Sewage Sludge as N-Fertilizers for Crop Production Enabling the Circular Bioeconomy in Agriculture: A Challenge for the New EU Regulation 1009/2019
Abstract
:1. Introduction
2. Materials and Methods
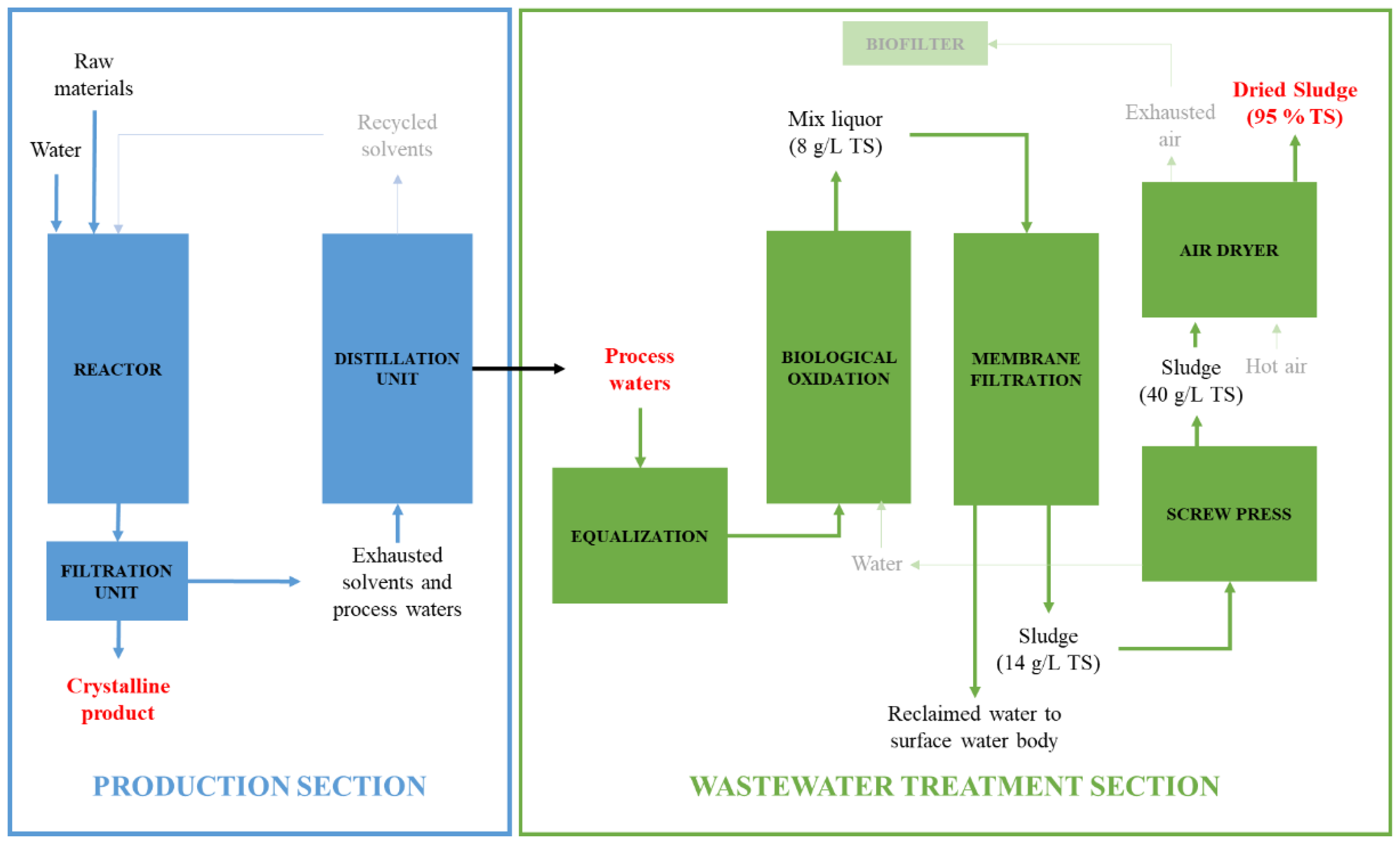
2.1. Production and Collection of the Pharmaceutical Dried Sewage Sludge
2.2. Analytical Methods
2.2.1. Physico-Chemical Analysis
2.2.2. Heavy Metals, Pathogens and Organic Contaminants
2.3. Plant Growth Trials
2.3.1. Experimental Design
2.3.2. Physiological Status and Plant Yields
2.3.3. Leachate Collection and Characterization
2.4. Statistics
3. Results and Discussion
3.1. Characterization of the Dried Pharmaceutical Sewage Sludge and Agronomic Value
3.2. Environmental Aspects
3.2.1. Heavy Metals
3.2.2. Pathogens
3.2.3. Organic Contaminants
3.3. Agronomic Trials
3.3.1. Yields, Physiological Status and the Composition of the Plants
3.3.2. Leachate Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Organization of the United Nations. Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 6 October 2021).
- Organization of the United Nations. Take Action for the Sustainable Development Goals—United Nations Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 6 October 2021).
- FAO. Food Outlook—Biannual Report on Global Food Markets, Food Outlook—Biannual Report on Global Food Markets. Available online: https://doi.org/10.4060/cb1993en (accessed on 6 October 2021).
- FAO. World Fertilizer Trends and Outlook to 2022. Available online: https://doi.org/10.4060/ca6746en (accessed on 6 October 2021).
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- Günther, S.; Grunert, M.; Müller, S. Overview of recent advances in phosphorus recovery for fertilizer production. Eng. Life Sci. 2018, 18, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mohammad, F. Eutrophication: Challenges and solutions. In Eutrophication: Causes, Consequences and Control; Abid, A.A., Saravajeet, S.G., Eds.; Springer Science & Business Media: Berlin, Germany, 2014; pp. 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, S.; Wang, Y. What could promote farmers to replace chemical fertilizers with organic fertilizers? J. Clean. Prod. 2018, 199, 882–890. [Google Scholar] [CrossRef]
- Pigoli, A.; Zilio, M.; Tambone, F.; Mazzini, S.; Schepis, M.; Meers, E.; Schoumans, O.; Giordano, A.; Adani, F. Thermophilic anaerobic digestion as suitable bioprocess producing organic and chemical renewable fertilizers: A full-scale approach. Waste Manag. 2021, 124, 356–367. [Google Scholar] [CrossRef]
- Zilio, M.; Pigoli, A.; Rizzi, B.; Geromel, G.; Meers, E.; Schoumans, O.; Giordano, A.; Adani, F. Measuring ammonia and odours emissions during full field digestate use in agriculture. Sci. Total Environ. 2021, 782, 146882. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2019/1009 Fertilizer Products. Off. J. Eur. Union 2019, 170. [Google Scholar]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge—The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- Nissim, W.G.; Cincinelli, A.; Martellini, T.; Alvisi, L.; Palm, E.; Mancuso, S.; Azzarello, E. Phytoremediation of sewage sludge contaminated by trace elements and organic compounds. Environ. Res. 2018, 164, 356–366. [Google Scholar] [CrossRef]
- Meng, X.Z.; Venkatesan, A.K.; Ni, Y.L.; Steele, J.C.; Wu, L.L.; Bignert, A.; Bergman, Å.; Halden, R.U. Organic Contaminants in Chinese Sewage Sludge: A Meta-Analysis of the Literature of the Past 30 Years. Environ. Sci. Technol. 2016, 50, 5454–5466. [Google Scholar] [CrossRef] [Green Version]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2019, 14, 16–22. [Google Scholar] [CrossRef]
- Yang, G.H.; Zhu, G.Y.; Li, H.L.; Han, X.M.; Li, J.M. Accumulation and bioavailability of heavy metals in a soil-wheat/maize system with long-term sewage sludge amendments. J. Integr. Agric. 2018, 17, 1861–1870. [Google Scholar] [CrossRef] [Green Version]
- Campo, G.; Cerutti, A.; Lastella, C.; Leo, A.; Panepinto, D.; Zanetti, M.; Ruffino, B. Production and destination of sewage sludge in the Piemonte region (Italy): The results of a survey for a future sustainable management. Int. J. Environ. Res. Public Health 2021, 18, 3556. [Google Scholar] [CrossRef] [PubMed]
- Zittel, R.; da Silva, C.P.; Domingues, C.E.; Seremeta, D.C.H.; da Cunha, K.M.; de Campos, S.X. Availability of nutrients, removal of nicotine, heavy metals and pathogens in compounds obtained from smuggled cigarette tobacco compost associated with industrial sewage sludge. Sci. Total Environ. 2019, 699, 134377. [Google Scholar] [CrossRef]
- Alvarenga, P.; Mourinha, C.; Farto, M.; Santos, T.; Palma, P.; Sengo, J.; Morais, M.C.; Cunha-Queda, C. Sewage sludge, compost and other representative organic wastes as agricultural soil amendments: Benefits versus limiting factors. Waste Manag. 2015, 40, 44–52. [Google Scholar] [CrossRef]
- Cucina, M.; Tacconi, C.; Ricci, A.; Pezzolla, D.; Sordi, S.; Zadra, C.; Gigliotti, G. Evaluation of benefits and risks associated with the agricultural use of organic wastes of pharmaceutical origin. Sci. Total Environ. 2018, 613–614, 773–782. [Google Scholar] [CrossRef]
- Cucina, M.; Ricci, A.; Zadra, C.; Pezzolla, D.; Tacconi, C.; Sordi, S.; Gigliotti, G. Benefits and risks of long-term recycling of pharmaceutical sewage sludge on agricultural soil. Sci. Total Environ. 2019, 695, 133762. [Google Scholar] [CrossRef] [PubMed]
- Scaglia, B.; Tambone, F.; Corno, L.; Orzi, V.; Lazzarini, Y.; Garuti, G.; Adani, F. Potential agronomic and environmental properties of thermophilic anaerobically digested municipal sewage sludge measured by an unsupervised and a supervised chemometric approach. Sci. Total Environ. 2018, 637–638, 791–802. [Google Scholar] [CrossRef]
- Congilosi, J.L.; Aga, D.S. Review on the fate of antimicrobials, antimicrobial resistance genes, and other micropollutants in manure during enhanced anaerobic digestion and composting. J. Hazard. Mater. 2020, 405, 123634. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 amending Directive 2008/98/EC on waste (Text with EEA relevance). Off. J. Eur. Union 2018, 150, 109–140. [Google Scholar]
- Presidency of the Council of Ministers. Implementation of Directive No. 86/278/EEC Concerning the Protection of the Environment, in Particular of the Soil, in the Use of Purification Sludge in Agriculture; Gazzetta Ufficiale: Rome, Italy, 2010. [Google Scholar]
- Presidency of the Council of Ministers. Legislative Decree 29 April 2010, No. 75—Reorganization and Revision of the Regulations on Fertilizers, Pursuant to Article 13 of the Law of 7 July 2009, No. 88; Gazzetta Ufficiale: Rome, Italy, 2010. [Google Scholar]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Lombardi, L.; Nocita, C.; Bettazzi, E.; Fibbi, D.; Carnevale, E. Environmental comparison of alternative treatments for sewage sludge: An Italian case study. Waste Manag. 2017, 69, 365–376. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- ANPA. Metodi di Analisi del Compost; ISPRA, Manuali e Linee Guida: Rome, Italy, 2001. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Method 3051A—Microwave Assisted Acid Digestion of Sediments, Sludges, Soils And Oils; United States Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- Gu, Y.G. Calculation of beryllium toxic factor for potential ecological risk evaluation: A case study. Environ. Technol. Innov. 2021, 21, 101361. [Google Scholar] [CrossRef]
- APAT. Metodi microbiologici di analisi del compost. In Manuali e Linee Guida 20/2003. APAT—Agenzia per la Protezione Dell’ambiente e per i Servizi Tecnici; APAT, Manuali e Linee Guida: Rome, Italy, 2003. [Google Scholar]
- International Organization for Standardization (ISO). Water Quality—Determination of 16 Polycyclic Aromatic Hydrocarbons (PAH) in Water—Method Using Gas Chromatography with Mass Spectrometric Detection (GC-MS); ISO: 28540:2011; International Organization for Standardization (ISO): Geneva, Switzerland, 2011. [Google Scholar]
- International Organization for Standardization (ISO). Water Quality—Determination of Benzene and Some Derivatives—Part 1: Head-Space Gas Chromatographic Method; ISO: 11423-1:1997; International Organization for Standardization (ISO): Geneva, Switzerland, 1997. [Google Scholar]
- International Organization for Standardization (ISO). Sludge, Treated Biowaste and Soil—Digestion of Aqua Regia Soluble Fractions of Elements; ISO: 54321:2020; International Organization for Standardization (ISO): Geneva, Switzerland, 2020. [Google Scholar]
- International Organization for Standardization (ISO). Soil, Treated Biowaste and Sludge—Determination of Polychlorinated Biphenyls (PCB) by Gas Chromatography with Mass Selective Detection (GC-MS) and Gas Chromatography with Electron-Capture Detection (GC-ECD); ISO: 13876:2013; International Organization for Standardization (ISO): Geneva, Switzerland, 2013. [Google Scholar]
- Ente Nazionale Italiano di Unificazione (UNI). Caratterizzazione dei Rifiuti—Determinazione di Policlorodibenzo-P-Diossine (PCDD) e Policlorodibenzofurani (PCDF) in Rifiuti Solidi; UNI 11199:2007; Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 2007. [Google Scholar]
- Ente Nazionale Italiano di Unificazione (UNI). Characterization of Waste—Determination of Hydrocarbon Content in the Range of C10 to C40 by Gas Chromatography; UNI EN 14039:2005; Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 2005. [Google Scholar]
- International Organization for Standardization (ISO). Soil Quality–Determination of Water Retention Characteristics–Laboratory Methods; ISO 11274:2019; International Organization for Standardization (ISO): Geneva, Switzerland, 2019. [Google Scholar]
- Garfí, M.; Martí-Herrero, J.; Garwood, A.; Ferrer, I. Household anaerobic digesters for biogas production in Latin America: A review. Renew. Sust. Energ. Rev. 2016, 60, 599–614. [Google Scholar] [CrossRef] [Green Version]
- Manirakiza, E.; Ziadi, N.; St. Luce, M.; Hamel, C.; Antoun, H.; Karam, A. Nitrogen mineralization and microbial biomass carbon and nitrogen in response to co-application of biochar and paper mill biosolids. App. Soil Ecol. 2019, 142, 90–98. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Cucina, M.; Tacconi, C.; Sordi, S.; Pezzolla, D.; Gigliotti, G.; Zadra, C. Valorization of a pharmaceutical organic sludge through different composting treatments. Waste Manag. 2018, 74, 203–212. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y. Effects of sewage sludge compost application on crops and cropland in a 3-year field study. Chemosphere 2005, 59, 1257–1265. [Google Scholar] [CrossRef]
- Romdhana, M.H.; Lecomte, D.; Ladevie, B.; Sablayrolles, C. Monitoring of pathogenic microorganisms contamination during heat drying process of sewage sludge. Process Saf. Environ. Prot. 2009, 87, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Heinonen-Tanski, H.; Mohaibes, M.; Karinen, P.; Koivunen, J. Methods to reduce pathogen microorganisms in manure. Livest. Sci. 2006, 102, 248–255. [Google Scholar] [CrossRef]
- Avery, L.M.; Booth, P.; Campbell, C.; Tompkins, D.; Hough, R.L. Prevalence and survival of potential pathogens in source-segregated green waste compost. Sci. Total Environ. 2012, 431, 128–138. [Google Scholar] [CrossRef]
- Perera, M.A.D.N.; Qin, W.; Yandeau-Nelson, M.; Fan, L.; Dixon, P.; Nikolau, B.J. Biological origins of normal-chain hydrocarbons: A pathway model based on cuticular wax analyses of maize silks. Plant J. 2010, 64, 618–632. [Google Scholar] [CrossRef]
- Agenzia Regionale per la protezione dell’ambiente del Veneto. Risultati del Monitoraggio Eseguito nel 2013 Delle Caratteristiche dei Digestati Prodotti da Impianti per la Produzione di Biogas che Trattano Liquami Zootecnici Situati nel Veneto; ARPA Veneto: Padova, Italy, 2013. [Google Scholar]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil—A critical review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.A.; Akdeniz, H.; Keskin, B.; Yilmaz, I.H. Possibilities of using sewage sludge as nitrogen fertilizer for maize. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2006, 56, 143–149. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient management in aquaponics: Comparison of three approaches for cultivating lettuce, mint and mushroom herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Samaras, V.; Tsadilas, C.D.; Stamatiadis, S. Effects of repeated application of municipal sewage sludge on soil fertility, cotton yield, and nitrate leaching. Agron. J. 2008, 100, 477–483. [Google Scholar] [CrossRef]
- Presidency of the Council of Ministers. Decreto Legislativo 3 Aprile 2006, N. 152: Norme in Materia Ambientale; Gazzetta Ufficiale: Rome, Italy, 2006. [Google Scholar]
- Sogn, T.A.; Dragicevic, I.; Linjordet, R.; Krogstad, T.; Eijsink, V.G.H.; Eich-Greatorex, S. Recycling of biogas digestates in plant production: NPK fertilizer value and risk of leaching. Int. J. Recyc. Org. Waste Agric. 2018, 7, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Forge, T.; Kenney, E.; Hashimoto, N.; Neilsen, D.; Zebarth, B. Compost and poultry manure as preplant soil amendments for red raspberry: Comparative effects on root lesion nematodes, soil quality and risk of nitrate leaching. Agric. Ecosyst. Environ. 2016, 223, 48–58. [Google Scholar] [CrossRef]
- Fang, W.; Wei, Y.; Liu, J. Comparative characterization of sewage sludge compost and soil: Heavy metal leaching characteristics. J. Hazard. Mater. 2016, 310, 1–10. [Google Scholar] [CrossRef]

| Parameter | Unit a | Soil | PDSS b | Soil + PDSS | DCM c | Soil + DCM |
|---|---|---|---|---|---|---|
| pH | - | 6.0 d ± 0.1 | 8.0 ± 0.1 | 6.1 ± 0.1 | 7.2 ± 0.1 | 6.0 ± 0.2 |
| Total solids | % | n.d. e | 96.1 ± 1.0 | n.d. | 52.3 ± 1.0 | n.d. |
| Total organic C | % | 1.5 ± 0.1 | 35.5 ± 1.2 | 1.6 ± 0.1 | 41.2 ± 1.7 | 1.4 ± 0.2 |
| Total N | % | 0.18 ± 0.01 | 6.6 ± 0.2 | 0.22 ± 0.00 | 4.2 ± 0.4 | 0.21 ± 0.01 |
| Organic N/Total N | % | n.d. | 100 | n.d. | 82.3 ± 0.2 | n.d. |
| C/N | - | 8.3 ± 0.7 | 5.4 ± 0.4 | 7.3 ± 0.1 | 9.8 ± 0.1 | 6.7 ± 0.2 |
| Total P | % | n.d. | 0.6 ± 0.1 | n.d. | 1.1 ± 0.2 | n.d. |
| Total P | Mg P2O5 kg−1 | 109 ± 5 | n.d. | 106 ± 8 | n.d. | 110 ± 4 |
| Total K | % | 2.1 ± 0.0 | 0.4 ± 0.1 | 2.1 ± 0.1 | 3.0 ± 0.3 | 2.3 ± 0.1 |
| Total Ca | % | n.d. | 4.6 ± 0.2 | n.d. | 1.4 ± 0.0 | n.d. |
| Total Mg | % | n.d. | 0.2 ± 0.1 | n.d. | 0.6 ± 0.1 | n.d. |
| Total Na | % | n.d. | 1.1 ± 0.1 | n.d. | 1.3 ± 0.1 | n.d. |
| Water-holding capacity | % | 30 ± 1 | n.a. f | n.d. | n.a. | n.d. |
| Cation exchange capacity | cmol kg−1 | 18.6 ± 1.3 | n.a. | n.d. | n.a. | n.d. |
| Parameter | Unit a | PDSS b | DCM b | AISS c | MSS c | GC d | OWC d | Straw d | ECD d | MSSD e | Limit Value f | Limit Value g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb | mg kg−1 | 1.3 ± 0.5 h | 5.0 ± 0.4 | 52.2 ± 3.7 | <5.6 | 24 ± 0.2 | 12.4 ± 1.0 | 3.2 ± 0.1 | 3.0 ± 0.0 | 64 ± 11 | 140 | 120 |
| Cu | mg kg−1 | 13.0 ± 5.6 | 58.3 ± 1.8 | 30.6 ± 1.3 | 140.8 ± 2.4 | 53.5 ± 1.6 | 37.6 ± 1.5 | 18.3 ± 1.2 | 83.3 ± 1.1 | 408 ± 60 | 230 | 300 |
| Zn | mg kg−1 | 79 ± 16 | 179 ± 8 | 84 ± 3 | 757 ± 12 | 151 ± 3 | 106 ± 3 | 54 ± 2 | 393 ± 4 | 1020 ± 120 | 500 | 800 |
| Ni | mg kg−1 | 8.6 ± 1.9 | 6.1 ± 0.2 | 44.3 ± 3.1 | 22.6 ± 0.9 | 41.8 ± 1.0 | 7.1 ± 1.0 | 4.4 ± 0.6 | 9.6 ± 0.5 | 61.0 ± 13.0 | 100 | 50 |
| Cd | mg kg−1 | <0.1 i | <0.1 | 4.6 ± 0.2 | 1.0 ± 0.1 | 0.17 ± 0.03 | 0.33 ± 0.09 | 0.12 ± 0.02 | 0.37 ± 0.05 | 1 ± 0.5 | 1.5 | 1.5 |
| Hg | mg kg−1 | 0.6 ± 0.2 | 0.4 ± 0.0 | <0.2 | <1.3 | 0.8 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.3 | 1.5 | 1 |
| Cr VI | mg kg−1 | <0.2 | <0.2 | - | - | <0.2 | <0.2 | <0.2 | <0.2 | - | 0.5 | 2 |
| Total Cr | mg kg−1 | 19.5 ± 5.3 | 7.4 ± 0.4 | <5.6 | <5.6 | 88.8 ± 0.9 | 11.8 ± 1.0 | 6.1 ± 0.3 | 17.2 ± 0.4 | 95.0 ± 22.0 | - | - |
| As | mg kg−1 | 0.9 ± 0.2 | 1.1 ± 0.1 | - | - | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.2 ± 0.0 | 1.1 ± 0.2 | 9.0 ± 2.2 | - | 40 |
| Se | mg kg−1 | 0.2 ± 0.1 | 0.6 ± 0.1 | - | - | - | - | - | - | 3.7 ± 2.1 | - | - |
| Be | mg kg−1 | <0.5 | <0.5 | - | - | - | - | - | - | - | - | - |
| Salmonella spp. | Faecal Coliform | Escherichia coli | |
|---|---|---|---|
| Unit a | Presence-absence in 25 g | CFU b g−1 | CFU g−1 |
| PDSS | Absent | <10 c | <10 |
| DCM | Absent | <10 | <10 |
| AISS d | Absent | - | <10 |
| MSS d | Absent | - | 8.9 × 102 |
| GC e | Absent | 7 × 104 ± 3.6 × 102 | 1 × 102 ± 0.4 × 102 |
| OWC e | Absent | 0 ± 0 | 0 ± 0 |
| ECD e | Absent | 0 ± 0 | 0 ± 0 |
| MSSD f | Absent | - | Absent |
| Straw e | Absent | 0 ± 0 | 0 ± 0 |
| Limit value g | Absent in 25 g | - | - |
| Limit value h | Absent in 25 g | - | 103 g−1 |
| Parameter | Unit | PDSS a | DCM a | AISS b | MSS b | GC c | OWC c | ECD c | MSSD d | Straw c |
|---|---|---|---|---|---|---|---|---|---|---|
| Hydrocarbons (C < 12) | mg kg−1 f.w. | 21 ± 8 e | 35 ± 2 | - | - | - | - | - | - | - |
| Hydrocarbons (C > 12) | mg kg−1 f.w. | 697 ± 337 | 1120 ± 142 | - | - | - | - | - | - | - |
| Total hydrocarbons | mg kg−1 f.w. | 613 ± 380 | 1155 ± 99 | - | - | - | - | - | 284 ± 251 | - |
| Total PAH | mg kg−1 d.m. | <0.6 f | <0.6 | 0.2 | 0.9 | 0.04 | <0.01 | 1.1 | 0.5 ± 0.5 | 0.08 |
| PCDD/PCDF + PCB DL | ngWHO-TEQ kg−1 d.m. | 0.3 ± 0.0 | <0.1 | 5.3 | 4.8 | 1.0 | 0.3 | 0.9 | 10.6 ± 2.9 | 0.2 |
| PCB | mg kg−1 d.m. | <0.1 | <0.1 | <d.l.m. | <d.l.m. | 0.01 | 0.02 | 0.12 | <0.1 | 0.00 |
| Toluene | mg kg−1 d.m. | <10 | <10 | - | - | - | - | - | - | - |
| 7-ZACA | mg kg−1 d.m. | <0.01 | - | - | - | - | - | - | - | - |
| Parameter | Plant | Unit | Control | PDSS a | DCM b | |
|---|---|---|---|---|---|---|
| Productive parameters | Fresh weight | Lettuce | g | 53.5 c± 4.3 a | 70.0 ± 3.2 b | 71.5 ± 3.0 b |
| Carrot | g | 40.5 ± 4.7 a | 56.2 ± 2.1 b | 45.6 ± 6.4 a | ||
| Dry weight | Lettuce | g | 7.3 ± 0.6 a | 9.1 ± 0.2 b | 9.3 ± 0.3 b | |
| Carrot | g | 5.8 ± 0.9 a | 8.5 ± 0.5 b | 7.1 ± 1.0 a | ||
| Physiological parameters | NBI d | Lettuce | - | 13.6 ± 0.6 a | 17.4 ± 0.4 b | 17.5 ± 0.8 b |
| CHL e | - | 25.0 ± 0.3 a | 29.2 ± 0.6 b | 28.1 ± 0.2 b | ||
| FLAV f | - | 2.0 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.1 |
| Plant | Parameter | Unit | Control | PDSS a | DCM b | Limit Value c |
|---|---|---|---|---|---|---|
| Lettuce | pH | - | 7.3 ± 0.0 d | 7.3 ± 0.0 | 7.4 ± 0.0 | 5.5–9.5 |
| EC | mS cm−1 | 0.8 ± 0.0 | 0.9 ± 0.1 | 0.8 ± 0.1 | - | |
| N-NO3- | mg L−1 | 6 ± 1 a | 9 ± 2 a | 15 ± 2 b | 20 | |
| Cu | µg L−1 | 4.5 ± 1.0 | 3.4 ± 0.5 | 4.4 ± 1.6 | 100 | |
| Zn | µg L−1 | 16 ± 3 | 15 ± 4 | 27 ± 7 | 500 | |
| Pb | µg L−1 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 200 | |
| Ni | µg L−1 | 80 ± 5 | 96 ± 5 | 85 ± 2 | 2000 | |
| Total Cr | µg L−1 | 118 ± 0 | 122 ± 3 | 121 ± 1 | 2000 | |
| Cd | µg L−1 | <0.1 e | <0.1 | 0.1 ± 0.0 | 20 | |
| As | µg L−1 | 0.6 ± 0.2 | 1.0 ± 0.4 | 0.6 ± 0.3 | 500 | |
| Se | µg L−1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 | 30 | |
| Carrot | pH | - | 7.2 ± 0.1 | 7.2 ± 0.0 | 7.3 ± 0.0 | 5.5–9.5 |
| EC | mS cm−1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | - | |
| N-NO3- | mg L−1 | 10 ± 0 a | 11 ± 3 a | 13 ± 1 b | 20 | |
| Cu | µg L−1 | 4.0 ± 0.6 | 4.0 ± 0.6 | 4.2 ± 1.0 | 100 | |
| Zn | µg L−1 | 13 ± 3 | 18 ± 2 | 24 ± 7 | 500 | |
| Pb | µg L−1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 200 | |
| Ni | µg L−1 | 54 ± 6 | 92 ± 10 | 85 ± 4 | 2000 | |
| Total Cr | µg L−1 | 118 ± 1 | 122 ± 1 | 119 ± 1 | 2000 | |
| Cd | µg L−1 | 0.1 ± 0.0 | <0.1 | 0.1 ± 0.0 | 20 | |
| As | µg L−1 | 0.7 ± 0.1 | 0.9 ± 0.2 | 0.7 ± 0.1 | 500 | |
| Se | µg L−1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucina, M.; De Nisi, P.; Sordi, S.; Adani, F. Sewage Sludge as N-Fertilizers for Crop Production Enabling the Circular Bioeconomy in Agriculture: A Challenge for the New EU Regulation 1009/2019. Sustainability 2021, 13, 13165. https://doi.org/10.3390/su132313165
Cucina M, De Nisi P, Sordi S, Adani F. Sewage Sludge as N-Fertilizers for Crop Production Enabling the Circular Bioeconomy in Agriculture: A Challenge for the New EU Regulation 1009/2019. Sustainability. 2021; 13(23):13165. https://doi.org/10.3390/su132313165
Chicago/Turabian StyleCucina, Mirko, Patrizia De Nisi, Simone Sordi, and Fabrizio Adani. 2021. "Sewage Sludge as N-Fertilizers for Crop Production Enabling the Circular Bioeconomy in Agriculture: A Challenge for the New EU Regulation 1009/2019" Sustainability 13, no. 23: 13165. https://doi.org/10.3390/su132313165
APA StyleCucina, M., De Nisi, P., Sordi, S., & Adani, F. (2021). Sewage Sludge as N-Fertilizers for Crop Production Enabling the Circular Bioeconomy in Agriculture: A Challenge for the New EU Regulation 1009/2019. Sustainability, 13(23), 13165. https://doi.org/10.3390/su132313165







