Magnetoelastic Materials for Monitoring and Controlling Cells and Tissues
Abstract
:1. Introduction
2. ME System Fabrication and Design Considerations for In Vitro Applications
2.1. ME Substrate Materials
2.2. ME Substrate Geometry
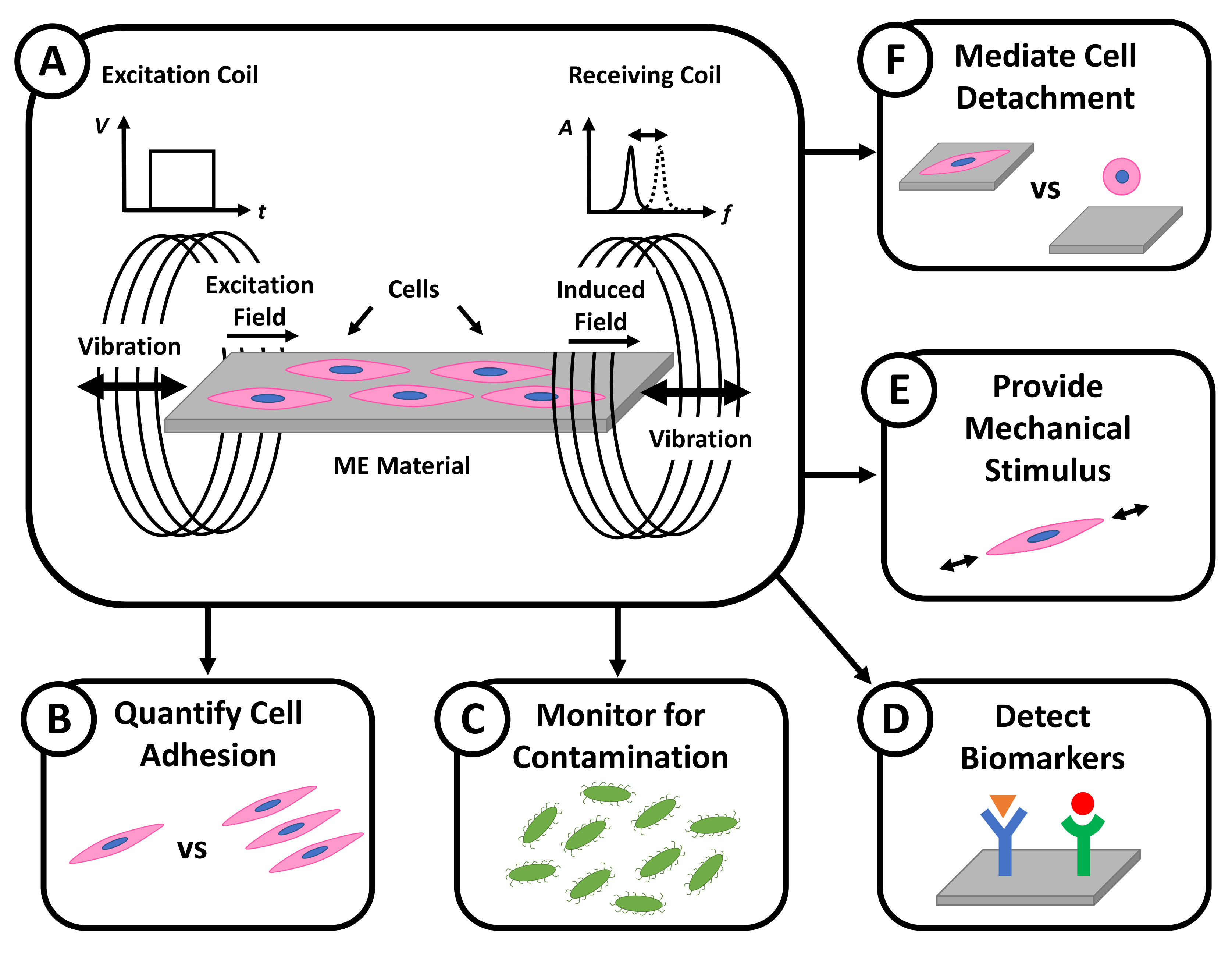
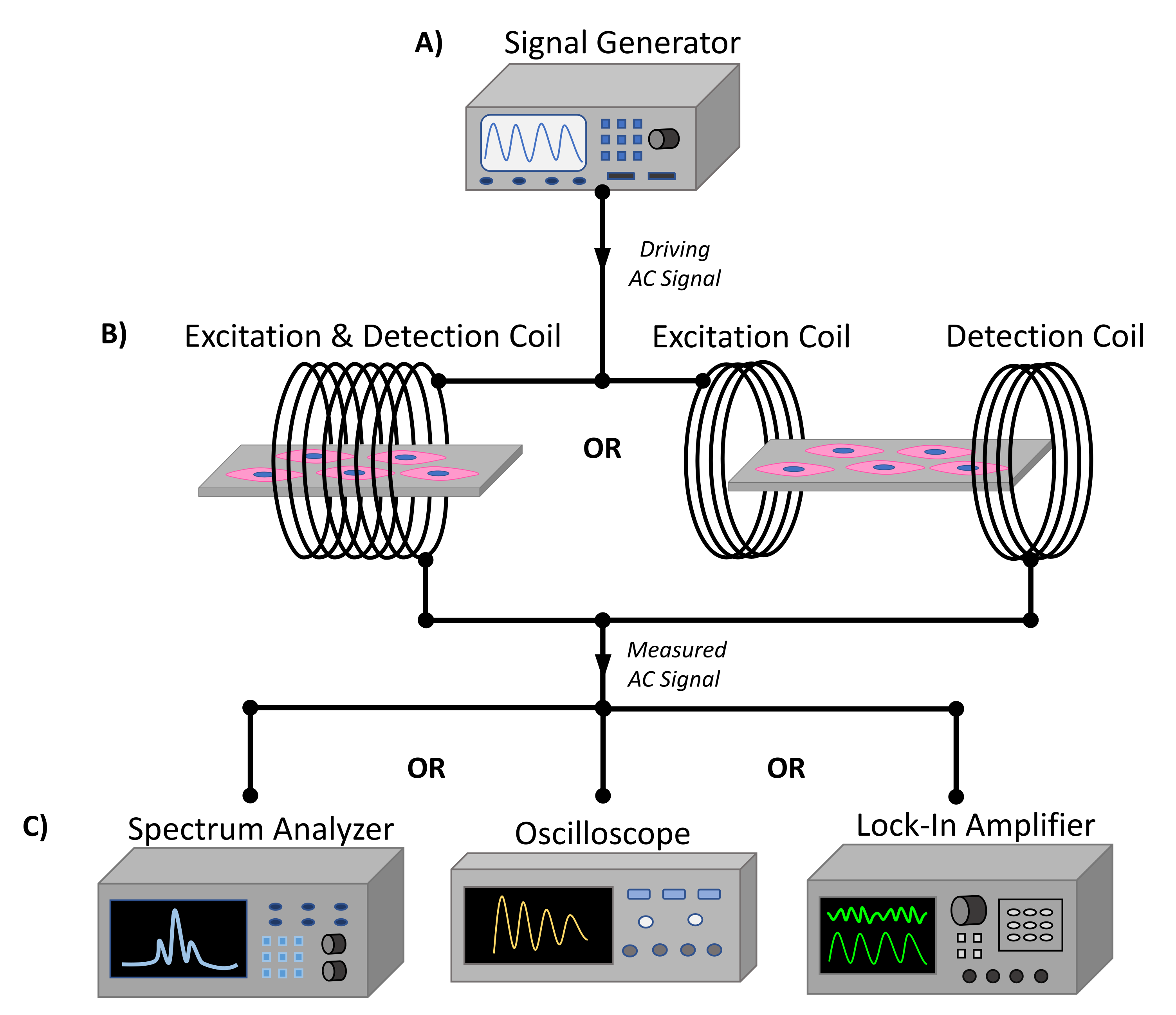
2.3. ME Excitation and Receiving System
2.4. Additional In Vitro Design Considerations
3. ME Materials for Cell Sensing
3.1. ME Material Sensing Mechanisms
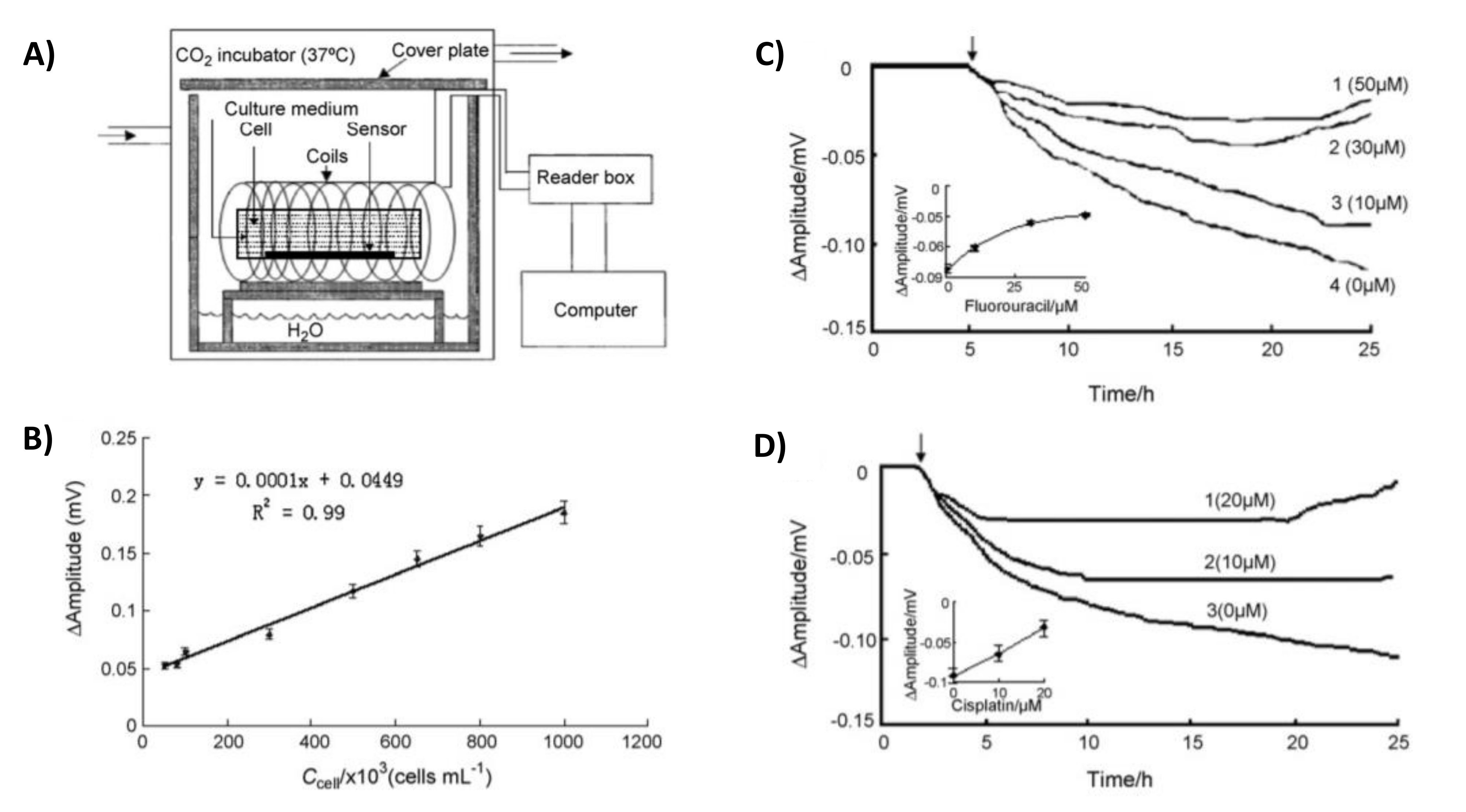
3.2. Sensing Mammalian Cells
3.3. Sensing Bacterial Cells
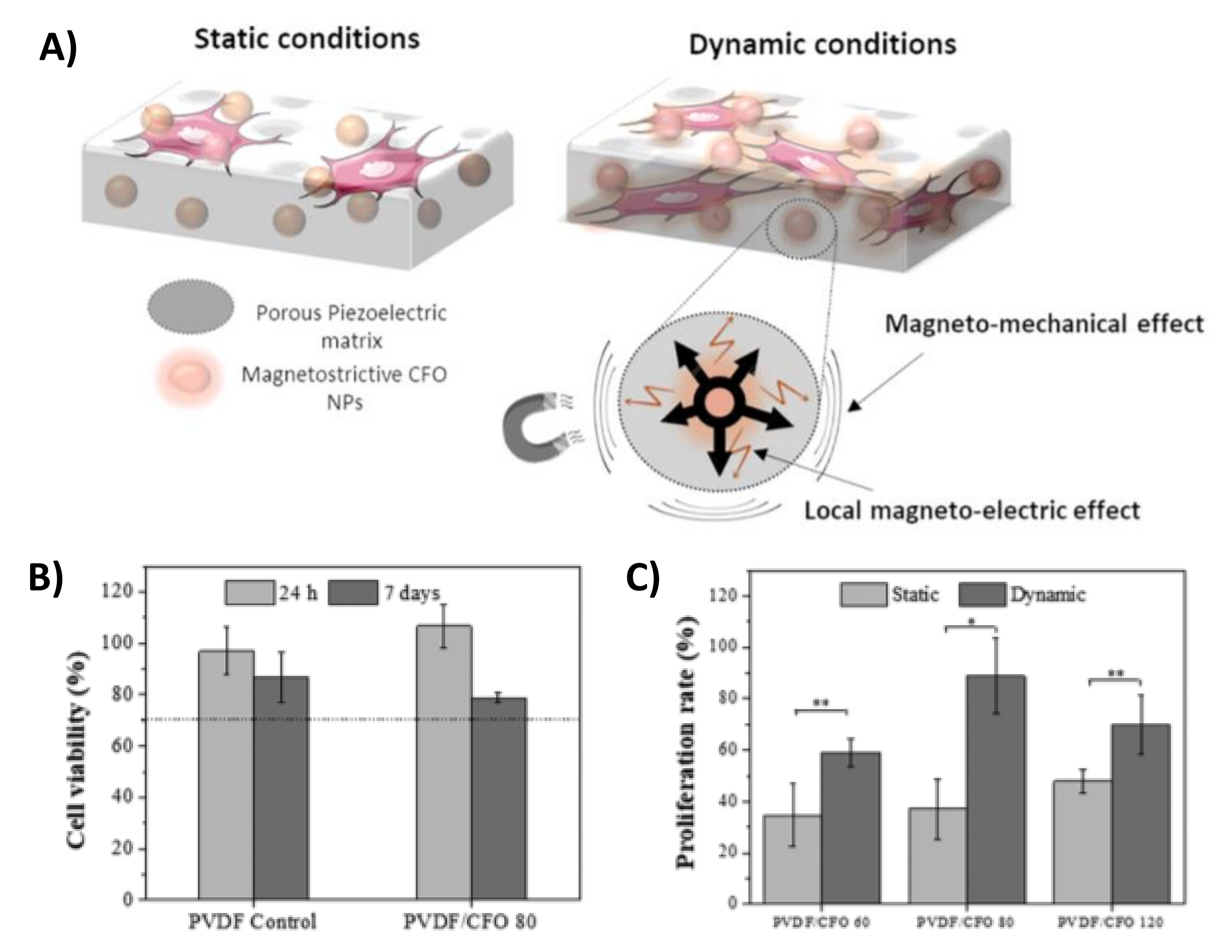
4. ME Materials for Cell Mechanical Stimulation
4.1. ME Material Vibration Mechanisms
4.2. Controlling Biomechanical Cues
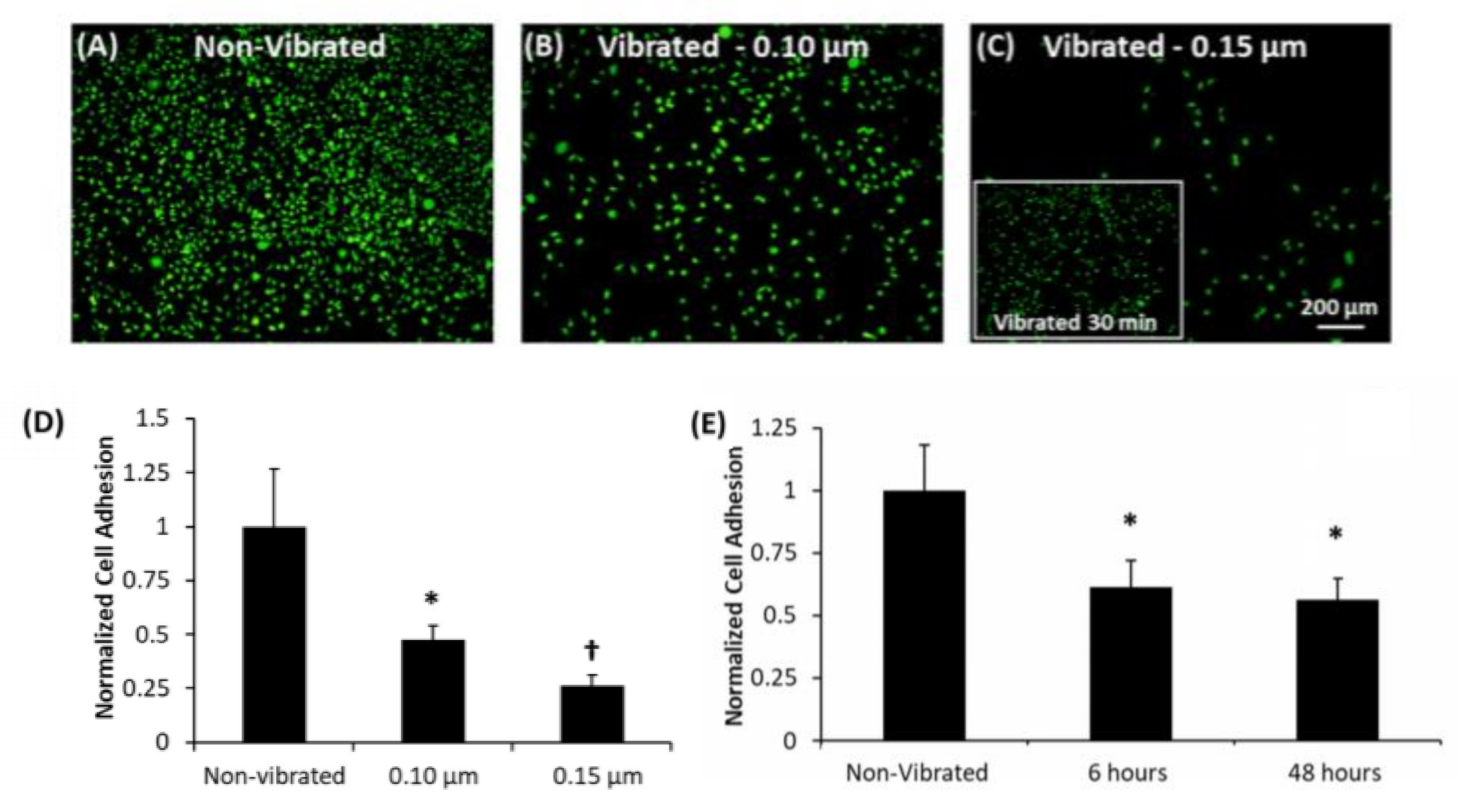
4.3. Controlling Cell Detachment
5. Future Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bastani, B. The present and future of transplant organ shortage: Some potential remedies. J. Nephrol. 2020, 33, 277–288. [Google Scholar] [CrossRef]
- Goradel, N.H.; Hour, F.G.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J. Cell. Biochem. 2018, 119, 95–104. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Robb, K.P.; Fitzgerald, J.C.; Barry, F.; Viswanathan, S. Mesenchymal stromal cell therapy: Progress in manufacturing and assessments of potency. Cytotherapy 2019, 21, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.; Farnia, S.; Kefalas, P.; Maziarz, R.T. Concise Review: The High Cost of High Tech Medicine: Planning Ahead for Market Access. Stem Cells Transl. Med. 2017, 6, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Galipeau, J.; MSC Committee of the International Society of Cell and Gene Therapy. Manufacturing mesenchymal stromal cells for clinical applications: A survey of Good Manufacturing Practices at U.S. academic centers. Cytotherapy 2019, 21, 782–792. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef] [Green Version]
- Pigeau, G.M.; Csaszar, E.; Dulgar-Tulloch, A. Commercial Scale Manufacturing of Allogeneic Cell Therapy. Front. Med. 2018, 5, 233. [Google Scholar] [CrossRef]
- Velasco-Garcia, M.N. Optical biosensors for probing at the cellular level: A review of recent progress and future prospects. Semin. Cell Dev. Biol. 2009, 20, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Couniot, N. Capacitive biosensing of bacterial cells: Analytical model and numerical simulations. Sens. Actuators B Chem. 2015, 211, 428–438. [Google Scholar] [CrossRef]
- Tan, E.L.; DeRouin, A.J.; Ong, K.G. Magnetoelastic-Harmonic Stress Sensors With Tunable Sensitivity. IEEE Sens. J. 2012, 12, 1878–1883. [Google Scholar] [CrossRef]
- Tan, E.L.; DeRouin, A.J.; Pereles, B.D.; Ong, K.G. Design, fabrication, and implementation of a wireless, passive implantable pressure sensor based on magnetic higher-order harmonic fields. Biosensors 2011, 1, 134–152. [Google Scholar] [CrossRef] [Green Version]
- Bras, Y.L. Magneto-Elastic Resonance: Principles, Modeling and Applications; IntechOpen: London, UK, 2017. [Google Scholar]
- Grimes, C. Wireless Magnetoelastic Resonance Sensors: A Critical Review. Sensors 2002, 2, 294–313. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Yu, K.; Tan, Y. Applications and Advances of Magnetoelastic Sensors in Biomedical Engineering: A Review. Materials 2019, 12, 1135. [Google Scholar] [CrossRef] [Green Version]
- Zeng, K. Wireless Magnetoelastic Physical, Chemical, and Biological Sensors. IEEE Trans. Magn. 2007, 43, 2358–2363. [Google Scholar] [CrossRef]
- D’Angelo, F.; Tiribuzi, R.; Armentano, I.; Kenny, J.M.; Martino, S.; Orlacchio, A. Mechanotransduction: Tuning stem cells fate. J. Funct. Biomater. 2011, 2, 67–87. [Google Scholar] [CrossRef] [Green Version]
- Holmes, H. Biodegradation and biocompatibility of mechanically active magnetoelastic materials. Smart Mater. Struct. 2014, 23, 095036. [Google Scholar] [CrossRef]
- Liu, R. High sensitivity detection of human serum albumin using a novel magnetoelastic immunosensor. J. Mater. Sci. 2019, 54, 9679–9688. [Google Scholar] [CrossRef]
- Beltrami, L.V.; Kunst, S.R.; Birriel, E.J.; Malfatti, C.D. Magnetoelastic biosensors: Corrosion protection of an FeNiMoB alloy from alkoxide precursors. Thin Solid Film. 2017, 624, 83–94. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Janka, L.P.; Ong, K.G.; Rajachar, R.M. Magnetoelastic Materials as Novel Bioactive Coatings for the Control of Cell Adhesion. IEEE Trans. Bio-Med. Eng. 2011, 58, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hu, J.; Wan, J.; Johnson, M.L.; Shu, H.; Chin, B.A. The effect of annealing and gold deposition on the performance of magnetoelastic biosensors. Mater. Sci. Eng. C 2008, 28, 380–386. [Google Scholar] [CrossRef]
- DeRouin, A.; Guillory, R.; He, W.L.; Frost, M.; Goldman, J.; Ong, K.G. Magnetoelastic galfenol as a stent material for wirelessly controlled degradation rates. J. Biomed. Mater. Res. B 2019, 107, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Menti, C.; Beltrami, M.; Possan, A.L.; Martins, S.T.; Henriques, J.A.P.; Santos, A.D.; Missell, F.P.; Roesch-Ely, M. Biocompatibility and degradation of gold-covered magneto-elastic biosensors exposed to cell culture. Colloids Surf. B Biointerfaces 2016, 143, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Golda-Cepa, M.; Engvall, K.; Hakkarainen, M.; Kotarba, A. Recent progress on parylene C polymer for biomedical applications: A review. Prog. Org. Coat. 2020, 140, 105493. [Google Scholar] [CrossRef]
- Das, A.M.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Huang, S.J.; Wang, Y.J.; Ge, S.T.; Cai, Q.Y.; Grimes, C.A. Quantification of Staphylococcus epidermidis using a wireless, mass-responsive sensor. Sens. Actuators B Chem. 2010, 150, 412–416. [Google Scholar] [CrossRef]
- Pang, P.; Cai, Q.; Yao, S.; Grimes, C.A. The detection of Mycobacterium tuberculosis in sputum sample based on a wireless magnetoelastic-sensing device. Talanta 2008, 76, 360–364. [Google Scholar] [CrossRef]
- Holmes, H.R.; Tan, E.L.; Ong, K.G.; Rajachar, R.M. Fabrication of biocompatible, vibrational magnetoelastic materials for controlling cellular adhesion. Biosensors 2012, 2, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Golda, M.; Brzychczy-Wloch, M.; Faryna, M.; Engvall, K.; Kotarba, A. Oxygen plasma functionalization of parylene C coating for implants surface: Nanotopography and active sites for drug anchoring. Mater. Sci. Eng. C 2013, 33, 4221–4227. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Kulig, W.; Cwiklik, L.; Kotarba, A. Molecular Dynamics Insights into Water-Parylene C Interface: Relevance of Oxygen Plasma Treatment for Biocompatibility. ACS Appl. Mater. Interfaces 2017, 9, 16685–16693. [Google Scholar] [CrossRef]
- Lakshmanan, R.S.; Guntupalli, R.; Hu, J.; Petrenko, V.A.; Barbaree, J.M.; Chin, B.A. Detection of Salmonella typhimurium in fat free milk using a phage immobilized magnetoelastic sensor. Sens. Actuators B Chem. 2007, 126, 544–550. [Google Scholar] [CrossRef]
- Pang, P. Determination of glucose using bienzyme layered assembly magnetoelastic sensing device. Sens. Actuators B Chem. 2009, 136, 310–314. [Google Scholar] [CrossRef]
- Grimes, C.A.; Roy, S.C.; Rani, S.; Cai, Q. Theory, instrumentation and applications of magnetoelastic resonance sensors: A review. Sensors 2011, 11, 2809–2844. [Google Scholar] [CrossRef]
- Shen, W.; Li, S.Q.; Park, M.K.; Zhang, Z.W.; Cheng, Z.Y.; Petrenko, V.A.; Chin, B.A. Blocking Agent Optimization for Nonspecific Binding on Phage Based Magnetoelastic Biosensors. J. Electrochem. Soc. 2012, 159, B818–B823. [Google Scholar] [CrossRef]
- Riquelme, M.V. Optimizing blocking of nonspecific bacterial attachment to impedimetric biosensors. Sens. Bio-Sens. Res. 2016, 8, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [Green Version]
- Park, M.K.; Chin, B.A. Novel Approach of a Phage-Based Magnetoelastic Biosensor for the Detection of Salmonella enterica serovar Typhimurium in Soil. J. Microbiol. Biotechnol. 2016, 26, 2051–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekhar, S.; Karipott, S.S.; Guldberg, R.E.; Ong, K.G. Magnetoelastic sensors for real-time tracking of cell growth. Biotechnol. Bioeng. 2021, 118, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Guo, M.; Li, Q.; Cai, Q.; Yao, S.; Grimes, C.A. In-situ monitoring of breast cancer cell (MCF-7) growth and quantification of the cytotoxicity of anticancer drugs fluorouracil and cisplatin. Biosens. Bioelectron. 2008, 24, 247–252. [Google Scholar] [CrossRef]
- Guntupalli, R.; Hu, J.; Lakshmanan, R.S.; Huang, T.S.; Barbaree, J.M.; Chin, B.A. A magnetoelastic resonance biosensor immobilized with polyclonal antibody for the detection of Salmonella typhimurium. Biosens. Bioelectron. 2007, 22, 1474–1479. [Google Scholar] [CrossRef]
- Guntupalli, R.; Lakshmanan, R.S.; Hu, J.; Huang, T.S.; Barbaree, J.M.; Vodyanoy, V.; Chin, B.A. Rapid and sensitive magnetoelastic biosensors for the detection of Salmonella typhimurium in a mixed microbial population. J. Microbiol. Methods 2007, 70, 112–118. [Google Scholar] [CrossRef]
- Ruan, C.; Zeng, K.; Varghese, O.K.; Grimes, C.A. Magnetoelastic immunosensors: Amplified mass immunosorbent assay for detection of Escherichia coli O157:H7. Anal. Chem. 2003, 75, 6494–6498. [Google Scholar] [CrossRef]
- Pang, P.; Huang, S.; Cai, Q.; Yao, S.; Zeng, K.; Grimes, C.A. Detection of Pseudomonas aeruginosa using a wireless magnetoelastic sensing device. Biosens. Bioelectron. 2007, 23, 295–299. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Correia, D.M.; Ribeiro, C.; Castro, N.; Correia, V.; Lanceros-Mendez, S. Bioinspired Three-Dimensional Magnetoactive Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 45265–45275. [Google Scholar] [CrossRef]
- Popa, E.G.; Santo, V.E.; Rodrigues, M.T.; Gomes, M.E. Magnetically-Responsive Hydrogels for Modulation of Chondrogenic Commitment of Human Adipose-Derived Stem Cells. Polymers 2016, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Holmes, H.R.; Vlaisavljevich, E.; Tan, E.L.; Snyder, K.L.; Ong, K.G.; Rajachar, R.M. Control of cellular adhesion and myofibroblastic character with sub-micrometer magnetoelastic vibrations. J. Biomech. 2018, 71, 199–207. [Google Scholar] [CrossRef]
- Paces, W.R.; Holmes, H.R.; Vlaisavljevich, E.; Snyder, K.L.; Tan, E.L.; Rajachar, R.M.; Ong, K.G. Application of sub-micrometer vibrations to mitigate bacterial adhesion. J. Funct. Biomater. 2014, 5, 15–26. [Google Scholar] [CrossRef]
- DeRouin, A. Multi-parameter sensing with a single magnetoelastic sensor by applying loads on the null locations of multiple resonant modes. Smart Mater. Struct. 2016, 25, 035044. [Google Scholar] [CrossRef]
- Pacella, N. Geometrical modification of magnetoelastic sensors to enhance sensitivity. Smart Mater. Struct. 2015, 24, 025018. [Google Scholar] [CrossRef]
- Karipott, S. An Embedded Wireless Temperature Sensor for Orthopedic Implants. IEEE Sens. J. 2018, 18, 1265–1272. [Google Scholar] [CrossRef]
- Ong, K.G. Control of a Magnetoelastic Sensor Temperature Response by Magnetic Field Tuning. IEEE Trans. Magn. 2003, 39, 3319–3321. [Google Scholar] [CrossRef]
- Ong, K.G. Quantification of Multiple Bioagents With Wireless, Remote-Query Magnetoelastic Microsensors. IEEE Sens. J. 2006, 6, 514–523. [Google Scholar] [CrossRef]
- Horikawa, S.; Bedi, D.; Li, S.; Shen, W.; Huang, S.; Chen, I.H.; Chai, Y.; Auad, M.L.; Bozack, M.J.; Barbaree, J.M.; et al. Effects of surface functionalization on the surface phage coverage and the subsequent performance of phage-immobilized magnetoelastic biosensors. Biosens. Bioelectron. 2011, 26, 2361–2367. [Google Scholar] [CrossRef]
- Wang, C.; Shan, S.; Wang, C.; Wang, J.; Li, J.; Hu, G.; Dai, K.; Li, Q.; Zhang, X. Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Exp. Cell Res. 2017, 352, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Delaine-Smith, R.M.; Reilly, G.C. Mesenchymal stem cell responses to mechanical stimuli. Muscles Ligaments Tendons J. 2012, 2, 169–180. [Google Scholar]
- Chen, J.C.; Jacobs, C.R. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res. Ther. 2013, 4, 107. [Google Scholar] [CrossRef] [Green Version]
- Brito-Pereira, R. Silk fibroin-magnetic hybrid composite electrospun fibers for tissue engineering applications. Compos. Part B 2018, 141, 70–75. [Google Scholar] [CrossRef]
- De Stefano, D.; Carnuccio, R.; Maiuri, M.C. Nanomaterials toxicity and cell death modalities. J. Drug Deliv. 2012, 2012, 167896. [Google Scholar] [CrossRef] [Green Version]
| Intended Use | Cell Type | ME Material | Functionalization Method | Detection Range | Reference |
|---|---|---|---|---|---|
| Sensor | L929 fibroblasts | Metglas 2826MB | Parylene and plasma etching | 10 × 103–75 × 103 cells/sensor | [39] |
| Human breast cancer cells (MCF-7) | Metglas 2826MB | Bayhydrol 110 | 50 × 103–1 × 106 cells/mL | [40] | |
| Salmonella typhimurium | Metglas 2826MB | Chromium, gold, and rabbit polyclonal antibody to S. typhimurium | 105–109 colony forming units/mL | [41,42] | |
| Escherichia coli | Metglas 2826MB | Gold and anti-E. coli antibodies | 102–106 cells/mL | [43] | |
| Pseudomonas aeruginosa | Metglas 2826MB | Bayhydrol 110 | 103–106 cells/mL | [44] | |
| Actuator | MC3T3 preosteoblasts | Cobalt ferrite | Polyvinylidene fluoride scaffold | [45] | |
| Human adipose derived stem cells | Magnetite | Kappa-carrageenan hydrogel | [46] | ||
| L929 fibroblasts | Metglas 2826MB | Parylene and plasma etching | [29,47] | ||
| Escherichia coli, Staphylococcus aureus, and Staphylococcus epidermidis | Metglas 2826MB | Parylene and plasma etching | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyers, K.M.; Ong, K.G. Magnetoelastic Materials for Monitoring and Controlling Cells and Tissues. Sustainability 2021, 13, 13655. https://doi.org/10.3390/su132413655
Meyers KM, Ong KG. Magnetoelastic Materials for Monitoring and Controlling Cells and Tissues. Sustainability. 2021; 13(24):13655. https://doi.org/10.3390/su132413655
Chicago/Turabian StyleMeyers, Kaylee Marie, and Keat Ghee Ong. 2021. "Magnetoelastic Materials for Monitoring and Controlling Cells and Tissues" Sustainability 13, no. 24: 13655. https://doi.org/10.3390/su132413655
APA StyleMeyers, K. M., & Ong, K. G. (2021). Magnetoelastic Materials for Monitoring and Controlling Cells and Tissues. Sustainability, 13(24), 13655. https://doi.org/10.3390/su132413655







