Pathogens Removal in a Sustainable and Economic High-Rate Algal Pond Wastewater Treatment System
Abstract
:1. Introduction
2. Materials and Methods
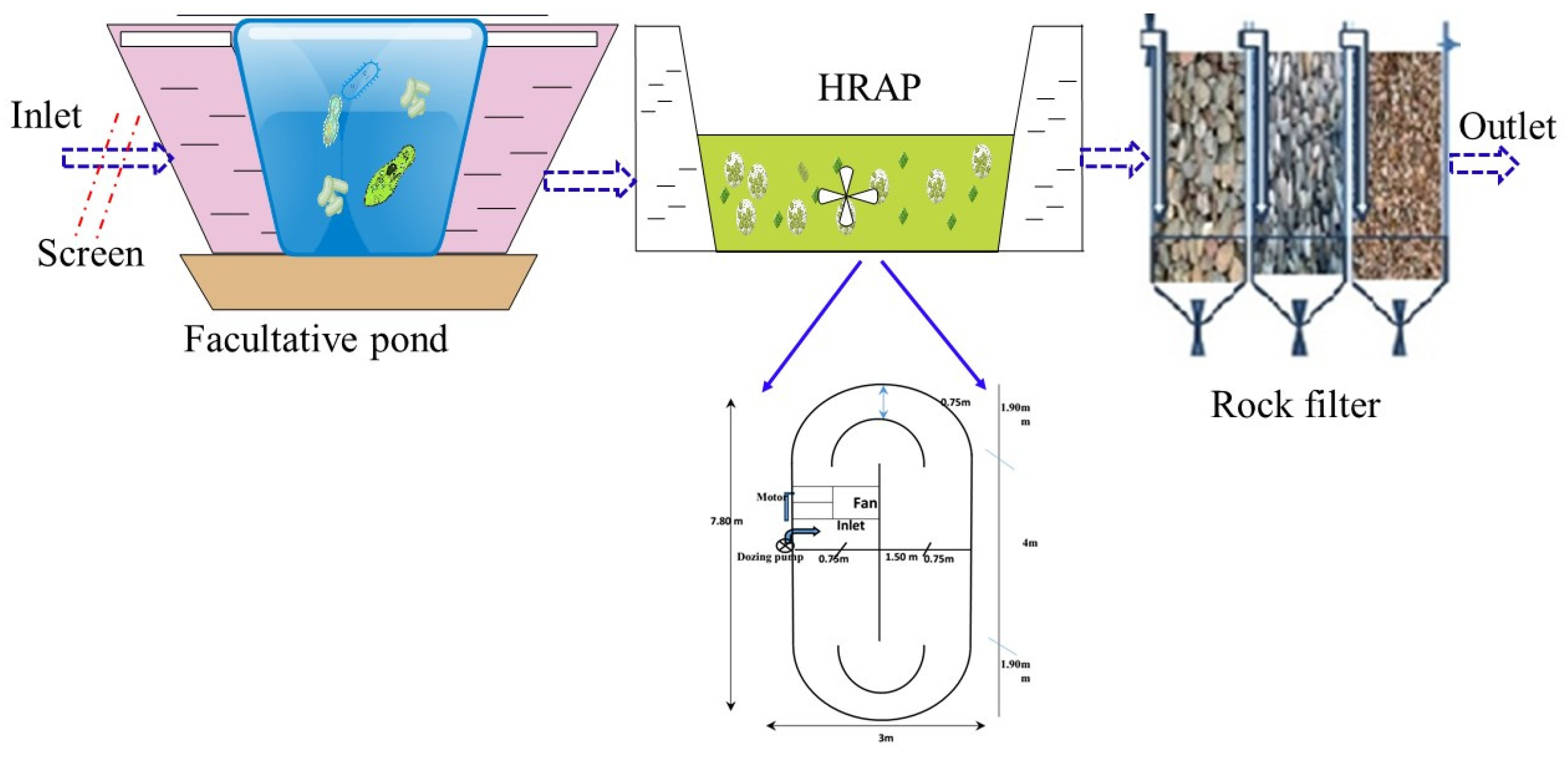
2.1. The Design of HRAP System and Samples Collection
2.2. Physicochemical, Bacteriological, and Algal Community Analysis
2.3. Virological and Parasitological Analyses
2.4. Statistical Analysis
3. Results and Discussion
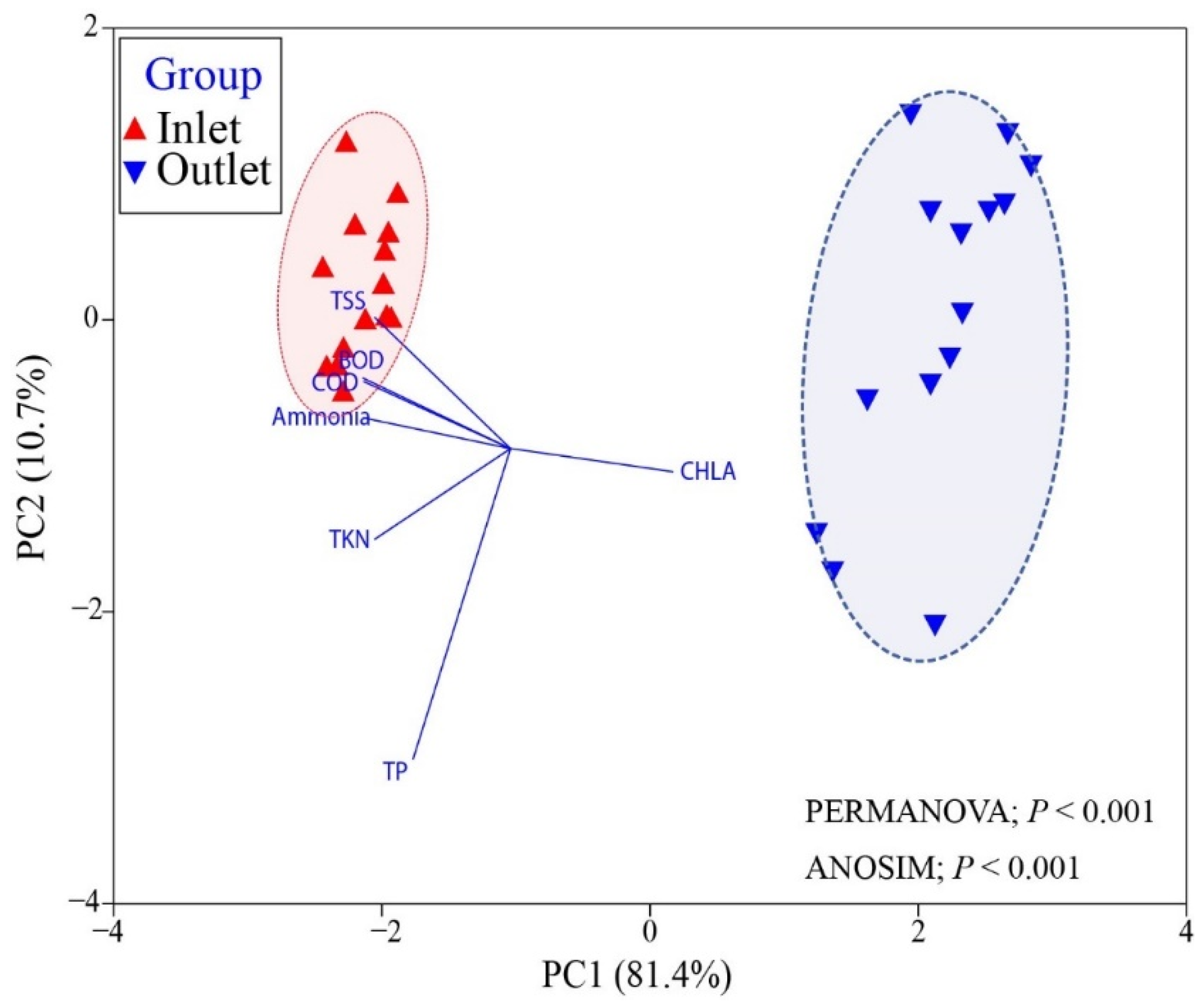
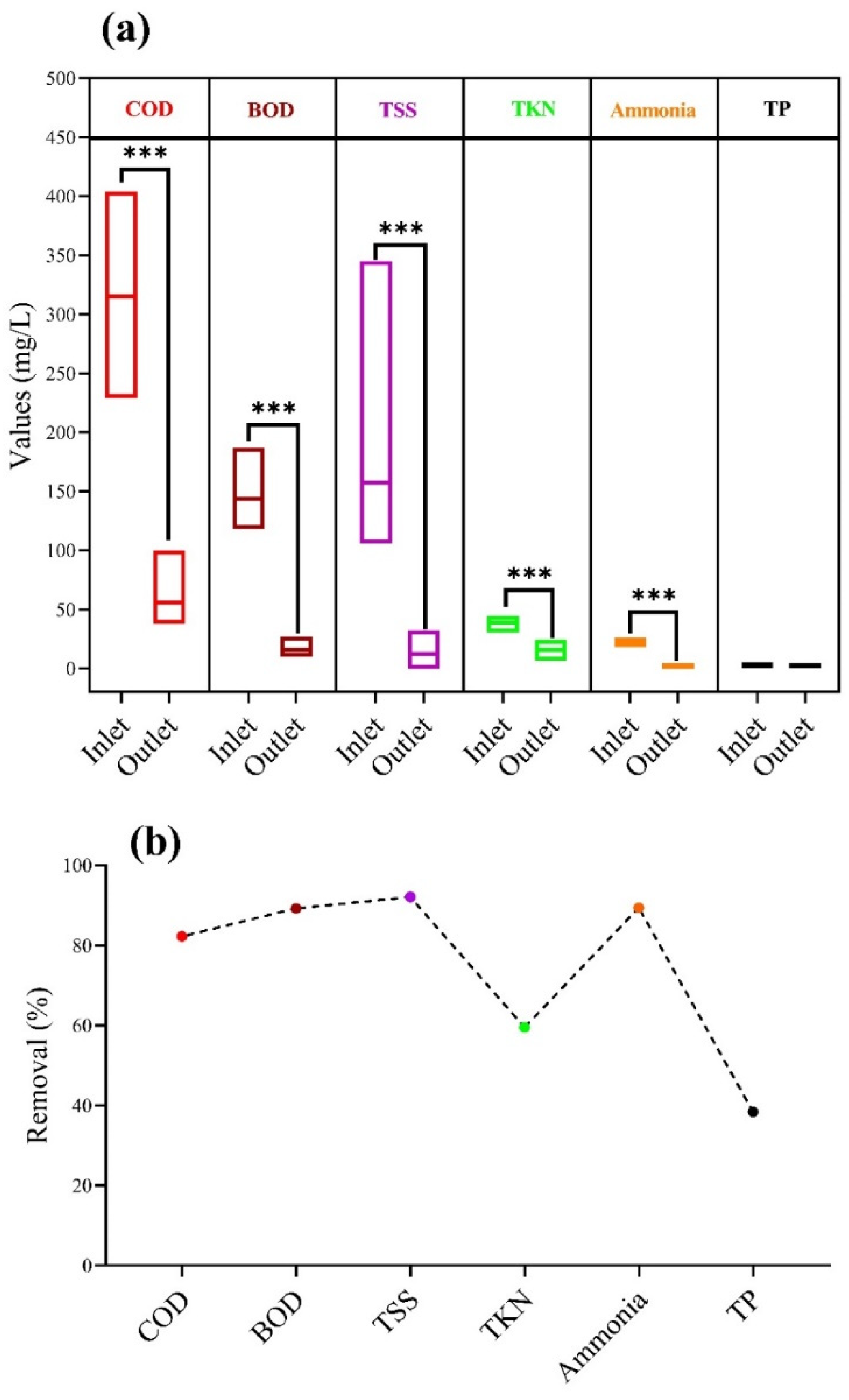
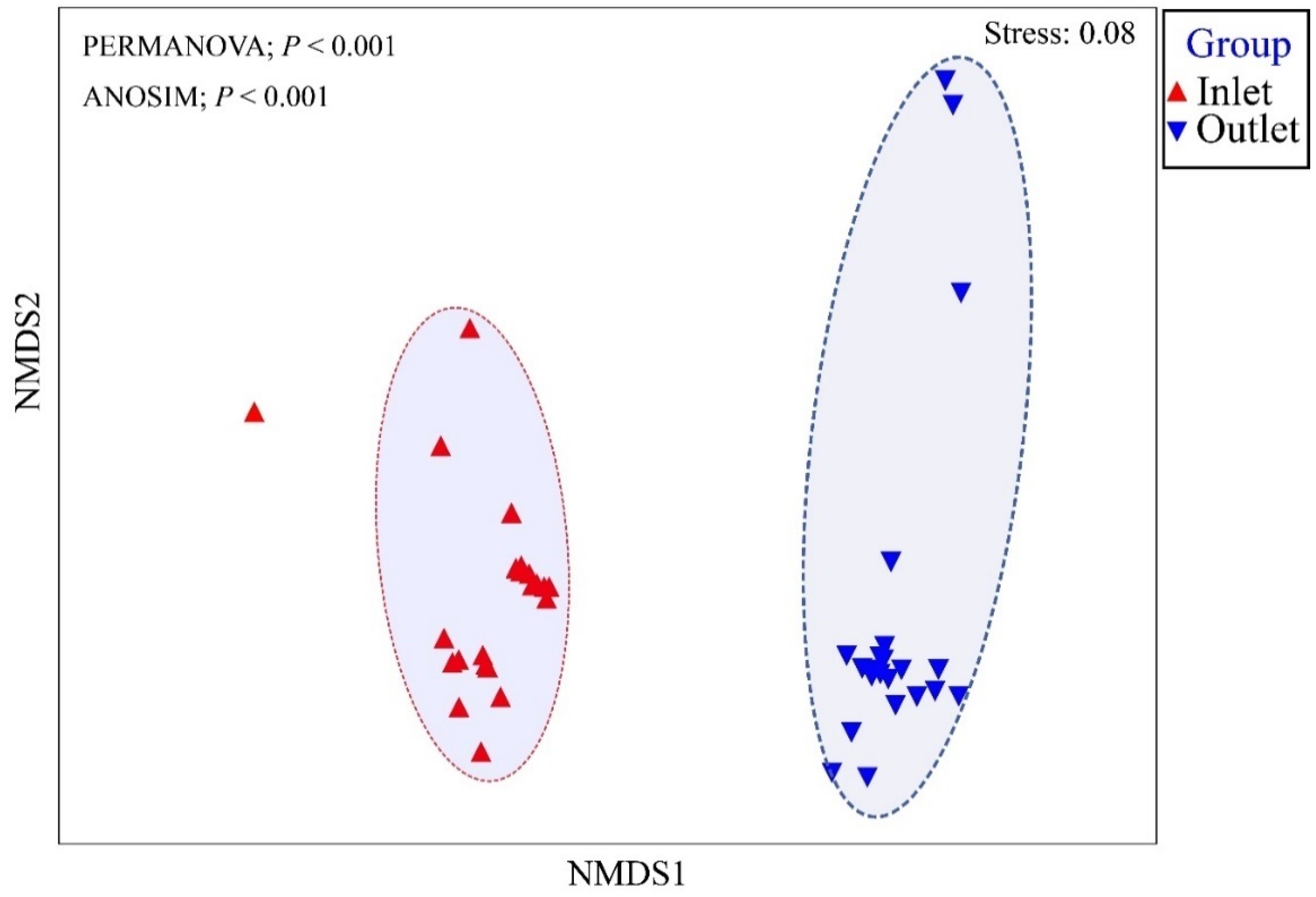
3.1. The Performance of the HRAP System
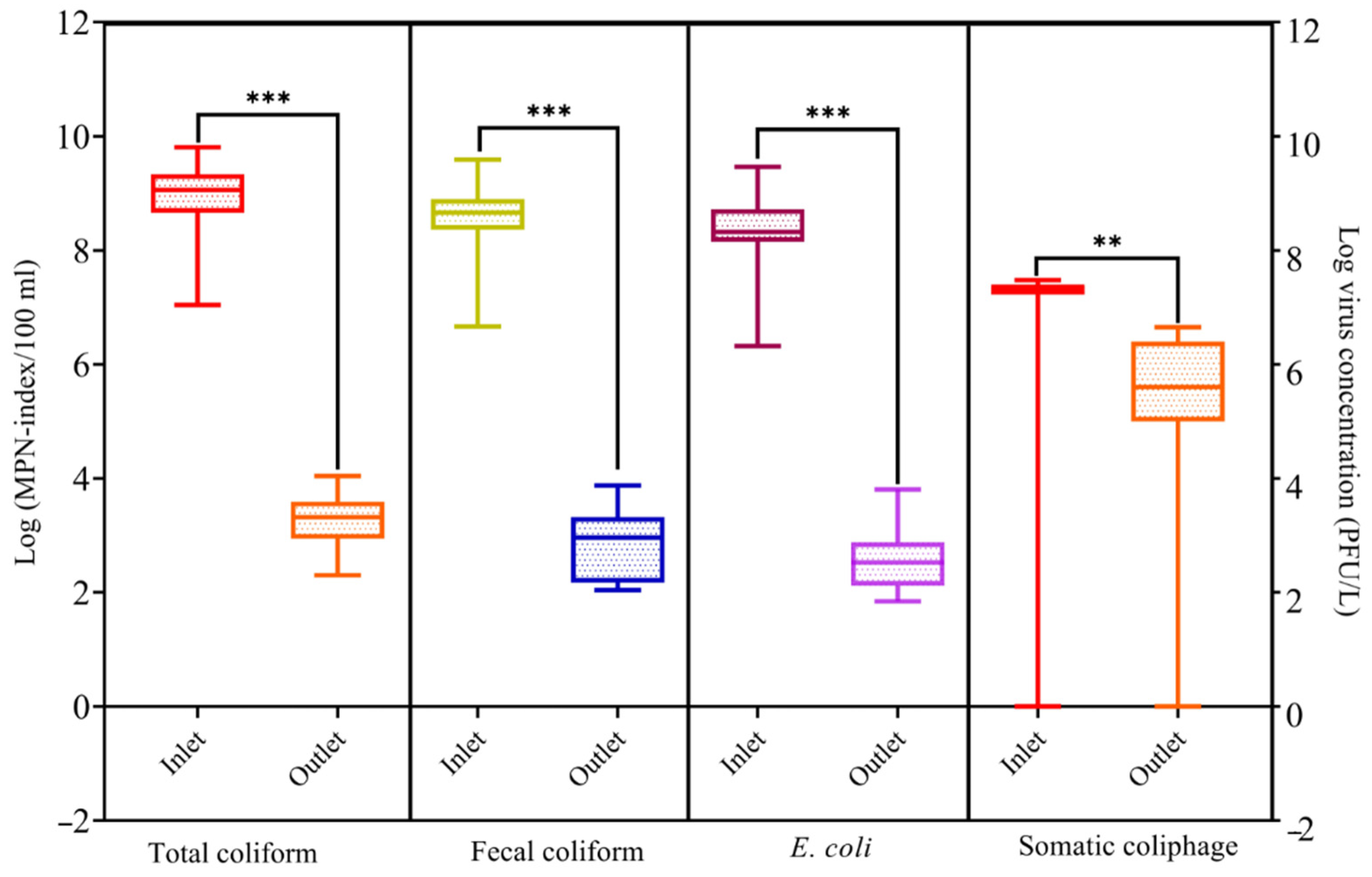
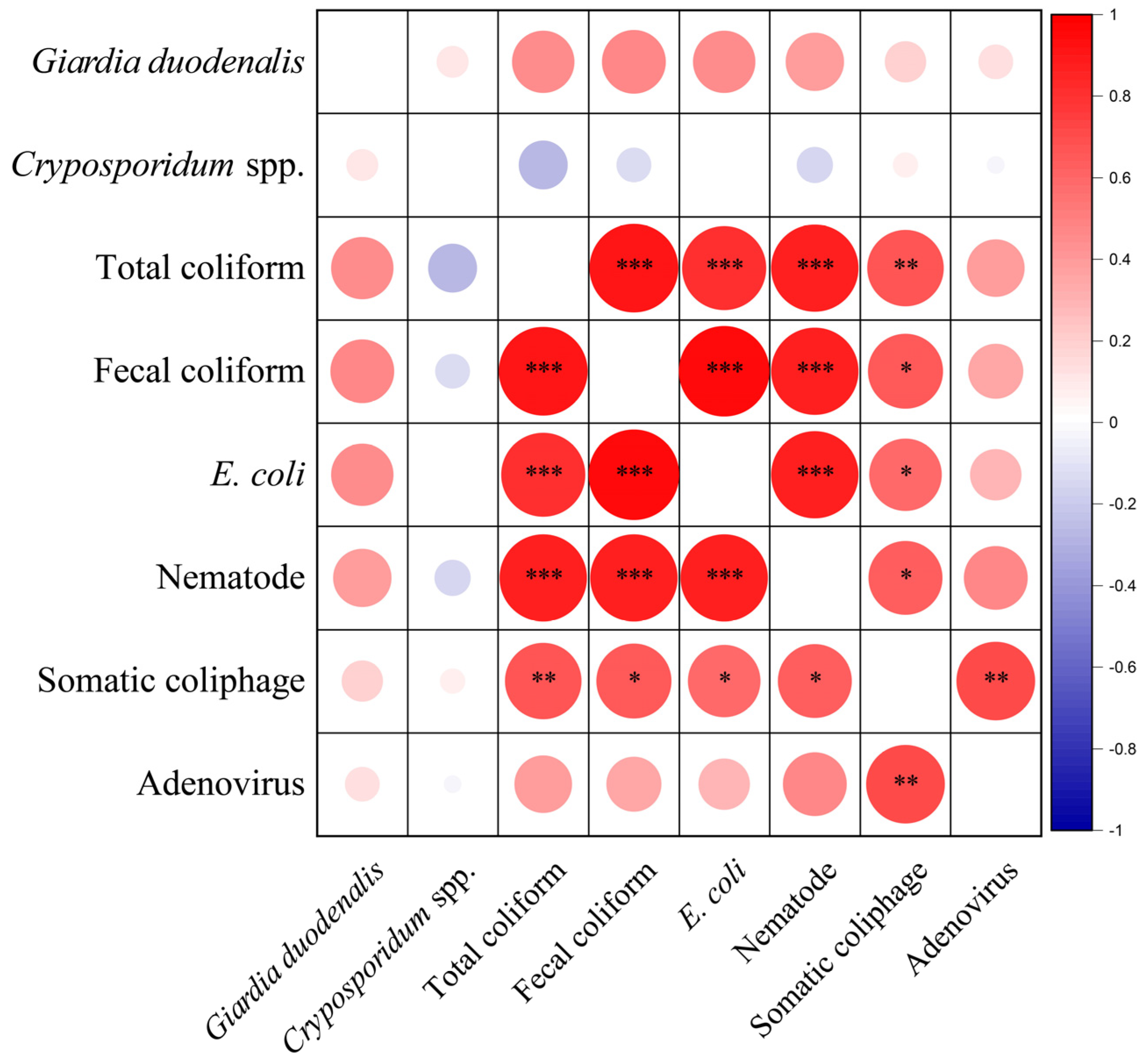
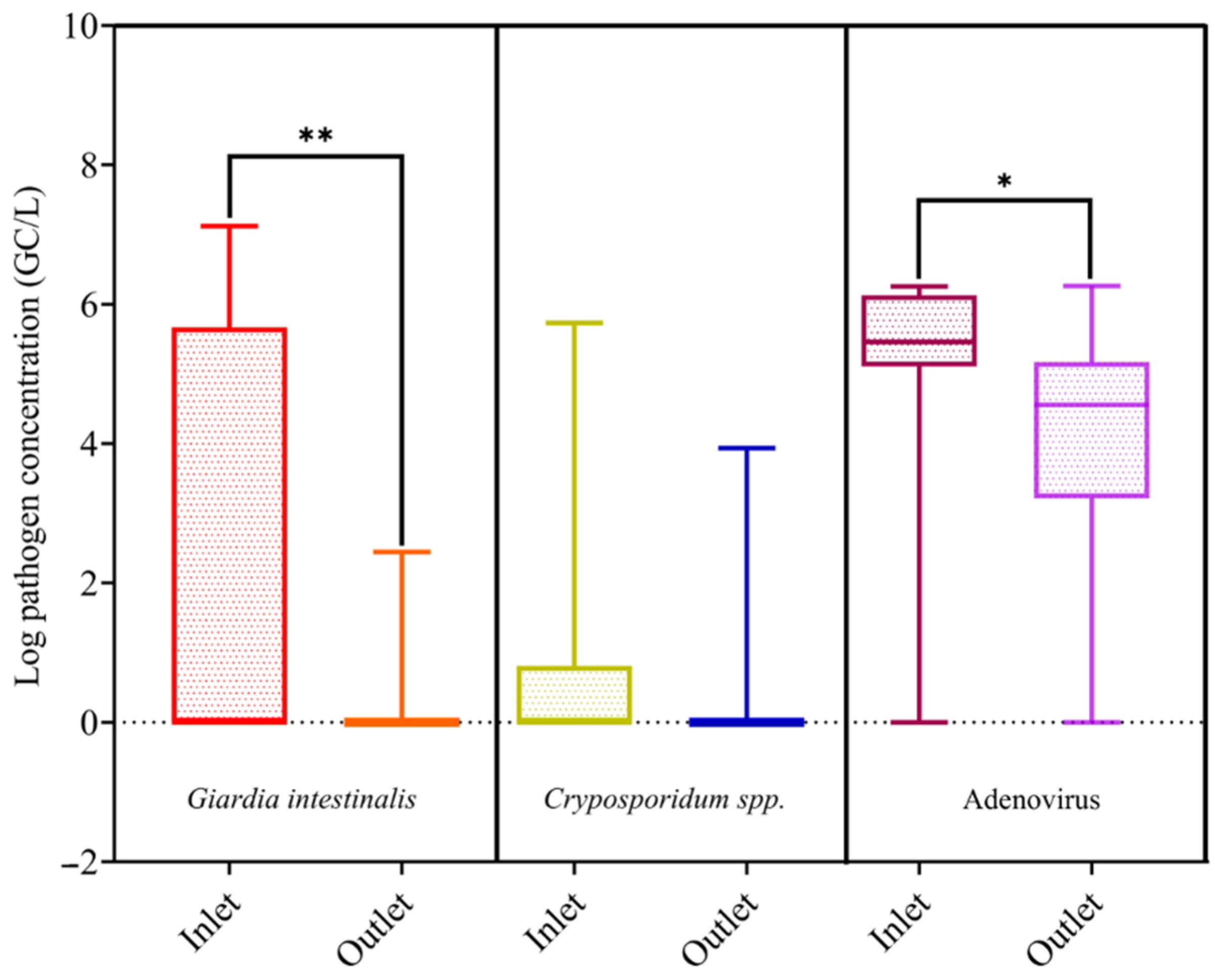
3.2. Pathogens Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Statistical Commission (UNSC). United Nations Technical Report by the Bureau of the United Nations Statistical Commission (UNSC) on the Process of the Development of an Indicator Framework for the Goals and Targets of the Post-2015 Development Agenda (Working Draft). 19 March 2015. Available online: https://sustainabledevelopment.un.org/index.php?page=view&type=111&nr=6754&menu=35 (accessed on 10 November 2021).
- United Nations. The Millennium Development Goals Report; United Nations: New York, NY, USA, 2015; p. 72. ISBN 978-92-1-101320-7. [Google Scholar]
- WHO. Sanitation Sheet Fact; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Baum, R.; Luh, J.; Bartram, J. Sanitation: A global estimate of sewerage connections without treatment and the resulting impact on MDG progress. Environ. Sci. Technol. 2013, 47, 1994–2000. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Blanch, A.R.; Campos, C.; Jofre, J.; Lucena, F. Pathogens, faecal indicators and human-specific microbial source-tracking markers in sewage. J. Appl. Microbiol. 2019, 126, 701–717. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: Design, performance and mechanism. Sci. Total Environ. 2014, 468–469, 908–932. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, Y.; Zhou, Q.; Vymazal, J. Effects of plant biomass on denitrifying genes in subsurface-flow constructed wetlands. Bioresour. Technol. 2014, 157, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, J.; Li, C.; Fan, J.; Zou, Y. Mass Balance Study on Phosphorus Removal in Constructed Wetland Microcosms Treating Polluted River Water. CLEAN Soil Air Water 2013, 41, 844–850. [Google Scholar] [CrossRef]
- Noyola, A.; Padilla-Rivera, A.; Morgan-Sagastume, J.M.; Güereca, L.P.; Hernández-Padilla, F. Typology of Municipal Wastewater Treatment Technologies in Latin America. CLEAN Soil Air Water 2012, 40, 926–932. [Google Scholar] [CrossRef]
- Verbyla, M.E. Pathogen Removal in Natural Wastewater Treatment and Resource Recovery Systems: Solutions for Small Cities in an Urbanizing World; University of South Florida: Tampa, FL, USA, 2015; ISBN 1339288516. [Google Scholar]
- Bhuyar, P.; Trejo, M.; Dussadee, N.; Unpaprom, Y.; Ramaraj, R.; Whangchai, K. Microalgae cultivation in wastewater effluent from tilapia culture pond for enhanced bioethanol production. Water Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Bhuyar, P.; Hong, D.D.; Mandia, E.; Rahim, M.H.A.; Maniam, G.P.; Govindan, N. Desalination of polymer and chemical industrial wastewater by using green photosynthetic microalgae, Chlorella sp. Maejo Int. J. Energy Environ. Commun. 2019, 1, 9–19. [Google Scholar] [CrossRef]
- Buchanan, N.A.; Young, P.; Cromar, N.J.; Fallowfield, H.J. Performance of a high rate algal pond treating septic tank effluent from a community wastewater management scheme in rural South Australia. Algal Res. 2018, 35, 325–332. [Google Scholar] [CrossRef]
- Fallowfield, H.J.; Young, P.; Taylor, M.J.; Buchanan, N.; Cromar, N.; Keegan, A.; Monis, P. Independent validation and regulatory agency approval for high rate algal ponds to treat wastewater from rural communities. Environ. Sci. Water Res. Technol. 2018, 4, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Vassalle, L.; Díez-Montero, R.; Machado, A.T.R.; Moreira, C.; Ferrer, I.; Mota, C.R.; Passos, F. Upflow anaerobic sludge blanket in microalgae-based sewage treatment: Co-digestion for improving biogas production. Bioresour. Technol. 2020, 300, 122677. [Google Scholar] [CrossRef] [PubMed]
- Young, P.; Buchanan, N.; Fallowfield, H.J. Inactivation of indicator organisms in wastewater treated by a high rate algal pond system. J. Appl. Microbiol. 2016, 121, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Alaton, I.; Olmez-Hanci, T. Green Technologies for Wastewater Treatment: Energy Recovery and Emerging Compounds Removal; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-94-007-1430-4. [Google Scholar]
- Muñoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Craggs, R.J. Advanced integrated wastewater ponds. In Pond Treatment Technology; IWA Scientific and Technical Report Series; IWA: London, UK, 2005; pp. 282–310. [Google Scholar]
- Mara, D. Domestic Wastewater Treatment in Developing Countries, Earthscan; James & James: London, UK, 2004; ISBN 1-84407-019-0. [Google Scholar]
- Araki, S.; Martın-Gomez, S.; Bécares, E.; De Luis-Calabuig, E.; Rojo-Vazquez, F. Effect of high-rate algal ponds on viability of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 2001, 67, 3322–3324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton, N.F.; Cromar, N.J.; Hallsworth, P.; Fallowfield, H.J. A review of the factors affecting sunlight inactivation of micro-organisms in waste stabilisation ponds: Preliminary results for enterococci. Water Sci. Technol. 2010, 61, 885–890. [Google Scholar] [CrossRef] [PubMed]
- El Hamouri, B.; Rami, A.; Vasel, J.-L. The reasons behind the performance superiority of a high rate algal pond over three facultative ponds in series. Water Sci. Technol. 2003, 48, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, N.; Cromar, N.; Bolton, N.; Fallowfield, H. Comparison of a high rate algal pond with a standard secondary facultative waste stabilisation pond in rural South Australia. In Proceedings of the 10th Specialised Conference on Small Water and Wastewater Treatment Systems, Venice, Italy, 18–22 April 2011; pp. 292–299. [Google Scholar]
- Espinosa, M.F.; Verbyla, M.E.; Vassalle, L.; Rosa-Machado, A.T.; Zhao, F.; Gaunin, A.; Mota, C.R. Reduction and partitioning of viral and bacterial indicators in a UASB reactor followed by high rate algal ponds treating domestic sewage. Sci. Total Environ. 2021, 760, 144309. [Google Scholar] [CrossRef]
- Picot, B.; Bahlaoui, A.; Moersidik, S.; Baleux, B.; Bontoux, J. Comparison of the purifying efficiency of high rate algal pond with stabilization pond. Water Sci. Technol. 1992, 25, 197–206. [Google Scholar] [CrossRef]
- Chambonniere, P.; Bronlund, J.; Guieysse, B. Pathogen removal in high-rate algae pond: State of the art and opportunities. Appl Phycol. 2021, 33, 1501–1511. [Google Scholar] [CrossRef]
- Hindiyeh, M.Y.; Al-Salem, S.; Malkawi, M.; Kofahi, A.; Al-Yaman, R.; Shugen, A. Integrated Guide to Sanitry Parasitology; World Health Organization: Geneva, Switzerland, 2004; pp. 1–124. [Google Scholar]
- Jamieson, R.; Gordon, R.; Joy, D.; Lee, H. Assessing microbial pollution of rural surface waters: A review of current watershed scale modeling approaches. Agric. Water Manag. 2004, 70, 1–17. [Google Scholar] [CrossRef]
- Boutilier, L.; Jamieson, R.; Gordon, R.; Lake, C.; Hart, W. Adsorption, sedimentation, and inactivation of E. coli within wastewater treatment wetlands. Water Res. 2009, 43, 4370–4380. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hall, G.; Champagne, P. The role of algae in the removal and inactivation of pathogenic indicator organisms in wastewater stabilization pond systems. Algal Res. 2020, 46, 101777. [Google Scholar] [CrossRef]
- Momba, M.; Ebdon, J.; Kamika, I.; Verbyla, M. Using indicators to assess microbial treatment and disinfection efficacy. In Global Water Pathogen Project; Michigan State University Press: East Lansing, MI, USA, 2019. [Google Scholar]
- Nasr, F.A.; Gad, M.A.; Al-Herrawy, A.Z.; Abdelfadil, A.S. Decentralized biological compact unit for the removal of parasitic helminth ova during sewage treatment. EnvironmentAsia 2019, 12, 178–186. [Google Scholar] [CrossRef]
- Rizk, N.M.; Hamza, I.A. Molecular Quantification of Human Bocavirus in Environmental Water Samples in Giza, Egypt. Egypt. J. Aquat. Biol. Fish. 2021, 25, 735–749. [Google Scholar] [CrossRef]
- Doma, H.S.; El-Liethy, M.A.; Abdo, S.M.; Ali, G.H. Potential of using high rate algal pond for algal biofuel production and wastewater treatment. Asian J. Chem. 2016, 28, 399. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D. APHA Standard Methods for Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 0875530478. [Google Scholar]
- Bhuyar, P.; Sundararaju, S.; Rahim, M.H.A.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Microalgae cultivation using palm oil mill effluent as growth medium for lipid production with the effect of CO2 supply and light intensity. Biomass Convers. Biorefinery 2021, 11, 1555–1563. [Google Scholar] [CrossRef]
- ISO 10705-2: Water Quality. Detection and Enumeration of Bacteriophages–Part 2: Enumeration of Somatic Coliphages; International Organization for Standardization: Geneva, Switzerland, 2000; pp. 1–16.
- Williams, F.P.; Stetler, R.E.; Safferman, R.S. USEPA Manual of Methods for Virology; U.S. Environmental Protection Agency: Washington, DC, USA, 2001; Volume 600, pp. 4–84. [Google Scholar]
- Moodley, P.; Archer, C.; Hawksworth, D. Standard Methods for the Recovery and Enumeration of Helminth Ova in Wastewater, Sludge, Compost and Urine–Diversion Waste in South Africa; Water Research Commission (WRC): Pretoria, South Africa, 2008; ISBN 9781770056480. [Google Scholar]
- Heim, A.; Ebnet, C.; Harste, G.; Pring-Åkerblom, P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 2003, 70, 228–239. [Google Scholar] [CrossRef]
- Haque, R.; Roy, S.; Siddique, A.; Mondal, U.; Rahman, S.M.M.; Mondal, D.; Houpt, E.; Petri, W.A. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am. J. Trop. Med. Hyg. 2007, 76, 713–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guy, R.A.; Payment, P.; Krull, U.J.; Horgen, P.A. Real-time PCR for quantification of Giardia and Cryptosporidium in environmental water samples and sewage. Appl. Environ. Microbiol. 2003, 69, 5178–5185. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.; Hou, L.; Li, J.; Wu, Y.; Rashid, A.; Chen, N.; Hu, A. Distinct mechanisms underlying the assembly of microeukaryotic generalists and specialists in an anthropogenically impacted river. Sci. Total Environ. 2020, 748, 1–12. [Google Scholar] [CrossRef]
- Reynolds, C.S. Environmental requirements and habitat preferences of phytoplankton: Chance and certainty in species selection. Bot. Mar. 2012, 55, 1–17. [Google Scholar] [CrossRef]
- Assemany, P.; Calijuri, M.L.; Couto, E.D.A.D.; de Souza, M.H.B.; Silva, N.C.; Santiago, A.; Castro, J. Algae/bacteria consortium in high rate ponds: Influence of solar radiation on the phytoplankton community. Ecol. Eng. 2015, 77, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Craggs, R.J.; Davies-Colley, R.J.; Tanner, C.C.; Sukias, J.P. Advanced pond system: Performance with high rate ponds of different depths and areas. Water Sci. Technol. 2003, 48, 259–267. [Google Scholar] [CrossRef]
- El Hafiane, F.; El Hamouri, B. Anaerobic reactor/high rate pond combined technology for sewage treatment in the Mediterranean area. Water Sci. Technol. 2005, 51, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, A.N. Comparing the Performance of a High Rate Algal Pond with a Waste Stabilisation Pond in Rural South Australia. Ph.D. Thesis, Flinders University, School of the Environment, Adelaide, SA, Australia, 2014. [Google Scholar]
- El Hamouri, B.; Jellal, J.; Outabiht, H.; Nebri, B.; Khallayoune, K.; Benkerroum, A.; Hajli, A.; Firadi, R. The performance of a high-rate algal pond in the Moroccan climate. Water Sci. Technol. 1995, 31, 67–74. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J. Wastewater treatment and algal production in high rate algal ponds with carbon dioxide addition. Water Sci. Technol. 2010, 633–639. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. Seasonal variation in light utilisation, biomass production and nutrient removal by wastewater microalgae in a full-scale high-rate algal pond. J. Appl. Phycol. 2014, 26, 1317–1329. [Google Scholar] [CrossRef]
- Santiago, A.F.; Calijuri, M.D.C.; Assemany, P.; Reis, A. Algal biomass production and wastewater treatment in high rate algal ponds receiving disinfected effluent. Environ. Technol. 2013, 34, 1877–1885. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Zhou, Q.; Paing, J.; Le, H.; Picot, B. Nutrient removal by the integrated use of high rate algal ponds and macrophyte systems in China. Water Sci. Technol. 2003, 48, 251–257. [Google Scholar] [CrossRef]
- El Hamouri, B. Rethinking natural, extensive systems for tertiary treatment purposes: The high-rate algae pond as an example. Desalination Water Treat. 2009, 4, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Young, P.; Taylor, M.; Fallowfield, H.J. Mini-review: High rate algal ponds, flexible systems for sustainable wastewater treatment. World J. Microbiol. Biotechnol. 2017, 33, 117. [Google Scholar] [CrossRef]
- Craggs, R.J.; Heubeck, S.; Lundquist, T.J.; Benemann, J.R. Algal biofuels from wastewater treatment high rate algal ponds. Water Sci. Technol. 2011, 63, 660–665. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Chambonniere, P.; Bronlund, J.; Guieysse, B. Escherichia coli removal during domestic wastewater treatment in outdoor high rate algae ponds: Long-term performance and mechanistic implications. Water Sci. Technol. 2020, 82, 1166–1175. [Google Scholar] [CrossRef]
- Harwood, V.; Shanks, O.; Koraijkic, A.; Verbyla, M.; Ahmed, W.; Iriate, M. General and host-associated bacterial indicators of faecal pollution. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management; Rose, J.B., Jiménez-Cisneros, B., Eds.; Michigan State University: East Lansing, MI, USA, 2017. [Google Scholar]
- WHO. Guidelines for the Safe Use of Wastewater, Excreta and Greywater: Vol. 1: Policy and Regulatory Aspects; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Fong, T.-T.; Griffin, D.W.; Lipp, E.K. Molecular assays for targeting human and bovine enteric viruses in coastal waters and their application for library-independent source tracking. Appl. Environ. Microbiol. 2005, 71, 2070–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hot, D.; Legeay, O.; Jacques, J.; Gantzer, C.; Caudrelier, Y.; Guyard, K.; Lange, M.; Andreoletti, L. Detection of somatic phages, infectious enteroviruses and enterovirus genomes as indicators of human enteric viral pollution in surface water. Water Res. 2003, 37, 4703–4710. [Google Scholar] [CrossRef]
- Ashbolt, N.J.; Grabow, W.O.K.; Snozzi, M. Indicators of microbial water quality. In Water Quality: Guidelines, Standards and Health; IWA Publishing: London, UK, 2001; pp. 289–316. [Google Scholar]
- Araki, S.; González, J.M.; De Luis, E.; Bécares, E. Viability of nematode eggs in high rate algal ponds: The effect of physico-chemical conditions. Water Sci. Technol. 2000, 42, 371–374. [Google Scholar] [CrossRef]
- Von Sperling, M.; Mascarenhas, L. Performance of very shallow ponds treating effluents from UASB reactors. Water Sci. Technol. 2005, 51, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.K.; Kazmi, A.A.; Chopra, A.K. Removal of fecal indicators and pathogens in a waste stabilization pond system treating municipal wastewater in India. Water Environ. Res. 2008, 80, 2111–2117. [Google Scholar] [CrossRef]
- Nelson, K.L.; Cisneros, B.J.; Tchobanoglous, G.; Darby, J.L. Sludge accumulation, characteristics, and pathogen inactivation in four primary waste stabilization ponds in central Mexico. Water Res. 2004, 38, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Sheludchenko, M.; Padovan, A.; Katouli, M.; Stratton, H. Removal of fecal indicators, pathogenic bacteria, Adenovirus, Cryptosporidium and Giardia (oo) cysts in waste stabilization ponds in Northern and Eastern Australia. Int. J. Environ. Res. Public Health 2016, 13, 96. [Google Scholar] [CrossRef] [Green Version]
- Armanious, A.; Aeppli, M.; Jacak, R.; Refardt, D.; Sigstam, T.; Kohn, T.; Sander, M. Viruses at Solid—Water Interfaces: A Systematic Assessment of Interactions Driving Adsorption. Environ. Sci. Technol. 2016, 50, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Voice, T.C.; Tarabara, V.V.; Xagoraraki, I. Sorption of human adenovirus to wastewater solids. J. Environ. Eng. 2018, 144, 6018008. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Craggs, R.J.; Park, J.; Sukias, J.P.S.; Nagels, J.W.; Stott, R. Virus removal in a pilot-scale “advanced” pond system as indicated by somatic and F-RNA bacteriophages. Water Sci. Technol. 2005, 51, 107–110. [Google Scholar] [CrossRef]
- Sinton, L.W.; Hall, C.H.; Lynch, P.A.; Davies-Colley, R.J. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 2002, 68, 1122–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattle, M.J.; Vione, D.; Kohn, T. Conceptual model and experimental framework to determine the contributions of direct and indirect photoreactions to the solar disinfection of MS2, phiX174, and adenovirus. Environ. Sci. Technol. 2015, 49, 334–342. [Google Scholar] [CrossRef]
- Kohn, T.; Mattle, M.J.; Minella, M.; Vione, D. A modeling approach to estimate the solar disinfection of viral indicator organisms in waste stabilization ponds and surface waters. Water Res. 2016, 88, 912–922. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence (5′-3′) | Reference | |
|---|---|---|---|
| Cryptosporidium spp. | COWP-F | CAAATTGATACCGTTTGTCCTTCTG | [42] |
| COWP-R | TGGTGCCATACATTGTTGTCCT | ||
| Adenovirus | Q1 | GCCACGGTGGGGTTTCTAAACTT | [41] |
| Q2 | GCCCCAGTGGTCTTACATGCACATC | ||
| Giardia duodenalis | β-Giardin P241-F | CATCCGCGAGGAGGTCAA | [43] |
| β-Giardin P241-R | GCAGCCATGGTGTCGATCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Elmaksoud, S.; Abdo, S.M.; Gad, M.; Hu, A.; El-Liethy, M.A.; Rizk, N.; Marouf, M.A.; Hamza, I.A.; Doma, H.S. Pathogens Removal in a Sustainable and Economic High-Rate Algal Pond Wastewater Treatment System. Sustainability 2021, 13, 13232. https://doi.org/10.3390/su132313232
Abd-Elmaksoud S, Abdo SM, Gad M, Hu A, El-Liethy MA, Rizk N, Marouf MA, Hamza IA, Doma HS. Pathogens Removal in a Sustainable and Economic High-Rate Algal Pond Wastewater Treatment System. Sustainability. 2021; 13(23):13232. https://doi.org/10.3390/su132313232
Chicago/Turabian StyleAbd-Elmaksoud, Sherif, Sayeda M. Abdo, Mahmoud Gad, Anyi Hu, Mohamed Azab El-Liethy, Neveen Rizk, Mohamed A. Marouf, Ibrahim A. Hamza, and Hala S. Doma. 2021. "Pathogens Removal in a Sustainable and Economic High-Rate Algal Pond Wastewater Treatment System" Sustainability 13, no. 23: 13232. https://doi.org/10.3390/su132313232
APA StyleAbd-Elmaksoud, S., Abdo, S. M., Gad, M., Hu, A., El-Liethy, M. A., Rizk, N., Marouf, M. A., Hamza, I. A., & Doma, H. S. (2021). Pathogens Removal in a Sustainable and Economic High-Rate Algal Pond Wastewater Treatment System. Sustainability, 13(23), 13232. https://doi.org/10.3390/su132313232






