Energy Potential of Oil Palm Empty Fruit Bunch (EFB) Fiber from Subsequent Cultivation of Volvariella volvacea (Bull.) Singer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
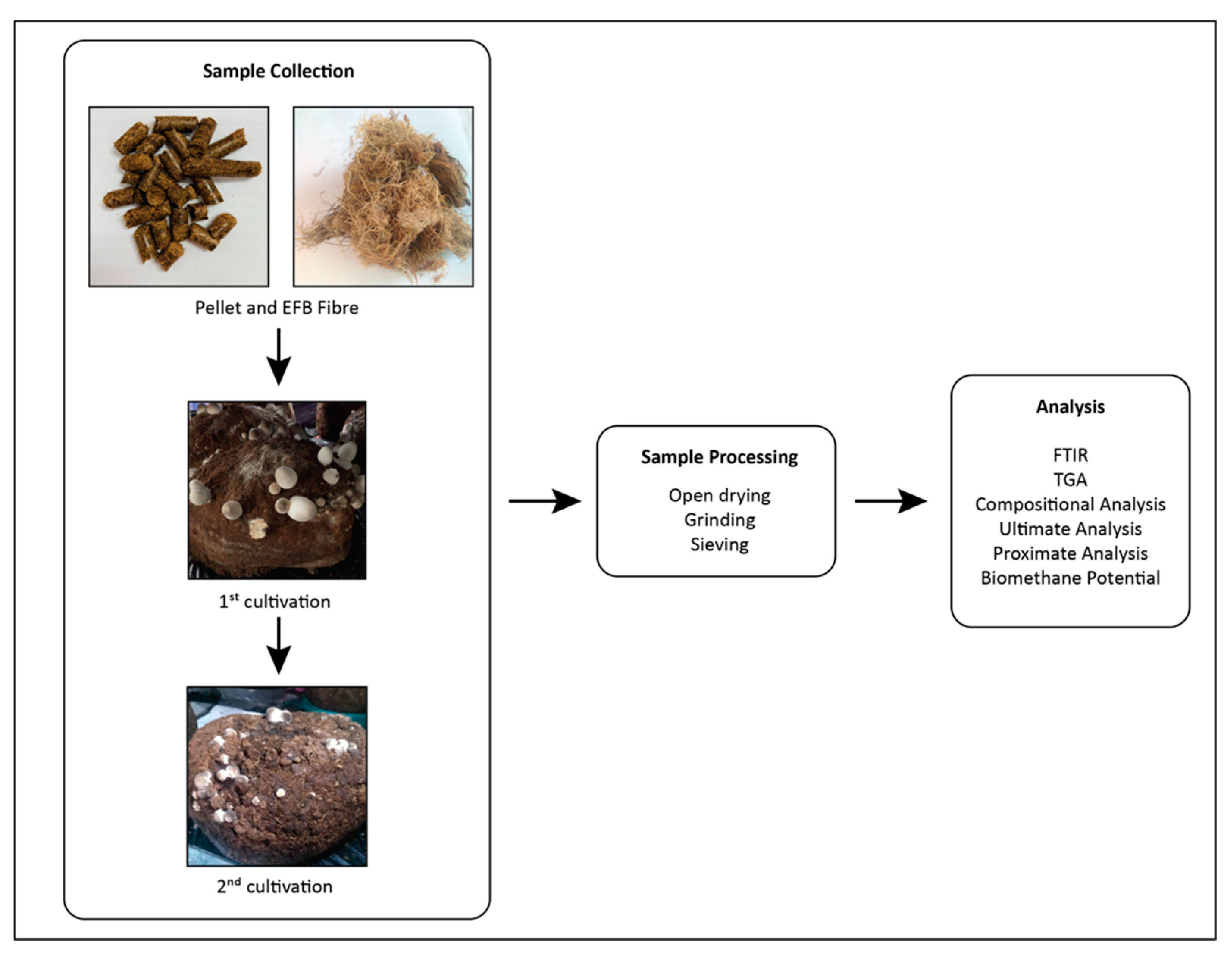
2.2. Biomass Samples
2.3. Volvariella volvacea Cultivation
2.4. Solid Analysis
2.5. Fiber Morphology and Composition Analysis
2.6. Calorific Value
2.7. Ultimate Analysis
2.8. Biomethane Potential Assay
2.9. Theoretical BMP and Biodegradability
2.10. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.11. Thermogravimetry Analysis (TGA)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Yield Performance, Physical Properties, and Compositional Analysis of the Biomass Sample
3.2. Proximate Analysis and Calorific Value
3.3. TGA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Symbols
| Abbreviation | Explanation |
| AD | Anaerobic digestion |
| BD | Biodegradability |
| BMP | Biomethane potential |
| CHNSO | Carbon, hydrogen, nitrogen, sulfur, and oxygen |
| CV | Calorific value |
| DFFH | Day for the first harvest |
| HD | Harvesting day |
| EFB | Empty fruit bunch |
| FTIR | Fourier-transform infrared spectroscopy |
| F | Fiber |
| FB | Fruiting body |
| FC | Fixed carbon |
| FS | Spent mushroom compost of EFB fiber |
| FS2 | Second spent mushroom compost of EFB fiber |
| HHV | High heating value |
| MC | Moisture content |
| SMC | Spent mushroom compost |
| TBMP | Theoretical biomethane potential |
| TGA | Thermogravimetry analysis |
| TG | Thermogravimetry |
| VM | Volatile matters |
References
- Fekete, B.M. Biomass. In Climate Vulnerability; Roger, A., Pieke, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 83–85. [Google Scholar] [CrossRef]
- Tustin, J.; Brown, A. Potential Contribution of Bioenergy to the World’s Future Energy Demand; IEA Bioenergy Secretariat: Rotarua, New Zealand, 2007; p. 2. [Google Scholar]
- Goh, K.J.; Wong, C.K.; Ng, P.H.C. Oil Palm. Encycl. Appl. Plant Sci. 2017, 382–390. [Google Scholar] [CrossRef]
- Nasrin, A.B.; Vijaya, S.; Loh, S.K.; Astimar, A.A.; Lim, W.S. Quality compliance and environmental impact assessments of commercial EFB pellet fuel in Malaysia. J. Oil Palm Res. 2017, 29, 570–578. [Google Scholar]
- Huzir, N.M.; Aziz, M.A.; Ismail, S.; Abdullah, B.; Mahmood, N.A.N.; Umor, N.; Muhammad, S.A.F.S. Agro-industrial waste to biobutanol production: Eco-friendly biofuels for next generation. Renew. Sustain. Energy Rev. 2018, 94, 476–485. [Google Scholar] [CrossRef]
- Umor, N.A.; Abdullah, S.; Mohamad, A.; Ismail, S.; Ismail, S.I.; Misran, A. Challenges and current state-of-art of the Volvariella volvacea cultivation using agriculture waste: A brief review. In Advance of Waste Processing Technology; Yaser, A.Z., Ed.; Springer Nature: Singapore, 2020; pp. 145–156. [Google Scholar]
- Williams, B.C.; McMullan, J.T.; McCahey, S. An initial assessment of spent mushroom compost as a potential energy feedstock. Bioresour Technol. 2001, 79, 227–230. [Google Scholar] [CrossRef]
- Phan, C.W.; Sabaratnam, V. Potential uses of SMC and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 2012, 96, 863–873. [Google Scholar] [CrossRef]
- Singh, A.D.; Vikineswary, S.; Abdullah, N.; Sekaran, M. Enzymes from spent mushroom substrate of Pleurotus sajor-caju for the decolourisation and detoxification of textile dyes. World J. Microbiol. Biotechnol. 2010, 27, 535–545. [Google Scholar] [CrossRef]
- United Nations. THE 17 GOALS|Sustainable Development. 2021. Available online: https://sdgs.un.org/goals (accessed on 28 July 2021).
- Ryu, C.; Finney, K.; Sharifi, V.N.; Swithenbank, J. Pelletised fuel production from coal tailings and spent mushroom compost —Part I: Identification of pelletisation parameters. Fuel Process. Technol. 2008, 89, 269–275. [Google Scholar] [CrossRef]
- Mamimin, C.; Chanthong, S.; Leamdum, C.; Thong, S.O.; Prasertsan, P. Improvement of empty palm fruit bunches biodegradability and biogas production by integrating the straw mushroom cultivation as a pretreatment in the solid-state anaerobic digestion. Bioresour Technol. 2021, 319, 124227. [Google Scholar] [CrossRef]
- Pérez-Chávez, A.M.; Mayer, L.; Albertó, E. Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy Sustain. Dev. 2019, 50, 50–60. [Google Scholar] [CrossRef]
- Purnomo, A.; Romli, M.; Hasanudin, U. Biogas production from oil palm empty fruit bunches of post mushroom cultivation media. IOP Conf. Ser. Earth Environ. Sci. 2018, 141, 012024. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef] [Green Version]
- Umor, N.A.; Ismail, S.; Abdullah, S.; Huzaifah, M.H.R.; Huzir, N.M.; Mahmood, N.A.N.; Zahrim, A.Y. Zero waste management of spent mushroom compost. J. Mater. Cycles Waste Manag. 2021, 23, 1726–1736. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. American Public Health Association (APHA) Method 9221: Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 1999. [Google Scholar]
- Perkebunan, M. Characteristic of oil palm empty fruit bunch pretreated with Pleurotus floridanus (Pretreatment biologi tandan kosong kelapa sawit menggunakan Pleurotus floridanus). E J. Menara Perkeb. 2017, 85, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Toscano, G.; Pedretti, E.F. Calorific value determination of solid biomass fuel by simplified method. J. Agric. Eng. 2009, 40, 1–6. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Q.; Wu, S.; Qi, D.; Li, W.; Zuo, Z.; Dong, R. Batch anaerobic co-digestion of pig manure with dewatered sewage sludge under mesophilic conditions. Appl. Energy 2014, 128, 175–183. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef]
- Saidu, M.; Yuzir, A.; Salim, M.R.; Salmiati; Azman, S.; Abdullah, N. Influence of palm oil mill effluent as inoculum on anaerobic digestion of cattle manure for biogas production. Bioresour. Technol. 2013, 141, 174–176. [Google Scholar] [CrossRef]
- Ugwu, S.N.; Enweremadu, C.C. Biodegradability and kinetic studies on biomethane production from okra (Abelmoschus esculentus) waste. S. Afr. J. Sci. 2019, 115. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.; Sulaiman, F.; Gerhauser, H. Characterisation of oil palm empty fruit bunches for fuel application. J. Phys. Sci. 2011, 22, 1–24. [Google Scholar]
- 2400 Series II CHNS/O Elemental Analysis. Available online: https://resources.perkinelmer.com/lab-solutions/resources/docs/BRO_2400_SeriesII_CHNSO_Elemental_Analysis.pdf (accessed on 1 August 2021).
- Anuar, N.I.S.; Zakaria, S.; Kaco, H.; Chia, C.H.; Wang, C.; Abdullah, H.S. Physico-Mechanical, Chemical Composition, Thermal Degradation and Crystallinity of Oil Palm Empty Fruit Bunch, Kenaf and Polypropylene Fibres: A Comparatives Study. Sains Malays. 2018, 47, 839–851. [Google Scholar] [CrossRef]
- Thiribhuvanamala, G. Improved techniques to enhance the yield of paddy straw mushroom (Volvariella volvacea) for commercial cultivation. Afr. J. Biotechnol. 2012, 11, 12740–12748. [Google Scholar] [CrossRef]
- Triyono, S.; Haryanto, A.; Telaumbanua, M.; Dermiyati; Lumbanraja, J.; To, F. Cultivation of straw mushroom (Volvariella volvacea) on oil palm empty fruit bunch growth medium. Int. J. Recycl. Org. Waste Agric. 2019, 8, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Li, Y.; Chen, M.; Li, Z. Improved fruiting of the straw mushroom (Volvariella volvacea) on cotton waste supplemented with sodium acetate. Appl. Microbiol. Biotechnol. 2017, 101, 8533–8541. [Google Scholar] [CrossRef]
- Abdul-Hamid, H.; Yusoff, M.-H.; Ab-Shukor, N.-A.; Zainal, B.; Musa, M.-H. Effects of Different Fertilizer Application Level on Growth and Physiology of Hibiscus cannabinus L. (Kenaf) Planted on BRIS Soil. J. Agric. Sci. 2009, 1, 121. [Google Scholar] [CrossRef]
- Idris, J.; Shirai, Y.; Anduo, Y.; Ali, A.A.M.; Othman, M.R.; Ibrahim, I.; Hassan, M. Improved yield and higher heating value of biochar from oil palm biomass at low retention time under self-sustained carbonisation. J. Clean. Prod. 2015, 104, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.; Salmiaton, A.; Azlina, W.W.; Amran, M.M. Gasification of oil palm empty fruit bunches: A characterization and kinetic study. Bioresour. Technol. 2012, 110, 628–636. [Google Scholar] [CrossRef]
- Sulaiman, F.; Abdullah, N. Optimum conditions for maximising pyrolysis liquids of oil palm empty fruit bunches. Energy 2011, 36, 2352–2359. [Google Scholar] [CrossRef]
- Apetorgbor, A.; Apetorgbor, M.; Derkyi, N. Comparative Studies on Growth and Yield of Oil Palm Mushroom, Volvariella volvacea (Bull. Ex. Fr.) Sing. on Different Substrates. Greener J. Agric. Sci. 2015, 5, 177–189. [Google Scholar] [CrossRef]
- Chang, S.; Miles, G.P. Mushrooms: Cultivation, Nutritional Value, Medicinal Effects and Environmental Impact; CRC Press: Boca Raton, FL, USA, 2004; p. 436. [Google Scholar]
- Jiang, H.; Cheng, Z.; Zhao, T.; Liu, M.; Zhang, M.; Li, J.; Hu, M.; Zhang, L.; Li, J. Pyrolysis kinetics of spent lark mushroom substrate and characterization of bio-oil obtained from the substrate. Energy Convers. Manag. 2014, 88, 259–266. [Google Scholar] [CrossRef]
- Yin, R.; Liu, R.; Mei, Y.; Fei, W.; Sun, X. Characterization of bio-oil and bio-char obtained from sweet sorghum bagasse fast pyrolysis with fractional condensers. Fuel 2013, 112, 96–104. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 2014, 128, 162–169. [Google Scholar] [CrossRef]
- Sumardi, I.; Hadiyane, A.; Rumidatul, A.; Melani, L. Characteristics of Empty Palm Bunch Fibers as Alternative Pulp Material. Am. J. Appl. Sci. 2020, 17, 129–134. [Google Scholar] [CrossRef]
- Jurak, E.; Punt, A.M.; Arts, W.; Kabel, M.; Gruppen, H. Fate of Carbohydrates and Lignin during Composting and Mycelium Growth of Agaricus bisporus on Wheat Straw Based Compost. PLoS ONE 2015, 10, e0138909. [Google Scholar] [CrossRef]
- Chin, K.L.; H’Ng, P.S.; Chai, E.W.; Tey, B.T.; Chin, M.J.; Paridah, M.T.; Luqman, A.C.; Maminski, M. Fuel Characteristics of Solid Biofuel Derived from Oil Palm Biomass and Fast Growing Timber Species in Malaysia. BioEnergy Res. 2012, 6, 75–82. [Google Scholar] [CrossRef]
- Loh, S.K. The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers. Manag. 2017, 141, 285–298. [Google Scholar] [CrossRef]
- Charis, G.; Danha, G.; Muzenda, E. Characterizations of Biomasses for Subsequent Thermochemical Conversion: A Comparative Study of Pine Sawdust and Acacia Tortilis. Processes 2020, 8, 546. [Google Scholar] [CrossRef]
- Chandrasekaran, S.R.; Hopke, P.K.; Rector, I.; Allen, G.; Lin, L. Chemical composition of wood chips and wood pellets. Energy Fuels 2012, 26, 4932–4937. [Google Scholar] [CrossRef]
- Finney, K.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. The reuse of spent 822 mushroom compost and coal tailings for energy recovery: Comparison of thermal 823 treatment technologies. Bioresour. Technol. 2009, 100, 310–315. [Google Scholar] [CrossRef]
- Chia, W.Y.; Chew, K.W.; Le, C.F.; Lam, S.S.; Chee, C.S.; Ooi, M.S.; Show, P.L. Sustainable utilization of biowaste compost for renewable energy and soil amendments. Environ. Pollut. 2020, 267, 115662. [Google Scholar] [CrossRef]
- Huzaifah, M.; Sapuan, S.; Leman, Z.; Ishak, M.; Maleque, M. A review of sugar palm (Arenga pinnata): Application, fibre characterisation and composites. Multidiscip. Model. Mater. Struct. 2017, 13, 678–698. [Google Scholar] [CrossRef]
- Lee, T.; Zubir, Z.A.; Jamil, F.M.; Matsumoto, A.; Yeoh, F.-Y. Combustion and pyrolysis of activated carbon fibre from oil palm empty fruit bunch fibre assisted through chemical activation with acid treatment. J. Anal. Appl. Pyrolysis 2014, 110, 408–418. [Google Scholar] [CrossRef]
- Idris, S.S.; Rahman, N.; Ismail, K.; Alias, A.B.; Rashid, Z.A.; Aris, M.J. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour. Technol. 2010, 101, 4584–4592. [Google Scholar] [CrossRef]
- Sivasangar, S.; Taufiq-Yap, Y.H.; Zainal, Z.; Kitagawa, K. Thermal behavior of lignocellulosic materials under aerobic/anaerobic environments. Int. J. Hydrogen Energy 2013, 38, 16011–16019. [Google Scholar] [CrossRef]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to Energy: A Focus on the Impact of Substrate Type in Biogas Production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- O-Thong, S.; Boe, K.; Angelidaki, I. Thermophilic anaerobic co-digestion of oil palm empty fruit bunches with palm oil mill effluent for efficient biogas production. Appl. Energy 2012, 93, 648–654. [Google Scholar] [CrossRef]
- Córdoba, V.; Colavolpe, M.B.; Fernández, M.; Santalla, E.; Albertó, E. Potential methane production of spent sawdust used in the cultivation of Gymnopilus pampeanus. J. Environ. Chem. Eng. 2016, 4, 4418–4425. [Google Scholar] [CrossRef] [Green Version]
- FTIR Basic Organic Functional Group Reference Chart. Available online: https://www.thermofisher.com/blog/materials/a-gift-for-you-an-ftir-basic-organic-functional-group-reference-chart/ (accessed on 5 August 2021).
- Rosli, N.S.; Harun, S.; Jahim, J.M.; Othaman, R. Chemical and Physical Characterization of Oil Palm Empty. Malays. J. Anal. Sci. 2017, 21, 188–196. [Google Scholar] [CrossRef]






| Samples | Yield (g) | BE (%) | DFFH | No. FB | HD |
|---|---|---|---|---|---|
| F | 196.67 ± 7.81 b | 9.83 | 16 ± 1.2 | 10 ± 1.2 | 5 ± 1.3 |
| P | 567.33 ± 58.4 c | 28.00 | 19 ± 0.5 | 32 ± 0.7 | 5 ± 1.5 |
| FS | 57.67 ± 20.1 a | 2.90 | 14 ± 0.0 | 6 ± 2.0 | 3 ± 0.0 |
| PS | 135.33 ± 13 bc | 6.76 | 17 + 1.5 | 10 ± 2.0 | 4 ± 0.0 |
| Sample | Moisture (%) | Length (mm) | Diameter (mm) | Bulk Density (kg m−3) | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|---|---|---|---|
| F | 4.21 | >50 | 0.33–0.71 | 0.0948 | 45.22 ±1.44 | 18.03 ± 2.12 | 35.9 ± 5.24 |
| FS | 13.5 | >50 | 0.19–0.44 | ND | 36.42 ± 2.08 | 22.31 ± 1.85 | 36.38 ± 4.31 |
| FS2 | 15.3 | >50 | 0.16–0.28 | ND | 23.41 ± 3.78 | 24.94 ± 1.54 | 33.51 ± 1.75 |
| P | 8.73 | 3–18 | Loose = 0.30–0.42 Intact = 8.85 | 0.615 | 45.37 ± 2.96 | 16.87 ± 3.24 | 31.33 ± 2.61 |
| PS | 50.1 | 3–18 | 0.23–0.29 | ND | 34.651 ± 3.35 | 10.67 ± 4.5 | 42.84 ± 2.62 |
| PS2 | 62.5 | 3–18 | 0.21–0.23 | ND | 27.3 ± 0.38 | 13.02 ± 4.3 | 35.29 ± 0.02 |
| Samples/Reference | Ultimate Analysis (%) | Proximate Analysis (%) | HHV (MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O1 | S | Ash | FC | VM | MC | ||
| F | 39.26 ± 1.2 | 6.15 ± 0.11 | 1.25 ± 0.01 | 53.34 | 0.016 ± 0.0 | 11.92 ± 1.2 | 22.98 ± 2.41 | 65.10 ± 1.11 | 3.80 ± 0.5 | 20.57 |
| FS | 42.04 ± 0.56 | 6.09 ± 0.09 | 0.72 ± 0.02 | 51.15 | 0.00 ± 0.0 | 19.11 ± 2.11 | 25.04 ± 2.34 | 55.85 ± 1.44 | 13.50 ± 0.45 | 16.77 |
| FS2 | 35.19 ± 0.87 | 4.68 ± 0.27 | 1.25 ± 0.03 | 58.88 | 0.23 ± 0.0 | 17.84 ± 1.75 | 25.88 ± 3.11 | 56.28 ± 1.98 | 15.30 ± 2.11 | 15.06 |
| P | 42.59 ± 1.31 | 5.40 ± 0.02 | 1.01 ± 0.08 | 51.00 | 0.055 ± 0.0 | 10.80 ± 0.97 | 21.30 ± 2.77 | 67.90 ± 1.45 | 10.10 ± 0.76 | 19.06 |
| PS | 41.65 ± 1.21 | 5.11 ± 0.22 | 0.6 ± 0.01 | 52.64 | 0.11 ± 0.01 | 8.02 ± 0.87 | 46.49 ± 2.55 | 45.49 ± 0.77 | 50.10 ± 2.51 | 15.53 |
| PS2 | 35.00 ± 0.77 | 3.95 ± 0.09 | 0.99 ± 0.03 | 60.05 | 0.13 ± 0.0 | 9.36 ± 0.71 | 54.64 ± 3.77 | 36.00 ± 1.98 | 62.50 ± 3.11 | 12.6 |
| EFB pellet [44] | 42.99 | 6.19 | 0.64 | 50.11 | 0.08 | - | - | - | - | - |
| EFB based SMC [12] | 51.00 | 6.40 | 0.65 | 40.20 | 0.00 | - | - | - | - | - |
| Sample | Initial Load (gVS/ L) | BMP (mLCH4/ gVS) | TBMP (mLCH4/ gVS) | BD (%) | Biogas | ||
|---|---|---|---|---|---|---|---|
| Total Vol (mL) | CH4 (%) | CO2 (%) | |||||
| Blank | ND | ND | ND | ND | 170 | ND | ND |
| F | 32.5 ± 2.11 | 28.15 ± 0.77 a | 101.4 ± 0.11 b | 28.2 | 349 | 50 | 50 |
| FS | 27.92 ± 0.92 | 17.2 ± 0.56 a | 141.28 ± 0.37 c | 12.17 | 260 | 50 | 50 |
| FS2 | 28.1 ± 0.77 | 19.75 ± 1.22 a | 44.08 ± 0.75 a | 44.8 | 234 | 75 | 25 |
| P | 33.95 ± 1.11 | 41.00 ± 0.39 b | 169.68 ± 0.55 d | 24.1 | 444 | 50 | 50 |
| PS | 17.4 ± 1.75 | 18.39 ± 0.21 a | 130.87 ± 0.56 c | 14.05 | 208 | 78 | 22 |
| PS2 | 18 ± 1.22 | 47.60 ± 0.22 b | 49.00 ± 0.12 a | 97 | 306 | 63 | 37 |
| Absorption Band F | Absorption Band FS | Absorption Band FS2 | Absorption Band P | Absorption Band PS | Absorption Band PS2 | Standard Band | Possible Compounds/Chains Rationale | Possible Bond |
|---|---|---|---|---|---|---|---|---|
| 3376.88 | 3291.5 | 3376.39 | 3356.38 | 3284.36 | 3281.18 | 3350 | Alcohol | O-H |
| ND | ND | ND | 2928.57 | 2928.57 | ND | 2930 | Methylene | C-H |
| ND | 1740.3 | 1736.53 | ND | ND | ND | 1730 | Aldehyde | C=O |
| 1648.09 | 1647.12 | 1646.87 | 1649.56 | 1649.35 | 1643.17 | 1640 | Amide/Alkenes | C=C |
| 1424.05 | 1418.63 | 1414.92 | 1428 | 1416.58 | 1413.68 | 1425 | Lignin and wood | C-H |
| 1244.73 | 1216.96 | 1217.26 | 1244.05 | ND | ND | 1200/1244 | Ester/aryl alkyl ether | C-O/C-O-C |
| 1036.15 | 1046.98 | 1044.15 | 1047.87 | 1044.66 | 1057.08 | 1100 | Ether/alcohol | C-O |
| ND | 874.73 | 873.68 | 899.77 | 872.93 | 873.49 | 890 | Alkenes (vinylidene) | C-O |
| ND | ND | ND | ND | 712.95 | 712.99 | 700 | ND | RING |
| SMC Application | Strength | Weakness | Measures to Overcome |
|---|---|---|---|
| Biogas |
|
|
|
| Fuel pellet |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umor, N.A.; Abdullah, S.; Mohamad, A.; Ismail, S.B.; Ismail, S.I.; Misran, A. Energy Potential of Oil Palm Empty Fruit Bunch (EFB) Fiber from Subsequent Cultivation of Volvariella volvacea (Bull.) Singer. Sustainability 2021, 13, 13008. https://doi.org/10.3390/su132313008
Umor NA, Abdullah S, Mohamad A, Ismail SB, Ismail SI, Misran A. Energy Potential of Oil Palm Empty Fruit Bunch (EFB) Fiber from Subsequent Cultivation of Volvariella volvacea (Bull.) Singer. Sustainability. 2021; 13(23):13008. https://doi.org/10.3390/su132313008
Chicago/Turabian StyleUmor, Noor Azrimi, Sumaiyah Abdullah, Azhar Mohamad, Shahrul Bin Ismail, Siti Izera Ismail, and Azizah Misran. 2021. "Energy Potential of Oil Palm Empty Fruit Bunch (EFB) Fiber from Subsequent Cultivation of Volvariella volvacea (Bull.) Singer" Sustainability 13, no. 23: 13008. https://doi.org/10.3390/su132313008
APA StyleUmor, N. A., Abdullah, S., Mohamad, A., Ismail, S. B., Ismail, S. I., & Misran, A. (2021). Energy Potential of Oil Palm Empty Fruit Bunch (EFB) Fiber from Subsequent Cultivation of Volvariella volvacea (Bull.) Singer. Sustainability, 13(23), 13008. https://doi.org/10.3390/su132313008






