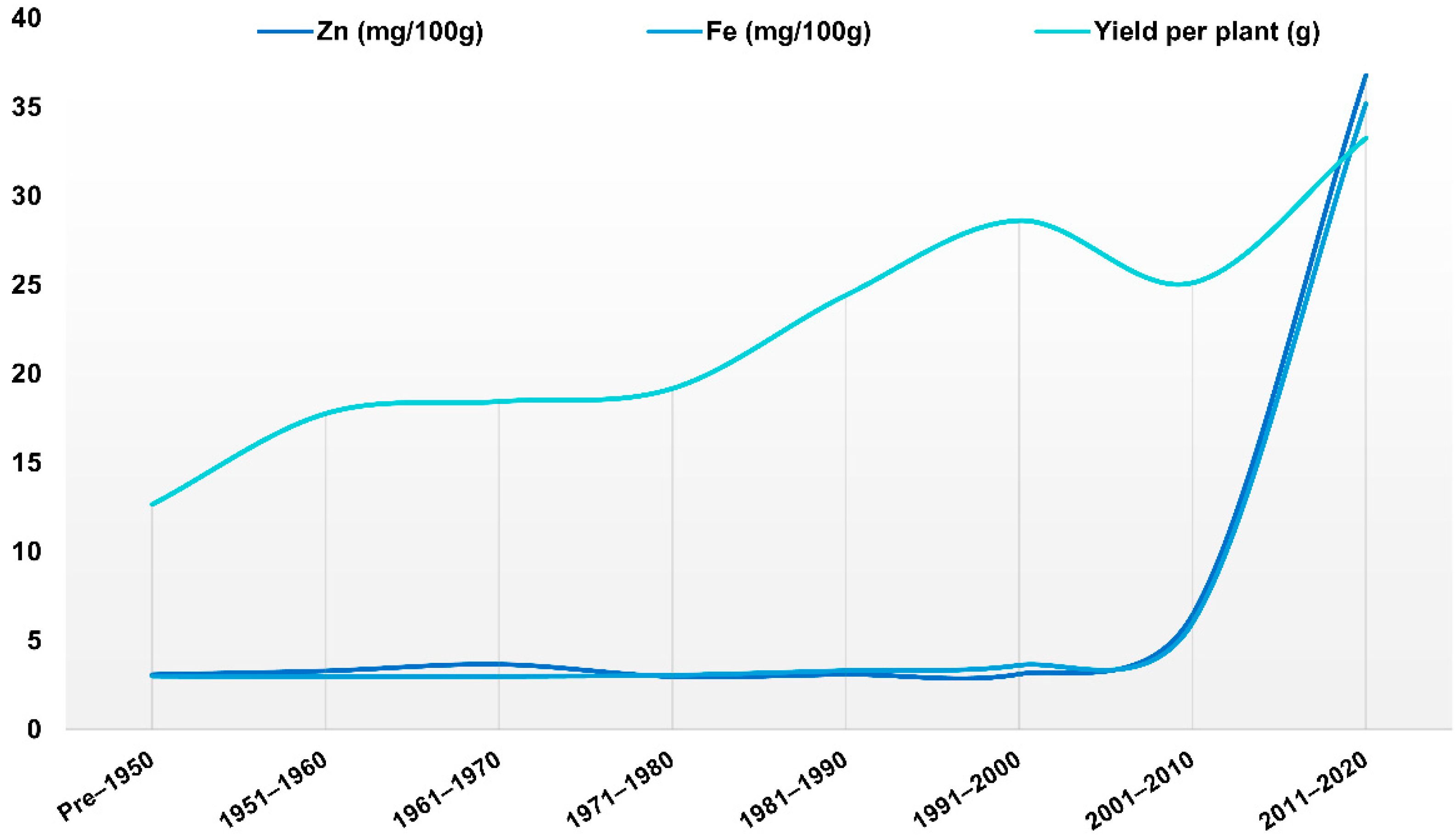
Nutritional Composition Status and Fe and Zn in Wheat
3. Genetic Variability, Heritability and Gene Action for Fe and Zn
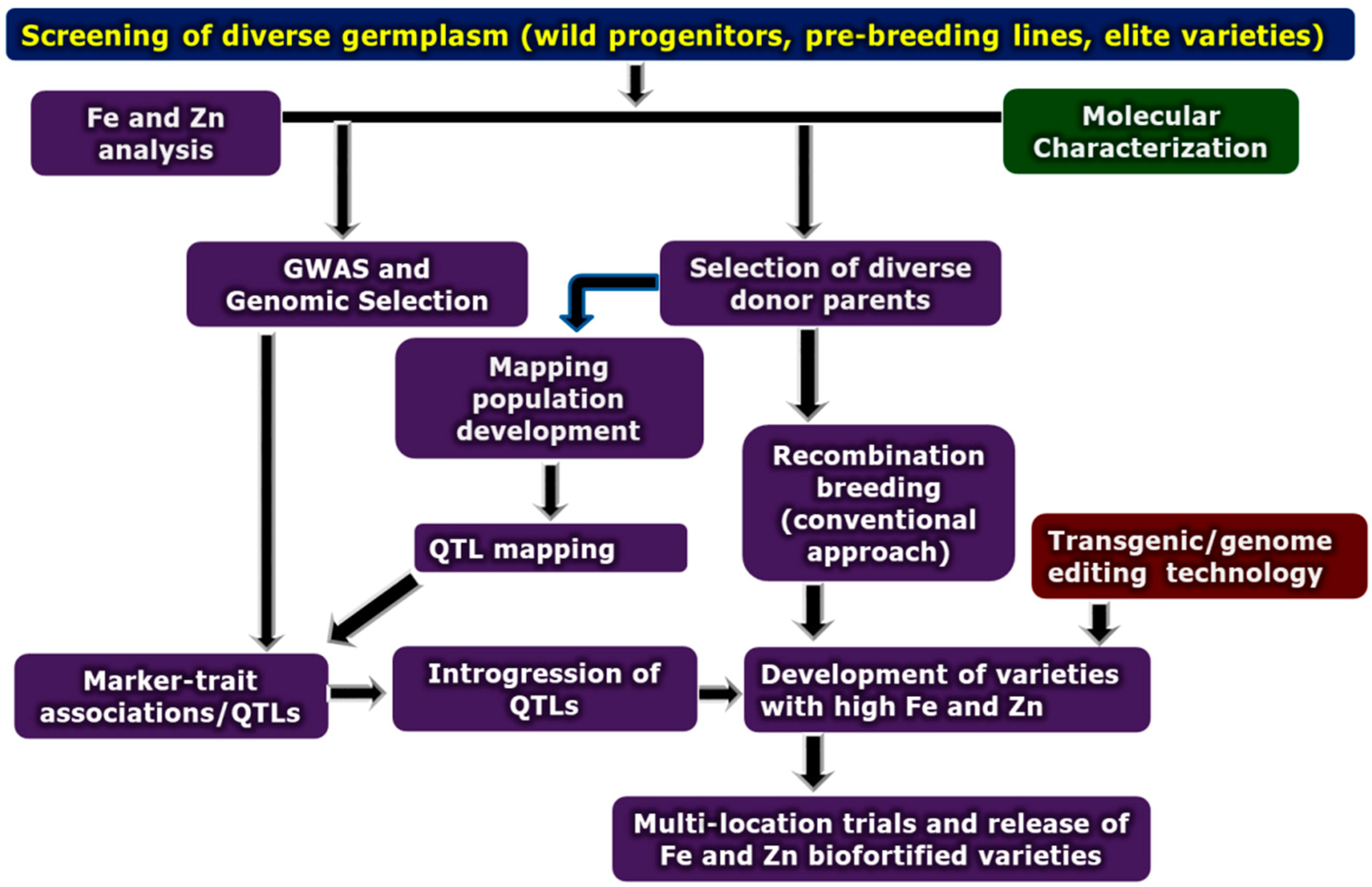
4. Genetic Biofortification via Conventional Breeding
5. Marker-Assisted Selection for Fe and Zn Biofortification
6. Promoters and Inhibitors in Fe and Zn Biofortification and Transgenic Approaches
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDRC Facts and Figures on Food and Biodiversity. Canada: IDRC Communications, International Development Research Centre. 2010. Available online: https://www.idrc.ca/en/research-in-action/facts-figures-food-and-biodiversity (accessed on 12 January 2021).
- Food and Agriculture Organization (FAO). FAOSTAT Statistical Database of the United Nation Food and Agriculture Organization; (FAO) Statistical Division: Rome, Italy, 2019. [Google Scholar]
- Wang, S.; Yin, L.; Tanaka, H.; Tanaka, K.; Tsujimoto, H. Wheat-Aegilops chromosome addition lines showing high iron and zinc contents in grains. Breed. Sci. 2011, 61, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Krishnaswamy, K. Diet and Nutrition in the Prevention of Non-Communicable Diseases. Proc. Indian Natl. Sci. Acad. 2016, 82, 1477–1494. [Google Scholar] [CrossRef]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. Review: Biofortification of Durum Wheat with Zinc and Iron. Cereal Chem. J. 2010, 87, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Black, R.E. Zinc deficiency, infectious disease and mortality in the developing world. J. Nutr. 2003, 133, 1485S–1489S. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Burgi, H.; Hurrell, R.F. Iron deficiency predicts poor maternal thyroid status during pregnancy. J. Clin. Endocrinol. Metab. 2007, 92, 3436–3440. [Google Scholar] [CrossRef] [Green Version]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Kundu, S.; Shoran, J.; Mishra, B.; Gupta, R.K. Indian Wheat Varieties at a Glance; Research Bulletin; Directorate of Wheat Research: Karnal, India, 2006. [Google Scholar]
- Sharma, V. Delineating Marker Trait Associations for Iron (Fe) and Zinc (Zn) Content in Seeds of Bread Wheat (Triticum aestivum L.). Master’s Thesis, Sher-e-Kashmir University of Agricultural Sciences & Technology, Jammu, India, 2016. [Google Scholar]
- Ganeshamurthy, A.N.; Kalaivanan, D.; Manjunath, B.L. Nutrients Removed from the Soil Decide the Nutritional Security of a Nation: The Case of Iron and Zinc in India. Curr. Sci. 2017, 113, 1167. Available online: https://www.jstor.org/stable/26494181 (accessed on 10 March 2020). [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, S.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Myers, S.S.; Wessells, K.R.; Kloog, I.; Zanobetti, A.; Schwartz, J. Effect of increased concentrations of atmospheric carbon dioxide on the global threat of zinc deficiency: A modelling study. Lancet Glob. Health 2015, 3, e639–e645. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef] [Green Version]
- DeValença, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Secur. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Gómez-Galera, S.; Rojas, E.; Sudhaka, D.; Zhu, C.; Pelacho, A.M.; Capell, T.; Christou, P. Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Res. 2010, 19, 165–180. [Google Scholar] [CrossRef]
- Muthusamy, V.; Hossain, F.; Thirunavukkarasu, N.; Choudhary, M.; Saha, S.; Bhat, J.S.; Gupta, H.S. Development of β-Carotene Rich Maize Hybrids through Marker-Assisted Introgression of β-carotene hydroxylase Allele. PLoS ONE 2014, 9, e113583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.R.L.E.; Ravichandran, K.; Antony, U. The impact of the Green Revolution on indigenous crops of India. J. Ethn. Food 2019, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; An, D.; Li, H.; Xu, H. Review: Breeding wheat for enhanced micronutrients. Can. J. Plant Sci. 2019, 91, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Khokhar, J.S.; King, J.; King, I.P.; Young, S.D.; Foulkes, M.J.; DeSilva, J.; Weerasinghe, M.; Mossa, A.; Griffiths, S.; Riche, A.B.; et al. Novel sources of variation in grain Zinc (Zn) concentration in bread wheat germplasm derived from Watkins landraces. PLoS ONE 2020, 15, e0229107. [Google Scholar] [CrossRef] [Green Version]
- Velu, G.; Singh, R.P. Genomic Approaches for Biofortification of Grain Zinc and Iron in Wheat. In Quality Breeding in Field Crops; Qureshi, A., Dar, Z., Wani, S., Eds.; Springer: Cham, Switzerland, 2019; pp. 193–198. [Google Scholar] [CrossRef]
- Weiss, E.; Zohary, D. The Neolithic Southwest Asian Founder Crops. Curr. Anthrop. 2011, 52, S237–S254. [Google Scholar] [CrossRef]
- Massawe, F.; Mayes, S.; Cheng, A. Crop Diversity: An Unexploited Treasure Trove for Food Security. Trends Plant Sci. 2016, 21, 365–368. [Google Scholar] [CrossRef]
- Singh, U.; Praharaj, C.S.; Singh, S.S.; Bohra, A. Biofortification: Introduction, approaches, limitations, and challenges. In Biofortification of Food Crops; Springer: New Delhi, India, 2016; pp. 3–18. [Google Scholar]
- Gomez, M.I.; Barrett, C.B.; Raney, T.; Pinstrup-Andersen, P.; Meerman, J.; Croppenstedt, A.; Carisma, B.; Thompson, B. Post-green revolution food systems and the triple burden of malnutrition. Food Policy 2013, 42, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Marambe, B.; Jayawardena, S.S.B.D.G.; Weerakoon, W.M.W.; Wijewardena, H. Input Intensification in Food Crops Production and Food Security. In Agricultural Research for Sustainable Food Systems in Sri Lanka; Marambe, B., Jeevika, W., Warshi, S.D., Eds.; Springer: Singapore, 2020; pp. 215–248. [Google Scholar] [CrossRef]
- Swaminathan, M.S.; Bhavani, R.V. Food production & availability-Essential prerequisites for sustainable food security. Ind. J. Med. Res. 2013, 138, 383–391. [Google Scholar]
- Pretty, J.; Bharucha, Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014, 114, 1571–1596. [Google Scholar] [CrossRef] [PubMed]
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Müller, J.V.; Toll, J. Adapting Agriculture to climate change: A global initiative to collect, conserve, and use crop wild relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Velu, G.; Ortiz-monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R.P. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Zhu, J.K. Developing naturally stress-resistant crops for a sustainable agriculture. Nat. Plants 2018, 4, 989–996. [Google Scholar] [CrossRef]
- Tadesse, W.; Sanchez-Garcia, M.; Thabet, A.S.; Tawkaz, S.; ElHanafi, S.; El-Baouchi, P.S.A.; Eddakir, K.; El-Shama, K.; Assefa, S.; Baum, M. Wheat Breeding Handbook at ICARDA. Beirut, Lebanon. 2019, pp. 91–92. Available online: https://hdl.handle.net/20.500.11766/10723 (accessed on 10 March 2020).
- Callejo, M.J.; Vargas-Kostiuk, M.E.; Ribeiro, M.; Rodríguez-Quijano, M. Triticum aestivum ssp. vulgare and ssp. spelta cultivars: 2. Bread-making optimisation. Eur. Food Res. Technol. 2019, 245, 1399–1408. [Google Scholar] [CrossRef]
- Cornell, H.J. The chemistry and biochemistry of wheat. In Breadmaking; Woodhead Publishing Ltd: Cambridge, UK, 2012; pp. 35–76. [Google Scholar]
- Borrill, P.; Connorton, J.M.; Balk, J.; Miller, A.J.; Sanders, D.; Uauy, C. Biofortification of wheat grain with iron and zinc: Integrating novel genomic resources and knowledge from model crops. Front. Plant Sci. 2014, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Peleg, Z.; Cakmak, I.; Ozturk, L.; Yazici, A.; Jun, Y.; Budak, H.; Saranga, Y. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat ×wild emmer wheat RIL population. Theor. Appl. Genet. 2009, 119, 353–369. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.A.T.; Kyriacou, B.; Callahan, D.L.; Carruthers, L.; Stangoulis, J.; Lombi, E.; Tester, M. Constitutive Overexpression of the OsNAS Gene Family Reveals Single-Gene Strategies for Effective Iron- and Zinc-Biofortification of Rice Endosperm. PLoS ONE 2011, 6, e24476. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.A.; Thapa, S. Biofortification of Food Crops: An Approach towards Improving Nutritional Security in South Asia. Int. J. Adv. Agric. Sci. Technol. 2019, 6, 23–33. [Google Scholar]
- Velu, G.; Tutus, Y.; Gomez-Becerra, H.F.; Hao, G. QTL mapping for grain zinc and iron concentrations and zinc efficiency in a tetraploid and hexaploid wheat mapping populations. Plant Soil 2017, 411, 81–99. [Google Scholar] [CrossRef]
- Amiri, R.; Bahraminejad, S.; Cheghamirza, K.; Arzani, A. Genetic analysis of iron and zinc concentrations in bread wheat grains. J. Cereal Sci. 2020, 95, 103077. [Google Scholar] [CrossRef]
- Oury, F.X.; Leenhardt, F.; Rémésy, C.; Chanliaud, E.; Duperrier, B.; Balfourier, F.; Charmet, G. Genetic variability and stability of grain magnesium, zinc and iron concentrations in bread wheat. Eur. J. Agron. 2006, 25, 177–185. [Google Scholar] [CrossRef]
- Peleg, Z.; Saranga, Y.; Yazici, A.; Fahima, T.; Ozturk, L.; Cakmak, I. Grain zinc, iron and protein concentrations and zinc-efficiency in wild emmer wheat under contrasting irrigation regimes. Plant Soil 2008, 306, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Khokhar, J.S.; Sareen, S.; Tyagi, B.S.; Singh, G.; Wilson, L.; King, I.P.; Young, S.D.; Broadley, M.R. Variation in grain Zn concentration, and the grain ionome, in field-grown Indian wheat. PLoS ONE 2018, 13, e0192026. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Torun, A.; Millet, E.; Feldman, M.; Fahima, T.; Korol, A.; Ozkan, H. Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci. Plant Nutr. 2004, 50, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Biofortification and bioavailability of Zn, Fe and Se in wheat: Present status and future prospects. Theor. Appl. Genet. 2020, 1–35. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hakki, E.E.; Thomas, G.; Hamurcu, M.; Gezgin, S.; Gizlenci, O.; Akkaya, M.S. Assessment of genetic variability for grain nutrients from diverse regions: Potential for wheat improvement. SpringerPlus 2016, 5, 1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velu, G.; Singh, R.P.; Crespo-Herrera, L.; Juliana, P.; Dreisigacker, S.; Valluru, R.; Stangoulis, J.; Sohu, V.S.; Mavi, G.S.; Mishra, V.K.; et al. Genetic dissection of grain zinc concentration in spring wheat for mainstreaming biofortification in CIMMYT wheat breeding. Sci. Rep. 2012, 8, 13526. [Google Scholar] [CrossRef] [PubMed]
- Morgounov, A.; Gómez-Becerra, H.F.; Abugalieva, A.; Dzhunusova, M.; Yessimbekova, M.; Muminjanov, H.; Zelenskiy, Y.; Ozturk, L.; Cakmak, I. Iron and zinc grain density in common wheat grown in Central Asia. Euphytica 2007, 155, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.; Lu, X.; Chen, Z. Zinc Fertilization Methods on Zinc Absorption and Translocation in Wheat. J. Agric. Sci. 2011, 3, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Saini, D.K.; Devi, P.; Kaushik, P. Advances in Genomic Interventions for Wheat Biofortification: A Review. Agronomy 2020, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, Z.H.; Li, F.; Li, K.; Yang, N.; Yang, Y.; Huang, D.; Liang, D.; Zhao, H.; Mao, H.; et al. Grain iron and zinc concentrations of wheat and their relationships to yield in major wheat production areas in China. Field Crop. Res. 2014, 156, 151–160. [Google Scholar] [CrossRef]
- Ozturk, L.; Yazici, M.A.; Yucel, C.; Torun, A.; Cekic, C.; Bagci, A.; Cakmak, I. Concentration and localization of zinc during seed development and germination in wheat. Physiol. Plant. 2006, 128, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Narwal, R.P.; Malik, R.S.; Dahiya, R.R. Addressing variations in status of a few nutritionally important micronutrients in wheat crop. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 141–143. Available online: https://www.iuss.org/19th%20WCSS/Symposium/pdf/1078.pdf (accessed on 10 March 2020).
- Srinivasa, J.; Balasubramaniam, A.; Mishra, V.K.; Singh, G.P.; Velu, G.; Babu, R.; Vasistha, N.K.; Joshi, A.K. Zinc and iron concentration QTL mapped in a Triticum spelta × T. aestivum cross. Theor. Appl. Genet. 2014, 127, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Alomari, D.Z.; Eggert, K.; VonWirén, N.; Polley, A.; Plieske, J.; Ganal, M.W.; Liu, F.; Pillen, K.; Röder, M.S. Whole-genome association mapping and genomic prediction for iron concentration in wheat grains. Int. J. Mol. Sci. 2019, 20, 76. [Google Scholar] [CrossRef] [Green Version]
- Manickavelu, A.; Hattori, T.; Yamaoka, S.; Yoshimura, K.; Kondou, Y.; Onogi, A.; Matsui, M.; Iwata, H.; Ban, T. Genetic nature of elemental contents in wheat grains and its genomic prediction: Toward the effective use of wheat landraces from Afghanistan. PLoS ONE 2017, 12, e0169416. [Google Scholar] [CrossRef]
- Younas, A.; Sadaqat, H.A.; Kashif, M.; Ahmed, N.; Farooq, M. Combining ability and heterosis for grain iron biofortification in bread wheat. J. Sci. Food Agric. 2020, 100, 1570–1576. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Rawat, N.; Neelam, K.; Kumar, S.; Randhawa, G.S.; Dhaliwal, H.S. Substitutions of 2S and 7U chromosomes of Aegilopskotschyi in wheat enhance grain iron and zinc concentration. Theor. Appl. Genet. 2010, 121, 259–269. [Google Scholar] [CrossRef]
- Cakmak, I.; Derici, R.; Torun, B. Role of rye chromosomes in improvement of zinc efficiency in wheat and triticale. Plant Soil 1997, 196, 249–253. [Google Scholar] [CrossRef]
- Kumar, A.; Kapoor, P.; Chunduri, V.; Sharma, S.; Garg, M. Potential of Aegilops sp. for improvement of grain processing and nutritional quality in wheat (Triticum aestivum). Front. Plant Sci. 2019, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Calderini, D.F.; Ortiz-Monasterio, I. Are synthetic hexaploids a means of increasing grain element concentrations in wheat? Euphytica 2003, 134, 169–178. [Google Scholar] [CrossRef]
- Singh, R.; Govindan, V.; Andersson, M.S. Zinc-Biofortified Wheat: Harnessing Genetic Diversity for Improved Nutritional Quality; Science Brief: Biofortification No. 1; HarvestPlus; Global Crop Diversity Trust: Bonn, Germany, 2017. [Google Scholar]
- Gupta, A.; Singh, C.; Kumar, V.; Tyagi, B.; Tiwari, V.; Chatrath, R.; Singh, G.P. Wheat Varieties Notified in India Since 1965; ICAR—Indian Institute of Wheat and Barley Research: Karnal, India, 2018. [Google Scholar]
- Velu, G.; Crespo Herrera, L.; Guzman, C.; Huerta, J.; Payne, T.; Singh, R.P. Assessing genetic diversity to breed competitive biofortified wheat with enhanced grain Zn and Fe concentrations. Front. Plant Sci. 2019, 9, 1971. [Google Scholar] [CrossRef]
- Trethowan, R.M.; Turner, M.A.; Chattha, T.M. Breeding Strategies to Adapt Crops to a Changing Climate. Adv. Glob. Chang. Res. 2010, 37, 155–174. [Google Scholar] [CrossRef]
- Badakhshan, H.; Moradi, N.; Mohammadzadeh, H.; Zakeri, M.R. Genetic variability analysis of grains Fe, Zn and beta-carotene concentration of prevalent wheat varieties in Iran. Int. J. Agric. Crop Sci. 2013, 6, 57. [Google Scholar]
- Virk, K. Zinc Rice. In Proceedings of the 2nd Global Conference on Biofortification: Getting Nutritious Foods to People, CIAT-HarvestPlus, Kigali, Rwanda, 31 March–2April 2014. [Google Scholar]
- Tiwari, V.K.; Rawat, N.; Chhuneja, P.; Neelam, K.; Aggarwal, R.; Randhawa, G.S.; Singh, K. Mapping of quantitative trait Loci for grain iron and zinc concentration in diploid A genome wheat. J. Hered. 2009, 100, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Herrera, L.A.; Velu, G.; Singh, R.P. Quantitative trait loci mapping reveals pleiotropic effect for grain iron and zinc concentrations in wheat. Ann. Appl. Biol. 2016, 169, 27–35. [Google Scholar] [CrossRef]
- Crespo-Herrera, L.; Govindan, V.; Stangoulis, J.; Hao, Y.; Singh, R.P. QTL Mapping of Grain Zn and Fe Concentrations in Two Hexaploid Wheat RIL Populations with Ample Transgressive Segregation. Front. Plant Sci. 2017, 8, 1800. [Google Scholar] [CrossRef] [Green Version]
- Shiri, M.; Mehraban, A.; Tobe, A. Effect of micronutrient foliar application on morphology, yield and iron and zinc grain concentration of durum wheat genotypes. J. Agric. Sci. 2019, 64, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Chandel, G.; Samuel, P.; Dubey, M.; Meena, R. In silico expression analysis of QTL specific candidate genes for grain micronutrient (Fe/Zn) content using ESTs and MPSS signature analysis in rice (Oryza sativa L.). J. Plant Genet. Transgenics 2011, 2, 11–22. [Google Scholar]
- Distelfeld, A.; Cakmak, I.; Peleg, Z.; Ozturk, L.; Yazici, A.M.; Budak, H.; Fahima, T. Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiol. Plant. 2007, 129, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC Gene Regulating Senescence Improves Grain Protein, Zinc, and Iron Content in Wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wu, B.; Singh, R.P.; Velu, G. QTL mapping for micronutrients concentration and yield component traits in a hexaploid wheat mapping population. J. Cereal Sci. 2019, 88, 57–64. [Google Scholar] [CrossRef]
- Krishnappa, G.; Rathan, N.D.; Sehgal, D.; Ahlawat, A.K.; Singh, S.K.; Singh, S.K.; Shukla, R.B.; Jaiswal, J.P.; Solanki, I.S.; Singh, G.P.; et al. Identification of Novel Genomic Regions for Biofortification Traits Using an SNP Marker-Enriched Linkage Map in Wheat (Triticum aestivum L.). Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Pu, Z.E.; Yu, M.; He, Q.Y.; Chen, G.Y.; Wang, J.R.; Liu, Y.X.; Zheng, Y.L. Quantitative trait loci associated with micronutrient concentrations in two recombinantinbred wheat lines. J. Integr. Agric. 2014, 13, 2322–2329. [Google Scholar] [CrossRef]
- Hao, Y.; Velu, G.; Peña, R.J.; Singh, S.; Singh, R.P. Genetic loci associated withhigh grain zinc concentration and pleiotropic effect on kernel weight in wheat (Triticum aestivum L.). Mol. Breed. 2014, 34, 1893–1902. [Google Scholar] [CrossRef]
- Rathan, N.D.; Sehgal, D.; Thiyagarajan, K.; Singh, R.; Singh, A.M.; Govindan, V. Identification of Genetic Loci and Candidate Genes Related to Grain Zinc and Iron Concentration Using a Zinc-Enriched Wheat “Zinc-Shaktia”. Front. Genet. 2021, 12, 756. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Rawat, N.; Neelam, K.; Randhawa, G.S.; Singh, K.; Chhuneja, P.; Dhaliwal, H.S. Development of Triticum turgidum subsp. durum—Aegilops longissimi amphiploids with high iron and zinc content through unreduced gamete formation in F1hybrids. Genome 2008, 51, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; An, D.; Liu, D.; Zhang, A.; Xu, H.; Li, B. Molecular mapping of QTLs for grain zinc, iron and protein concentration of wheat across two environments. Food Crop. Res. 2012, 138, 57–62. [Google Scholar] [CrossRef]
- Goffinet, B.; Gerber, S. Quantitative trait loci: A meta-analysis. Genetics 2000, 155, 463–473. [Google Scholar] [CrossRef]
- Shariatipour, N.; Heidari, B.; Richards, C.M. Meta-analysis of QTLome for grain zinc and iron contents in wheat (Triticum aestivum L.). Euphytica 2021, 217, 1–4. [Google Scholar] [CrossRef]
- Lau, W.C.P.; Latif, M.A. Current breeding approaches for developing rice with improved grain and nutritional qualities. In Quality Breeding in Field Crops; Iqbal Qureshi, A.M., Dar, Z.A., Wani, S.H., Eds.; Springer: Cham, Switzerland, 2019; pp. 199–216. [Google Scholar]
- Velu, G.; Crossa, J.; Singh, R.P.; Hao, Y.; Dreisigacker, S.; Perez-Rodriguez, P.; Joshi, A.K.; Chatrath, R.; Gupta, V.; Balasubramaniam, A.; et al. Genomic prediction for grain zinc and iron concentrations in spring wheat. Theor. Appl. Genet. 2016, 129, 1595–1605. [Google Scholar] [CrossRef]
- Cu, S.T.; Guild, G.; Nicolson, A.; Velu, G.; Singh, R.; Stangoulis, J. Genetic dissection of zinc, iron, copper, manganese and phosphorus in wheat (Triticum aestivum L.) grain and rachis at two developmental stages. Plant Sci. J. 2020, 291, 110–338. [Google Scholar] [CrossRef]
- Huynh, B.L.; Palmer, L.; Mather, D.; Wallwork, H.; Graham, R.D.; Welch, R.; Stangoulis, J.C.R. Genotypic variation in wheat grain fructan content revealed by a simplified HPLC method. J. Cereal Sci. 2008, 48, 369–378. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Greiner, R.; Konietzny, U.; Jany, K.D. Phytate-an undesirable constituent of plant-based foods? J. Ernährungsmedizin 2006, 8, 18–28. Available online: https://www.kup.at/kup/pdf/6239.pdf (accessed on 10 March 2020).
- Ram, S.; Verma, A.; Sharma, S. Large variability exits in phytase levels among Indian wheat varieties and synthetic hexaploids. J. Cereal Sci. 2010, 52, 486–490. [Google Scholar] [CrossRef]
- Lyons, G.; Ortiz-Monasterio, I.; Stangoulis, J.; Graham, R. Selenium concentration in wheat grain: Is there sufficient genotypic variation to use in breeding? Plant Soil 2005, 269, 369–380. [Google Scholar] [CrossRef]
- Velu, G.; Bhattacharjee, R.; Rai, K.N.; Sahrawat, K.L.; Longvah, T. A simple and rapid screening method for grain zinc content in pearl millet. J. SAT Agric. Res. 2008, 6, 1–4. [Google Scholar]
- Singh, S.P.; Keller, B.; Gruissem, W.; Bhullar, N.K. Rice Nicotianaminesynthase 2 expression improves dietary iron and zinc levels in wheat. Theor. Appl. Genet. 2017, 130, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 1–27. [Google Scholar] [CrossRef]
- Carvalho, S.M.P.; Vasconcelos, M.W. Producing more with less: Strategies and novel technologies for plant-based food biofortification. Food Res. Intern. 2013, 54, 961–971. [Google Scholar] [CrossRef]
- Abid, N.; Khatoon, A.; Maqbool, A.; Irfan, M.; Bashir, A.; Asif, I.; Malik, K.A. Transgenic expression of phytase in wheat endosperm increases bioavailability of iron and zinc in grains. Transgenic Res. 2017, 26, 109–122. [Google Scholar] [CrossRef]
- Bhati, K.K.; Alok, A.; Kumar, A.; Kaur, J.; Tiwari, S.; Pandey, A.K. Silencing of ABCC13 transporter in wheat reveals its involvement in grain development, phytic acid accumulation and lateral root formation. J. Exp. Bot. 2016, 67, 4379–4389. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.; Kumar, A.; Bhati, K.K.; Kaur, G.; Shukla, V.; Tiwari, S.; Pandey, A.K. RNAi-mediated downregulation of inositol pentakisphosphate kinase (IPK1) in wheat grains decreases phytic acid levels and increases Fe and Zn accumulation. Front. Plant Sci. 2018, 9, 259. [Google Scholar] [CrossRef]
- Borg, S.; Brinch-Pedersen, H.; Tauris, B.; Madsen, L.H.; Darbani, B.; Noeparvar, S.; Holm, P.B. Wheat ferritins: Improving the iron content of the wheat grain. J. Cereal Sci. 2012, 56, 204–213. [Google Scholar] [CrossRef]
- Connorton, J.M.; Jones, E.R.; Rodríguez-Ramiro, I.; Fairweather-Tait, S.; Uauy, C.; Balk, J. Wheat Vacuolar Iron Transporter TaVIT2 Transports Fe and Mn and Is Effective for Biofortification. Plant Physiol. 2017, 174, 2434–2444. [Google Scholar] [CrossRef] [Green Version]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; MarkCigan, A. Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nat. Commun. 2016, 7, 13274. [Google Scholar] [CrossRef]
- Ali, N.; Paul, S.; Gayen, D.; Sarkar, S.N.; Datta, K.; Datta, S.K. Development of Low Phytate Rice by RNAi Mediated Seed-Specific Silencing of Inositol 1,3,4,5,6-Pentakisphosphate 2-Kinase Gene (IPK1). PLoS ONE 2013, 8, e68161. [Google Scholar] [CrossRef] [Green Version]
- Balmer, Y.; Vensel, W.H.; DuPont, F.M.; Buchanan, W.J. Proteome of amyloplastsisolated from developing wheat endosperm presents evidence of broad metabolic capability. J. Exp. Bot. 2006, 57, 1591–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Kawakami, Y.; Bhullar, N.K. Molecular analysis of iron deficiency response in hexaploid wheat. Front. Sustain. Food Syst. 2019, 3, 67. [Google Scholar] [CrossRef]
- Kaur, G.; Shukla, V.; Kumar, A.; Kaur, M.; Goel, P.; Singh, P.; Shukla, A.; Meena, V.; Kaur, J.; Singh, J.; et al. Integrative analysis of hexaploid wheat roots identifies signature components during iron starvation. J. Exp. Bot. 2019, 70, 6141–6161. [Google Scholar] [CrossRef] [Green Version]
| Cross | QTLs | Chr. | Position (cM) | Flanking Markers | Confidence Interval (cM) | PVE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Iron (Fe) | |||||||
| T. durumSaricanak98 × T. dicoccon MM 5/4 | QGFe.sar_5BMCO | 5B | 97 | wPt-7400–wPt-8449 | 94.1–108.6 | 16.9 | [41] |
| T. durum Saricanak98 × T. dicoccon MM 5/4 | QGFe.sar_5BTKM | 5B | 28 | wPt-81.25–wPt-9504 | 27.5–40.2 | ~15 | |
| T. aestivum Adana 99 × T. sphaerococum 70711 | QGFe.ada-7B | 7B | 35.7 | wPt-5922 | 35.70 | 18.0 | |
| QGFe.ada-2Btsk | 2B | 77.1 | wPt-9812 | 77.10 | 17.0 | ||
| QGFe.ada-2Btkm | 2B | 52.4 | wPt-1394–wPt-7864 | 50.3–53.6 | 17.0 | ||
| T. spelta H+ 26 × T. aestivum cv. HUW 234 | QFe.bhu-3B | 3B | 1022 | 3022954|F|0–1102324|F|0 | 1015.23–1022.28 | 25.9 | [57] |
| T. spelta H+ 26 × T. aestivum cv. HUW 234 | QFe.bhu-1A.3 | 1A | 346 | 1708014|F|0–1000008|F|0 | 345.76–355.02 | 16.5 | |
| T. aestivum SeriM82 × T. dicoccoides/Ae. tauschii SHW CWI76364 | QFe.Across_7DS | 7DS | 5 | TP43715–TP37547 | 2.0–5.4 | ~15.0 | [72] |
| Synthetic hexaploid wheat Louries × T. spelta | QGFe.cimmyt-4A_P2 | 4A | 199 | 3385350–1211533 | 198.5–199.5 | 21.1 | [73] |
| Roelfs F 2007 × Chinese Parental Line | QGFe.co-3B.1 | 3B | 200 | 1089107 1127875|F|0 | 199.5–200.5 | 14.56 | [78] |
| WH 542 × Synthetic derivative (T.dicoccon/Ae. tauschii[409]//BCN) | QGFe.iari-7D.1 | 7D | 11 | Xwmc550–Xgwm350 | 9.5–12.5 | 42.13 | [79] |
| T. aestivum Chuanmai 42 × T. aestivum Chuannong 16 | QTL | 4D | 160 | Xgwm154–Xbarc108 | 160.00 | 19.1 | [80] |
| Zinc (Zn) | |||||||
| T. aestivum Adana 99 × T. sphaerococum 70711 | QGZn.ada-1D | 1D | 80.3 | wPt-6979–wPt-730,718 | 67.8–125.8 | 31.0 | [41] |
| QGZn.ada-6B | 6B | 65.1 | wPt-667,798–wPt-7065 | 62.0–66.1 | 27.0 | ||
| QGZn.ada-7B | 7B | 28.1 | wPt-733,112 | 28.10 | 25.0 | ||
| QGZn.ada-7A | 7A | 76.1 | wPt-2083–wPt-6083 | 74.0–77.3 | 15.0 | ||
| T. spelta H+ 26 × T. aestivum cv. HUW 234 | QZn.bhu-2B | 2B | 966 | 989092|F|0–1101425|F|0 | 948.23–966.68 | 16.5 | [57] |
| T. aestivum SeriM82 × T. dicoccoides/Ae. tauschii SHW CWI76364 | QZn.Y13-14_4BS | 4BS | 23.2 | TP91631–TP81797 | 23.1–23.7 | 19.6 | [72] |
| QZn.Across_4BS | 4BS | 23.2 | TP91631–TP81797 | 23.1–23.7 | 17.3 | ||
| T. spelta × synthetic hexaploid wheat | QGZn.cimmyt-7B_1P2 | 7B | 44 | 1079651–1262676 | 43.5–44.5 | 32.7 | |
| T. spelta × synthesized hexaploid wheat | QGZn.cimmyt-7B_1P1 | 7B | 62 | 3945822 -1132640F0-5CG | 61.5–62.5 | 16.8 | |
| QGZn.cimmyt-1B_P1 | 1B | 84 | 3934172–33934936 | 83.5–84.5 | 15.1 | ||
| Roelfs F 2007 × Chinese Parental Line | QGZn.co-5A | 5A | 54 | 1244217–1272027|F|0 | 53.5–54.5 | 14.22 | [78] |
| WH 542 × Synthetic derivative | QGZn.iari-7D.2 | 7D | 21 | Xgwm350–AX-94958668 | 4.5–41.5 | 13.07 | [79] |
| T. aestivum Chuanmai 42 × T. aestivum Chuannong 16 | QTL | 4D | 194 | Xcfa2149–Xbarc48 | 194.00 | 15.9 | [80] |
| T. aestivum PBW343 × T. aestivum Kenya Swara | QGzncpk.cimmyt-3AL | 3A | 79.1 | wPt-0286 | 74.4–84.0 | 15.0 | [81] |
| Zinc-Shakti × Kachu | QFeC-2A | 2A | 111 | 1074973–2253877 | 104.5–148.5 | 10.6 | [82] |
| Approaches/ Introduced Genes/Promoter | Cultivar/Species | Fold Increase in Fe and Zn Concentration Compared to Non-Transgenic Wheat | Remarks | Ref. |
|---|---|---|---|---|
| Approach 1: Improving Translocation of Fe and Zn from Root to Plant Parts | ||||
| Rice NICOTIANAMINE SYNTHASE 2 (OsNAS2) | T. aestivum L. | Fe 2.1-fold (grains) and 2.5-fold (flour) Zn 3.7-fold (grains) and 4.2-fold (flour) | HarvestPlus recommended target level of 30% dietary estimated average requirement (EAR) for Fe, and 40% of EAR for Zn, with lines containing 93.1 µg/g of Fe and 140.6 µg/g of Zn in the grains. | [65] |
| Bean FERRITIN (PvFERRITIN) | T. aestivum L. | Fe 1.6-fold (grains) and 1.7-fold (flour) Zn 1.7-fold (grains) and 1.5-fold (flour) | ||
| Approach 2: Holistic Approaches by Multiple Gene Family | ||||
| Both PvFERRITIN and OsNAS2 | T. aestivum L. | - | Fe 1.7-fold (whole grains) 1.8-fold (flour) | [65] |
| Approach 3: Improving Sink Size for Fe and Zn | ||||
| GPC-B1 | T. turgidum ssp. durum cultivar Langdon | 2-fold Fe and 1.6-fold Zn | NAC transcription factor (NAM-B1) that increase senescence and nutrient remobilization | [77] |
| Aspergillus japonicus phytase gene (phyA) | T. aestivum L. | Significant increase | 2 to 9-fold higher expression of phyAmRNA and 12–76% reduction in phytic acid in seed | [99] |
| TaFer1, TaFer2 | Wheat cultivar Bob White | 1.5 to 1.9-fold | Placed on chromosome 5 and 4 | [102] |
| Approach 4: Improving Transportation of Fe and Zn from Soil to Different Plant Part | ||||
| TaVIT2 | T. aestivum L. | 2-fold Fe in white flour | Stable in the next generation | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, V.; Choudhary, M.; Kumar, P.; Choudhary, J.R.; Khokhar, J.S.; Kaushik, P.; Goli, S. Harnessing the Wild Relatives and Landraces for Fe and Zn Biofortification in Wheat through Genetic Interventions—A Review. Sustainability 2021, 13, 12975. https://doi.org/10.3390/su132312975
Sharma V, Choudhary M, Kumar P, Choudhary JR, Khokhar JS, Kaushik P, Goli S. Harnessing the Wild Relatives and Landraces for Fe and Zn Biofortification in Wheat through Genetic Interventions—A Review. Sustainability. 2021; 13(23):12975. https://doi.org/10.3390/su132312975
Chicago/Turabian StyleSharma, Vivek, Mukesh Choudhary, Pawan Kumar, Jeet Ram Choudhary, Jaswant S. Khokhar, Prashant Kaushik, and Srinivas Goli. 2021. "Harnessing the Wild Relatives and Landraces for Fe and Zn Biofortification in Wheat through Genetic Interventions—A Review" Sustainability 13, no. 23: 12975. https://doi.org/10.3390/su132312975







