Levulinic Acid Production from Macroalgae: Production and Promising Potential in Industry
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Use of Levulinic Acid in Industry
3.2. Chemical Structure of Levulinic Acid (LA)
3.3. Levulinic Acid Production from Macroalgae
3.4. Biochemical of Potential Macroalgae Candidate
3.4.1. Pretreatment
3.4.2. Hydrolysis
Acid Hydrolysis
Enzymatic Hydrolysis
4. Future Needs and Challenges
4.1. Technology to Optimize the Conversion of Carbohydrates into Monosugar
4.2. Cultivation Technology
4.3. The Drawback of LA Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries-Industrial Processes and Products. In Ullmann’s Encycl. Ind. Chem; Wiley-VCH: Weinheim, Germany, 2016; pp. 1–38. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Kumar, B.R.; Mathimani, T.; Arunkumar, K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 2019, 228, 1320–1333. [Google Scholar] [CrossRef]
- Leal Silva, J.F.; Grekin, R.; Mariano, A.P.; Maciel Filho, R. Making Levulinic Acid and Ethyl Levulinate Economically Viable: A Worldwide Technoeconomic and Environmental Assessment of Possible Routes. Energy Technol. 2018, 6, 613–639. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I: Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar]
- Yan, L.; Yao, Q.; Fu, Y. Conversion of levulinic acid and alkyl levulinates into biofuels and high-value chemicals. Green Chem. 2017, 19, 5527–5547. [Google Scholar] [CrossRef]
- Chakraborti, T.; Desouza, A.; Adhikari, J. Prediction of Thermodynamic Properties of Levulinic Acid via Molecular Simulation Techniques. ACS Omega 2018, 37, 18877–18884. [Google Scholar] [CrossRef]
- Park, M.R.; Kim, S.K.; Jeong, G.T. Optimization of the levulinic acid production from the red macroalga, Gracilaria verrucosa using methanesulfonic acid. Algal Res. 2018, 31, 116–121. [Google Scholar] [CrossRef]
- Ghorpade, V.; Hanna, M. Industrial Application for Levulinic Acid. In Cereals: Novel Uses and Processes; Campbell, G.M., Webb, C., McKee, S.L., Eds.; Plenum Press: New York, NY, USA, 1997; pp. 49–55. [Google Scholar]
- Gao, G.; Burgess, J.G.; Wu, M.; Wang, S.; Gao, K. Using macroalgae as biofuel: Current opportunities and challenges. Bot. Mar. 2020, 63, 355–370. [Google Scholar] [CrossRef]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A review of macroalgae production, with potential applications in biofuels and bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Marhaeni, B.; Jeong, G.T.; Hong, Y.K. Sequential acid and enzymatic hydrolysis of carrageenan solid waste for bioethanol production: A biorefinery approach. J. Appl. Phycol. 2019, 31, 2507–2515. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transformation of levulinic acid: A platform for speciality chemicals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Wilson, L.D.; Bhanage, B.M. Recent advances for sustainable production of levulinic acid in ionic liquids from biomass: Current scenario, opportunities and challenges. Renew. Sustain. Energy Rev. 2019, 102, 266–284. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, S.K.; Hong, Y.K.; Jeong, G.T. Optimization of the production of platform chemicals and sugars from the red macroalga, Kappaphycus alvarezii. Algal Res. 2016, 13, 303–310. [Google Scholar] [CrossRef]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Galletti, A.M.R. New frontiers in the catalytic synthesis of levulinic acid: From sugars to raw and waste biomass as starting feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Cheng, J.J. Pretreatment of corn stover for sugar production with switchgrass-derived black liquor. Bioresour. Technol. 2012, 111, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Misurcova, L. Isolation and Chemical Properties of Molecules Derived from Seaweeds. Chemical Composition of Seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 171–192. [Google Scholar]
- Naseri, A.; Jacobsen, C.; Sejberg, J.J.P.; Pedersen, T.E.; Larsen, J.; Hansen, K.M.; Holdt, S.L. Multi-Extraction and Quality of Protein and Carrageenan from Commercial Spinosum (Eucheuma denticulatum). Foods 2020, 9, 1072. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Cho, D.W.; Wang, D.; Tsang, D.C.W.; Zhang, S.; Ding, S.; Wang, L.; Ok, Y.S. Microwave-assisted low-temperature hydrothermal treatment of red seaweed (Gracilaria lemaneiformis) for production of levulinic acid and algae hydrochar. Bioresour. Technol. 2019, 273, 251–258. [Google Scholar] [CrossRef]
- Yoon, J.J.; Kim, Y.J.; Kim, S.H.; Ryu, H.J.; Choi, J.Y.; Kim, G.S.; Shin, M.K. Production of polysaccharides and corresponding sugars from red seaweed. Adv. Mater. Res. 2010, 93–94, 463–466. [Google Scholar] [CrossRef]
- Tian, H.; Yin, X.; Zeng, Q.; Zhu, L.; Chen, J. Isolation, structure, and surfactant properties of polysaccharides from Ulva lactuca L. from South China Sea. Int. J. Biol. Macromol. 2015, 79, 577–582. [Google Scholar] [CrossRef]
- Li, X.; Xiong, F.; Liu, Y.; Liu, F.; Hao, Z.; Chen, H. Total fractionation and characterization of the water-soluble polysaccharides isolated from Enteromorpha intestinalis. Int. J. Biol. Macromol. 2018, 111, 319–325. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.G.; Dabaghian, E.H.; Surayot, U. Water-soluble polysaccharides from Ulva intestinalis: Molecular properties, structural elucidation and immunomodulatory activities. J. Food Drug Anal. 2018, 26, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cheng, C.; Zhao, H.; Jing, J.; Gong, N.; Lu, W. In vivo anti-radiation activities of the Ulva pertusa polysaccharides and polysaccharide-iron(III) complex. Int. J. Biol. Macromol. 2013, 60, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Kok, J.M.L.; Wong, C.L. Physicochemical properties of edible alginate film from Malaysian Sargassum polycystum C. Agardh. Sustain. Chem. Pharm. 2018, 9, 87–94. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Kim, H.S.; Lee, W.W.; Cui, Y.; Lee, H.G.; Kim, Y.T.; Ko, J.Y.; Jeon, Y.J. Protective effect of polysaccharides from Celluclast-assisted extract of Hizikia fusiforme against hydrogen peroxide-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish. Int. J. Biol. Macromol. 2018, 112, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Saraswaty, V.; Mozef, T.; Risdian, C.; Rasyid, A. Bioactivity of Polysaccharide from Gracilaria verrucosa as α-Glucosidase Inhibitor. Procedia Chem. 2015, 16, 687–693. [Google Scholar] [CrossRef]
- Sunwoo, I.Y.; Ra, C.H.; Jeong, G.T.; Kim, S.K. Evaluation of ethanol production and bioadsorption of heavy metals by various red seaweeds. Bioprocess Biosyst. Eng. 2016, 39, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Muraguri, E.N.; Wakibia, J.G.; Kinyuru, J.N. Chemical Composition and Functional Properties of Selected Seaweeds from the Kenya Coast. J. Food Res. 2016, 5, 114. [Google Scholar] [CrossRef]
- Jeong, G.T.; Ra, C.H.; Hong, Y.K.; Kim, J.K.; Kong, I.S.; Kim, S.K.; Park, D.H. Conversion of red-algae Gracilaria verrucosa to sugars, levulinic acid and 5-hydroxymethylfurfural. Bioprocess Biosyst. Eng. 2015, 38, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Marhaeni, B.; Oktaviani, D.F.; Jeong, G.T.; Hong, Y.K. Comparison of bioethanol production from cultivated versus wild Gracilaria verrucosa and Gracilaria gigas. J. Appl. Phycol. 2018, 30, 143–147. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chang, J.H.; Lee, S.B. Chemical composition, saccharification yield, and the potential of the green seaweed Ulva pertusa. Biotechnol. Bioprocess Eng. 2014, 19, 1022–1033. [Google Scholar] [CrossRef]
- Do, J.R.; Nam, Y.J.; Park, J.H.; Jo, J.H. Studies on chemical composition of red algae. J. Kor. Fish. Soc. 1997, 30, 428–431. [Google Scholar]
- Montville, J.B.; Ahuja, J.K.C.; Martin, C.L.; Heendeniya, K.Y.; Omolewa-Tomobi, G.; Steinfeldt, L.C.; Anand, J.; Adler, M.E.; LaComb, R.P.; Moshfegh, A. USDA Food and Nutrient Database for Dietary Studies (FNDDS), 5.0. Procedia Food Sci. 2013, 2, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Lee, S.B.; Kim, S.K.; Park, D.H.; Jeong, G.T. Optimization and Evaluation of Sugars and Chemicals Production from Green Macro-algae Enteromorpha intestinalis. Bioenergy Res. 2016, 9, 1155–1166. [Google Scholar] [CrossRef]
- Almodares, A.; Jafarinia, M.; Hadi, M.R. The Effects of Nitrogen Fertilizer on Chemical Compositions in Corn and Sweet Sorghum. Am. J. Agric. Environ. Sci. 2009, 6, 441–446. [Google Scholar]
- Liang, C.; Hu, Y.; Wang, Y.; Wu, L.; Zhang, W. Production of levulinic acid from corn cob residue in a fed-batch acid hydrolysis process. Process Biochem. 2018, 73, 124–131. [Google Scholar] [CrossRef]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef]
- Pulidindi, I.N.; Kim, T.H. Conversion of levulinic acid from various herbaceous biomass species using hydrochloric acid and effects of particle size and delignification. Energies 2018, 11, 621. [Google Scholar] [CrossRef] [Green Version]
- Nges, I.A.; Li, C.; Wang, B.; Xiao, L.; Yi, Z.; Liu, J. Physio-chemical pretreatments for improved methane potential of Miscanthus lutarioriparius. Fuel 2016, 166, 29–35. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Marhaeni, B.; Hong, Y.K.; Jeong, G.T. Enzymatic saccharification of agar waste from Gracilaria verrucosa and Gelidium latifolium for bioethanol production. J. Appl. Phycol. 2017, 29, 3201–3209. [Google Scholar] [CrossRef]
- Kang, M.; Kim, S.W.; Kim, J.W.; Kim, T.H.; Kim, J.S. Optimization of levulinic acid production from Gelidium amansii. Renew. Energy 2013, 54, 173–179. [Google Scholar] [CrossRef]
- Sukwong, P.; Ra, C.H.; Sunwoo, I.Y.; Tantratian, S.; Jeong, G.T.; Kim, S.K. Improved fermentation performance to produce bioethanol from Gelidium amansii using Pichia stipitis adapted to galactose. Bioprocess Biosyst. Eng. 2018, 41, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Marhaeni, B.; Winanto, T.; Setyaningsih, D.; Hong, Y.K. Catalytic efficiency of sulfuric and hydrochloric acids for the hydrolysis of Gelidium latifolium (Gelidiales, Rhodophyta) in bioethanol production. J. Ind. Eng. Chem. 2015, 27, 108–114. [Google Scholar] [CrossRef]
- Nunraksa, N.; Rattanasansri, S.; Praiboon, J.; Chirapart, A. Proximate composition and the production of fermentable sugars, levulinic acid, and HMF from Gracilaria fisheri and Gracilaria tenuistipitata cultivated in earthen ponds. J. Appl. Phycol. 2019, 31, 683–690. [Google Scholar] [CrossRef]
- Park, M.R.; Kim, H.S.; Kim, S.K.; Jeong, G.T. Thermo-chemical conversion for production of levulinic and formic acids from glucosamine. Fuel Process. Technol. 2018, 172, 115–124. [Google Scholar] [CrossRef]
- Thakkar, A.; Shell, K.M.; Bertosin, M.; Rodene, D.D.; Amar, V.; Bertucco, A.; Gupta, R.B.; Shende, R.; Kumar, S. Production of levulinic acid and biocarbon electrode material from corn stover through an integrated biorefinery process. Fuel Process. Technol. 2021, 213, 106644. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.K.; Jeong, G.T. Catalytic conversion of glucose into levulinic and formic acids using aqueous Brønsted acid. J. Ind. Eng. Chem. 2018, 63, 48–56. [Google Scholar] [CrossRef]
- Jeong, G.T.; Park, D.H. Production of Sugars and Levulinic Acid from Marine Biomass Gelidium amansii. Appl. Biochem. Biotechnol. 2010, 161, 41–52. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Licursi, D.; Mussi, L.; Balestri, E.; Lardicci, C. Levulinic acid production from the green macroalgae Chaetomorpha linum and Valonia aegagropila harvested in the orbetello lagoon. Chem. Eng. Trans. 2019, 74, 103–108. [Google Scholar] [CrossRef]
- Jeong, G.T.; Kim, S.K. Valorization of thermochemical conversion of lipid-extracted microalgae to levulinic acid. Bioresour. Technol. 2020, 313, 123684. [Google Scholar] [CrossRef]
- Smith, A.D.; Landoll, M.; Falls, M.; Holtzapple, M.T. Chemical production from lignocellulosic biomass: Thermochemical, sugar and carboxylate platforms. In Bioalcohol Production; Woodhead Publishing: Sawston, UK, 2010; pp. 391–414. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Hydrothermal pretreatment of rice straw biomass: A potential and promising method for enhanced methane production. Appl. Energy 2012, 94, 129–140. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Hong, Y.K.; Jeong, G.T. Comparison of sulfuric and hydrochloric acids as catalysts in hydrolysis of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst. Eng. 2012, 35, 123–128. [Google Scholar] [CrossRef]
- Morales-Delarosa, S.; Campos-Martin, J.M. Catalytic processes and catalyst development in biorefining. In Advances in Biorefineries; Woodhead Publishing: Sawston, UK, 2014; pp. 152–198. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Kang, J.Y.; Jeong, G.T.; Koo, H.M.; Park, S.M.; Hong, Y.K. Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii). J. Appl. Phycol. 2012, 24, 857–862. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Hong, Y.K.; Jeong, G.T. Detoxification of acidic catalyzed hydrolysate of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst. Eng. 2012, 35, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ra, C.H.; Seo, J.H.; Jeong, G.T.; Kim, S.K. Evaluation of 2,3-butanediol production from red seaweed Gelidium amansii Hydrolysates using engineered Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2021, 30, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Marhaeni, B.; Winanto, T.; Jeong, G.T.; Khan, M.N.A.; Hong, Y.K. Comparison of agarophytes (Gelidium, Gracilaria, and Gracilariopsis) as potential resources for bioethanol production. J. Appl. Phycol. 2013, 25, 1957–1961. [Google Scholar] [CrossRef]
- Jeong, G.-T.; Park, D.-H. Production of Levulinic Acid from Marine Algae Codium fragile Using Acid-Hydrolysis and Response Surface Methodology. KSBB J. 2011, 26, 341–346. [Google Scholar] [CrossRef]
- Park, M.R.; Kim, S.K.; Jeong, G.T. Production of levulinic acid from glucosamine using zirconium oxychloride. J. Ind. Eng. Chem. 2018, 61, 119–123. [Google Scholar] [CrossRef]
- Jeong, G.T. Production of levulinic acid from glucosamine by dilute-acid catalyzed hydrothermal process. Ind. Crop. Prod. 2014, 62, 77–83. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kawai, S.; Murata, K. Strategies for the production of high concentrations of bioethanol from seaweeds. Bioengineered 2013, 4, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Lee, S.B.; Jeong, G.T. Production of reducing sugar from Enteromorpha intestinalis by hydrothermal and enzymatic hydrolysis. Bioresour. Technol. 2014, 161, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.M.; Kim, D.H.; Kim, S.K.; Jeong, G.T. Production of sugars from macro-algae Gracilaria verrucosa using combined process of citric acid-catalyzed pretreatment and enzymatic hydrolysis. Algal Res. 2016, 13, 293–297. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; Food and Agriculture Organization of United Nations: Rome, Italy, 2020; ISBN 9789251326923. [Google Scholar]
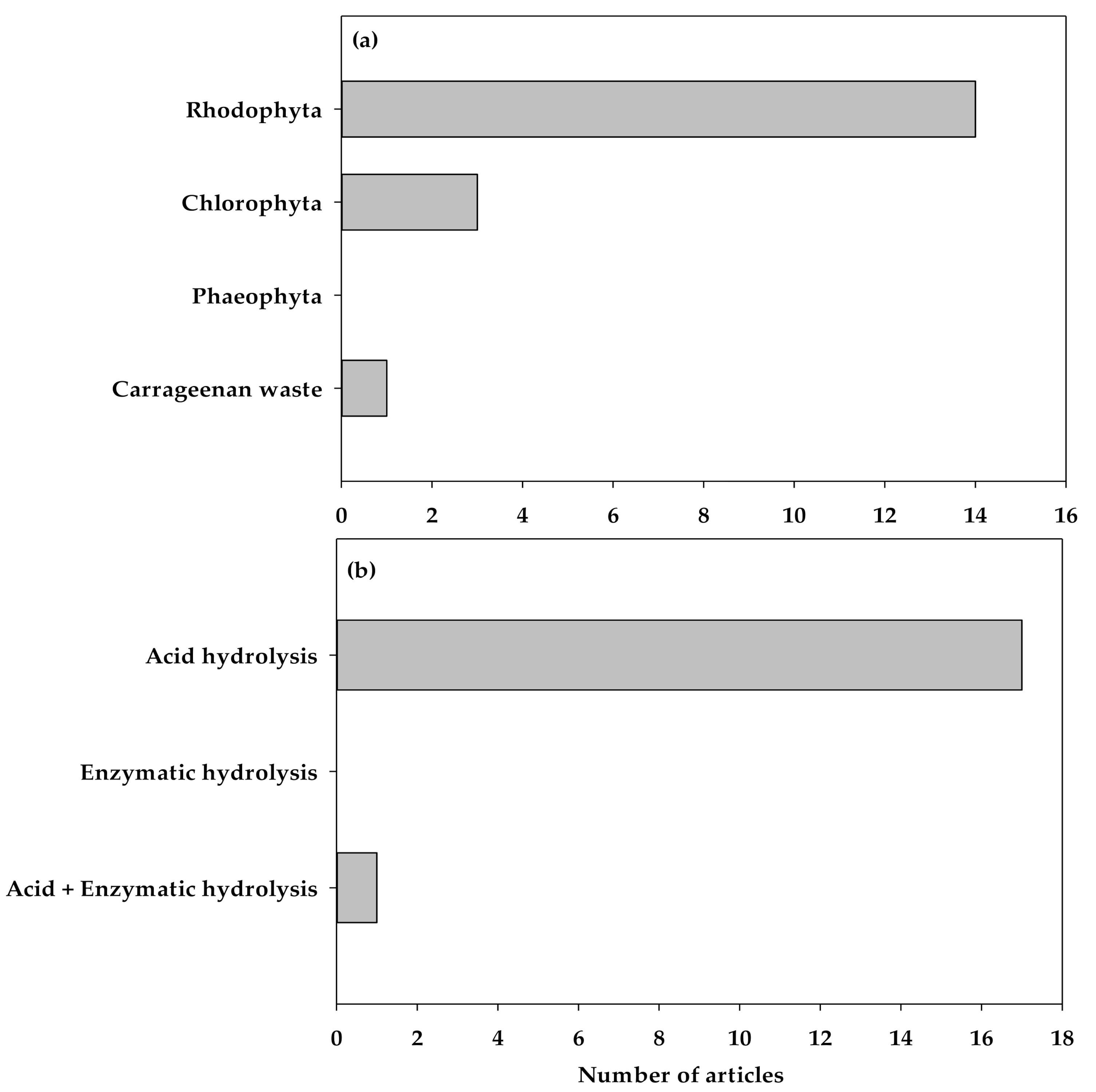
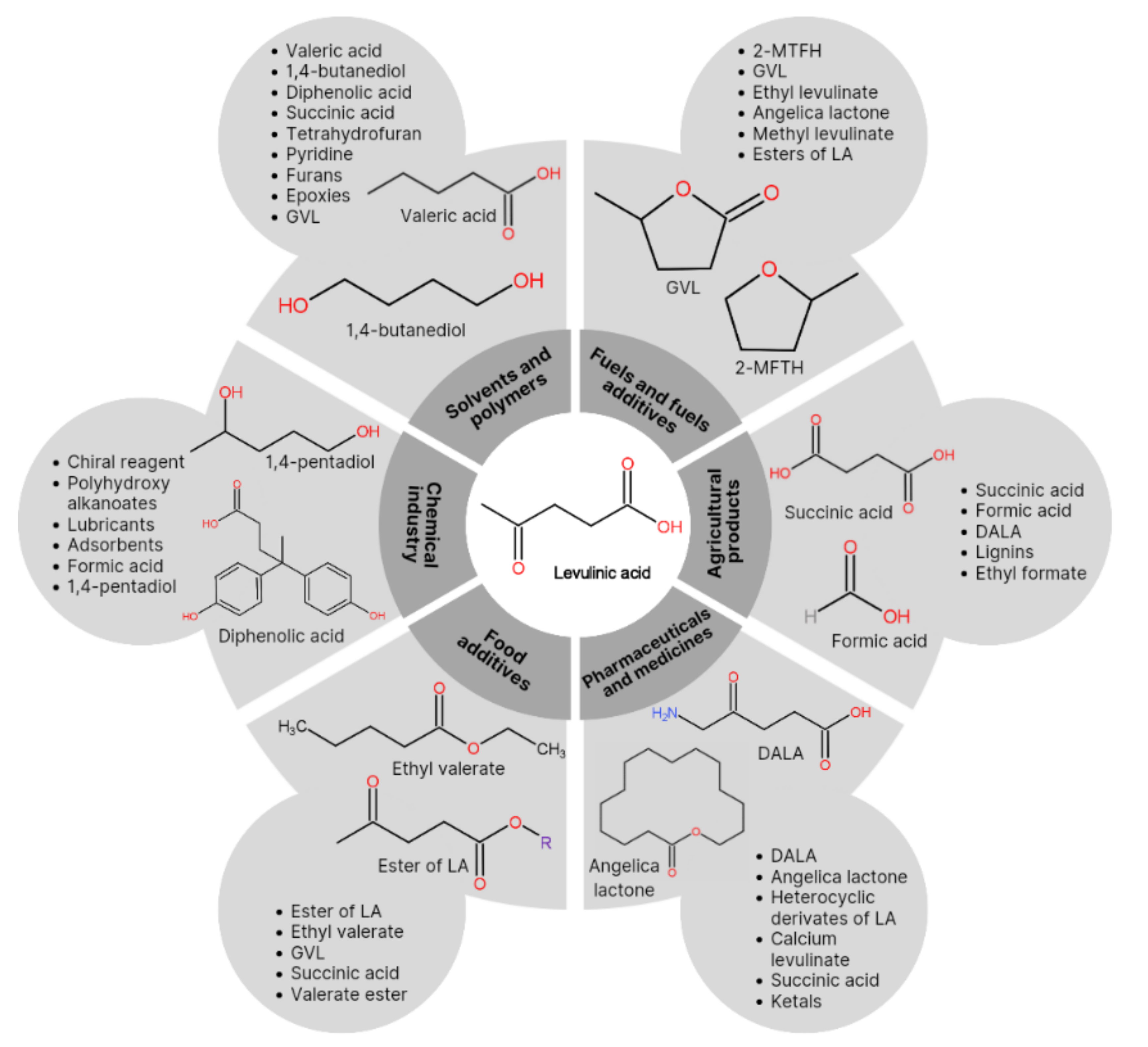
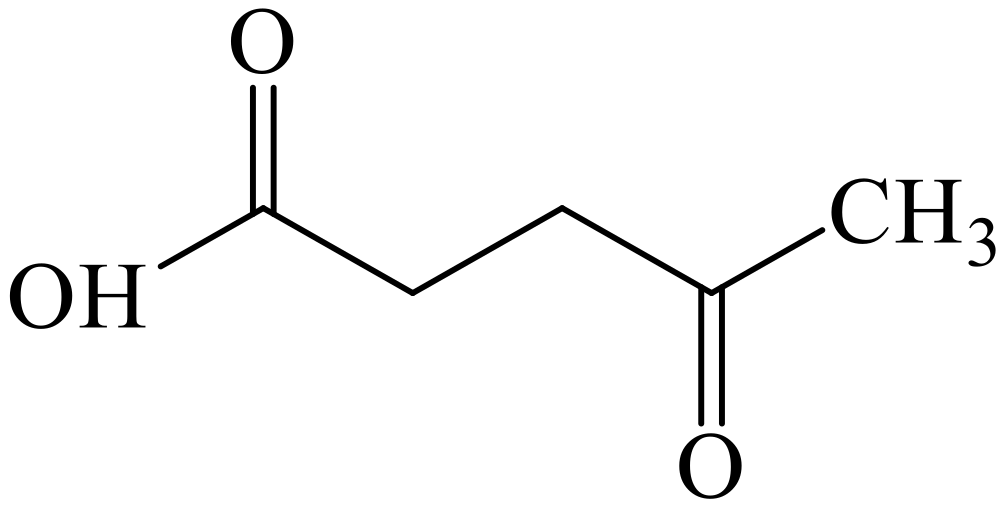

| Macroalgae | Types of Polysaccharide | Chemical Structure | Polysaccharide Content (%) | Ref. |
|---|---|---|---|---|
| Red Macroalgae | ||||
| Kappaphycus alvarezii | Carrageenan | α(1–4)-anhydro-D-galactose and β(1–3)-D-galactose | 32.95 ± 1.43 | [12] |
| Eucheuma denticulatum | Carrageenan | 17.8 to 35.5 | [21] | |
| Gracilaria verrucosa | Agar | D-galactose and 3,6-anhydro-L-galactopyranose | 0.135 to 35.11 | [30] |
| Gracilaria lemaneiformis | Agar | 64.80 | [22] | |
| Gelidium amansii | Agar | D-galactose and 3,6-anhydro-L-galactose | 58.60 | [23] |
| Green Macroalgae | ||||
| Enteromorpha intestinalis | Water-soluble sulfated polysaccharides | (1→2)-linked rhamnose and (1→2)-linked glucose residues | 11.38 to 59.1 | [25,26] |
| Ulva lactuca | Ulvan | 1→, 1→6, 1→2, 1→2, 6, or unoxidized glycosidic bonds | 17.57 | [24] |
| Ulva pertusa | Crude polysaccharide | 18.30 | [27] | |
| Brown Macroalgae | ||||
| Sargassum polycystum | Alginate | (1–4) -linked β-D-mannuronate (M) and α-L-guluronate (G) | 15.85 | [28] |
| Hizikia fusiforme | Alginate | 63.56 ± 0.32 | [29] |
| Biomass | Proximate Composition (g/100 g Dry Weight) | Ref. | |||
|---|---|---|---|---|---|
| Carbohydrate | Protein | Lipid | Ash | ||
| Red Macroalgae | |||||
| Kappaphycus alvarezii | 67.8 ± 10.40 | 3.60 ± 0.00 | 0.60 ± 0.00 | 18.40 ± 0.50 | [16] |
| Euchema denticulatum | 64.70 (a) | 4.50 (a), 5.06 (b) | 0.20 (a), 1.78 (b) | 30.6 (a), 27.13 (b) | [31] (a), [32] (b) |
| Gracilaria verrucosa | 66.95 | 9.4 | 0.65 | 7.42 | [33] |
| Gracilaria verrucosa | 38.38 to 60.81 | 6.64 to 9.86 | 0.80 to 0.58 | 13.85 to 12.51 | [34] |
| Gracilaria lemaneiformis | 71.5 | 9.30 | 0.92 | 18.2 | [22] |
| Gracilaria gigas | 47.31 to 64.71 | 8.14 to 12.63 | 0.60 to 1.31 | 17.86 to 19.59 | [34] |
| Gelidium amansii | 66.0 to 75.2 | 18.5 to 20.5 | 0.20 to 0.60 | 5.70 to 13.3 | [35] |
| Carpopeltis cornea | 60.7 | 23.4 | 0.4 | 15.6 | [36] |
| Chondrus crispus | 65.7 | 8.1 | 0.9 | 25.2 | [37] |
| Green Macroalgae | |||||
| Enteromorpha intestinalis | 42.8 | 31.6 | 1.30 | 24.3 | [38] |
| Ulva lactuca | 50.4 | 26.8 | 0.60 | 22.2 | [35] |
| Ulva pertusa | 52.3 | 25.1 | 0.10 | 22.5 | [35] |
| Brown Macroalgae | |||||
| Sargassum polycystum | 46.6 | 6.00 | 0.30 | 47.1 | [35] |
| Hizikia fusiforme | 47.5 | 9.80 | 1.20 | 41.5 | [35] |
| Undaria pinnatifida | 43.2 | 23.80 | 3.50 | 29.5 | [35] |
| Non-Macroalgae | |||||
| Corn cob | 10.4 (a) | 7.10 (a) | - | 3.00 (b) | [39] (a), [40] (b) |
| Rice straw | 58.3 (a) | - | - | 8.20 ± 0.10 (b) | [41] (a), [42] (b) |
| Corn stover | 71.7 (a) | - | - | 1.50 ± 0.10 (b) | [19] (a), [42] (b) |
| Sweet sorghum bagasse | 7.70 (a) | 5.40 (a) | - | 1.00 ± 0.10 (b) | [39] (a), [42] (b) |
| Miscanthus | 65.4 (a) | 3.2 (a) | - | 2.10 ± 0.30 (b) | [43] (a), [42] (b) |
| Biomass | Sugar, HMF and Levulinic Acid after Hydrolysis | Ref. | |
|---|---|---|---|
| Red Macroalgae | |||
| Kappaphycus alvarezii | Glucose Galactose 5-HMF | 0.215 g/L 1.447 g/L 0.302 g/L | [16] |
| Gracilaria verrucosa | Glucose Galactose 5-HMF | 0.27% 1.23% 0.47% | [8] |
| Gracilaria verrucosa | Glucose Galactose 5-HMF | 4.29 g/L 18.38 g/L 3.74 g/L | [33] |
| Gracilaria verrucosa | Glucose Galactose | 18.17 g/L 10.83 g/L | [44] |
| Gelidium amansii | Glucose Galactose | 3.76 g/100 g 1.36 g/100 g | [45] |
| Gelidium amansii | Glucose Galactose 5-HMF Formic acid | 8.4 g/L 20.3 g/L 3.8 g/L 1.6 g/L | [46] |
| Gelidium latifolium | Glucose Galactose 5-HMF | 2.4 g/L 34.43 g/L 5.7 g/L | [47] |
| Gracilaria fisheri | Glucose Galactose 5-HMF | 7.86 g/L 8.37 g/L 1.55 g/L | [48] |
| Gracilaria tenuistipitata | Glucose Galactose 5-HMF | 3.15 g/L 5.75 g/L 1.42 g/L | [48] |
| Green Macroalgae | |||
| Enteromorpha intestinalis | Glucose Xylose–mannose–galactose (XMG) Total reducing sugar (TRS) 5-HMF Furfural | 10.42% 18.08% 28.61% 1.71% 2.03% | [38] |
| Other biomasses | |||
| Glucosamine | Formic acid | 50.80% | [49] |
| Corn stover | Lignin Glucan Xylan Arabinan Others | 27.00% 28.20% 21.60% 2.50% 14.20% | [50] |
| Corn cob | Cellulose Lignin Hemicellulose Others | 60.70 g/L 31.40 g/L 2.70 g/L 2.20 g/L | [40] |
| Rice straw | Glucan Xylan Arabinan Acid-insoluble lignin (AIL) Acid-soluble lignin (ASL) | 36.30 ± 0.10 wt% 14.00 ± 1.00 wt% 3.70 ± 0.00 wt% 15.00 ± 0.70 wt% 2.10 ± 0.40 wt% | [42] |
| Corn stover | Glucan Xylan Arabinan Acid-insoluble lignin (AIL) Acid-soluble lignin (ASL) | 33.00 ± 0.90 wt% 18.40 ± 0.70 wt% 5.30 ± 0.10 wt% 15.20 ± 0.30 wt% 2.20 ± 0.10 wt% | [42] |
| Sweet sorghum bagasse | Glucan Xylan Arabinan Acid-insoluble lignin (AIL) Acid-soluble lignin (ASL) | 41.30 ± 0.20 wt% 11.70 ± 0.00 wt% 3.10 ± 0.10 wt% 12.00 ± 0.30 wt% 1.30 ± 0.10 wt% | [42] |
| Miscanthus | Glucan Xylan Arabinan Acid-insoluble lignin (AIL) Acid-soluble lignin (ASL) | 44.30 ± 0.30 wt% 18.40 ± 0.10 wt% 3.50 ± 0.00 wt% 18.90 ± 0.30 wt% 0.70 ± 0.00 wt% | [42] |
| Glucose | Formic acid Glucose 5-HMF | 50.79% 99.80% 0.06% | [51] |
| No. | Raw Material | Pretreatment | Ref. |
|---|---|---|---|
| 1 | Kappaphycus alvarezii (macroalgae) |
| [16] |
| 2 | Gracilaria verrucosa (macroalgae) |
| [8] |
| 3 | Gracilaria verrucosa (macroalgae) |
| [33] |
| 4 | Gracilaria lemaneiformis (macroalgae) |
| [22] |
| 5 | Gelidium amansii (macroalgae) |
| [52] |
| 6 | Gelidium amansii (macroalgae) |
| [45] |
| 7 | Enteromorpha intestinalis (macroalgae) |
| [38] |
| 8 | Chaetomorpha linum (macroalgae) |
| [53] |
| 9 | Valonia aegagropila (macroalgae) |
| [53] |
| 10 | Scenedesmus obliquus (microalgae) |
| [54] |
| 11 | Corn stover |
| [50] |
| 12 | Corn cob |
| [40] |
| 13 | Rice straw |
| [42] |
| 14 | Corn stover |
| [42] |
| 15 | Sweet sorghum bagasse |
| [42] |
| 16 | Miscanthus |
| [42] |
| Biomass | Hydrolysis | Reaction Condition | Yield of LA | Ref. | ||
|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Catalyst Concentration | ||||
| Red Macroalgae | ||||||
| Kappaphycus alvarezii | Acid | 178.2 | 39.3 | 2.87% H2SO4 | 1.17 g/L | [16] |
| Kappaphycus alvarezii | Acid | 130 | 15 | 0.2 M HCl | 2.8 g/L | [57] |
| Kappaphycus alvarezii | Acid | 130 | 15 | 0.2 M H2SO4 | 1.07 g/L | [59] |
| Kappaphycus alvarezii | Acid + enzyme | 120 | 15 | 0.2 M H2SO4 | 2.11:2.02% g/g | [12] |
| Kappaphycus alvarezii | Acid | 130 | 15 | H2SO4 | 0.96 g/L | [60] |
| Gracilaria verrucosa | Acid | 180 | 20 | 0.5 M MSA | 22.02% | [8] |
| Gracilaria verrucosa | Acid | 180.9 | 50 | 2.85% H2SO4 | 1.47 g/L | [33] |
| Gracilaria lemaneiformis | Acid | 180 | 20 | 0.2 M H2SO4 | 16.30 wt% | [22] |
| Gelidium amansii | Acid | 160 | 43.1 | 3% H2SO4 | 9.74 g/L | [52] |
| Gelidium amansii | Acid | 180 | 48.22 | 3% H2SO4 | 42.88% | [45] |
| Gelidium amansii | Acid | 142.6 | 11 | 358.3 mM H2SO4 | 6.3 g/L | [46] |
| Gelidium amansii | Acid | 180 | 20 | H3PO4:HNO3 = 5:5, mM | 7.87 g/L | [61] |
| Gracilaria fisheri | Acid | 96 | 150 | 1 M H2SO4 | 3.66 g/L | [48] |
| Gracilaria tenuistipitata | Acid | 96 | 150 | 1 M H2SO4 | 6.12 g/L | [48] |
| Gracilariopsis chorda | Acid | 130 | 15 | 0.2 M H2SO4 | 0.42 g/L | [62] |
| Gelidium latifolium | Acid | 130 | 15 | H2SO4 and HCl | 3.45 g/L and 1.88 g/L | [47] |
| Green Macroalgae | ||||||
| Enteromorpha intestinalis | Acid | 175 | 35 | 3.7% H2SO4 | 4.00% | [38] |
| Chaetomorpha linum | Acid | 190 | 45 | 4.7% H2SO4 | 19 wt% | [53] |
| Valonia aegagropila | Acid | 200 | 45 | 4.7% H2SO4 | 16 wt% | [53] |
| Codium fragile | Acid | 160.7 | 39.1 | 3.9% H2SO4 | 4.26 g/L | [63] |
| Other Biomasses | ||||||
| Scenedesmus obliquus (microalgae) | Acid | 180 | 10 | 0.85 M HCl | 45.63 wt% | [54] |
| Glucosamin (crustacean shell chitosan monomer from food waste) | Acid | 200 | 20 | 15 mol% ZrOCl2 | 21.29 mol% | [64] |
| Glucosamin (crustacean chitosan) | Acid | 188 | 49 | 4% H2SO4 | 30.30 g/L | [65] |
| Glucosamin (chitin/chitosan monomer) | Acid | 200 | 30 | 0.5 M MSA | 49.90% | [49] |
| Corn stover | Acid | 190 | 5 | 2% H2SO4 | 10–35 wt% | [50] |
| Corn cob | Acid | 180 | 50 | 0.5 mol/L H2SO4 | 107.93 g/L | [40] |
| Rice straw | Acid | 150 | 300 (5 h) | 1 M HCl | 60.20 wt% | [42] |
| Corn stover | Acid | 150 | 300 (5 h) | 1 M HCl | 75.10 wt% | [42] |
| Sweet sorghum bagasse | Acid | 150 | 300 (5 h) | 1 M HCl | 78.50 wt% | [42] |
| Miscanthus | Acid | 150 | 300 (5 h) | 1 M HCl | 61.70 wt% | [42] |
| Glucose | Acid | 181.2 | 44.4 | 0.35 M MSA | 48.95% | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meinita, M.D.N.; Amron, A.; Trianto, A.; Harwanto, D.; Caesarendra, W.; Jeong, G.-T.; Choi, J.-S. Levulinic Acid Production from Macroalgae: Production and Promising Potential in Industry. Sustainability 2021, 13, 13919. https://doi.org/10.3390/su132413919
Meinita MDN, Amron A, Trianto A, Harwanto D, Caesarendra W, Jeong G-T, Choi J-S. Levulinic Acid Production from Macroalgae: Production and Promising Potential in Industry. Sustainability. 2021; 13(24):13919. https://doi.org/10.3390/su132413919
Chicago/Turabian StyleMeinita, Maria Dyah Nur, Amron Amron, Agus Trianto, Dicky Harwanto, Wahyu Caesarendra, Gwi-Taek Jeong, and Jae-Suk Choi. 2021. "Levulinic Acid Production from Macroalgae: Production and Promising Potential in Industry" Sustainability 13, no. 24: 13919. https://doi.org/10.3390/su132413919







