Does Fish Conditioning in Aquaculture Increase Survival Success in the Wild? A Case Study on a Cyprinid Fish
Abstract
:1. Introduction
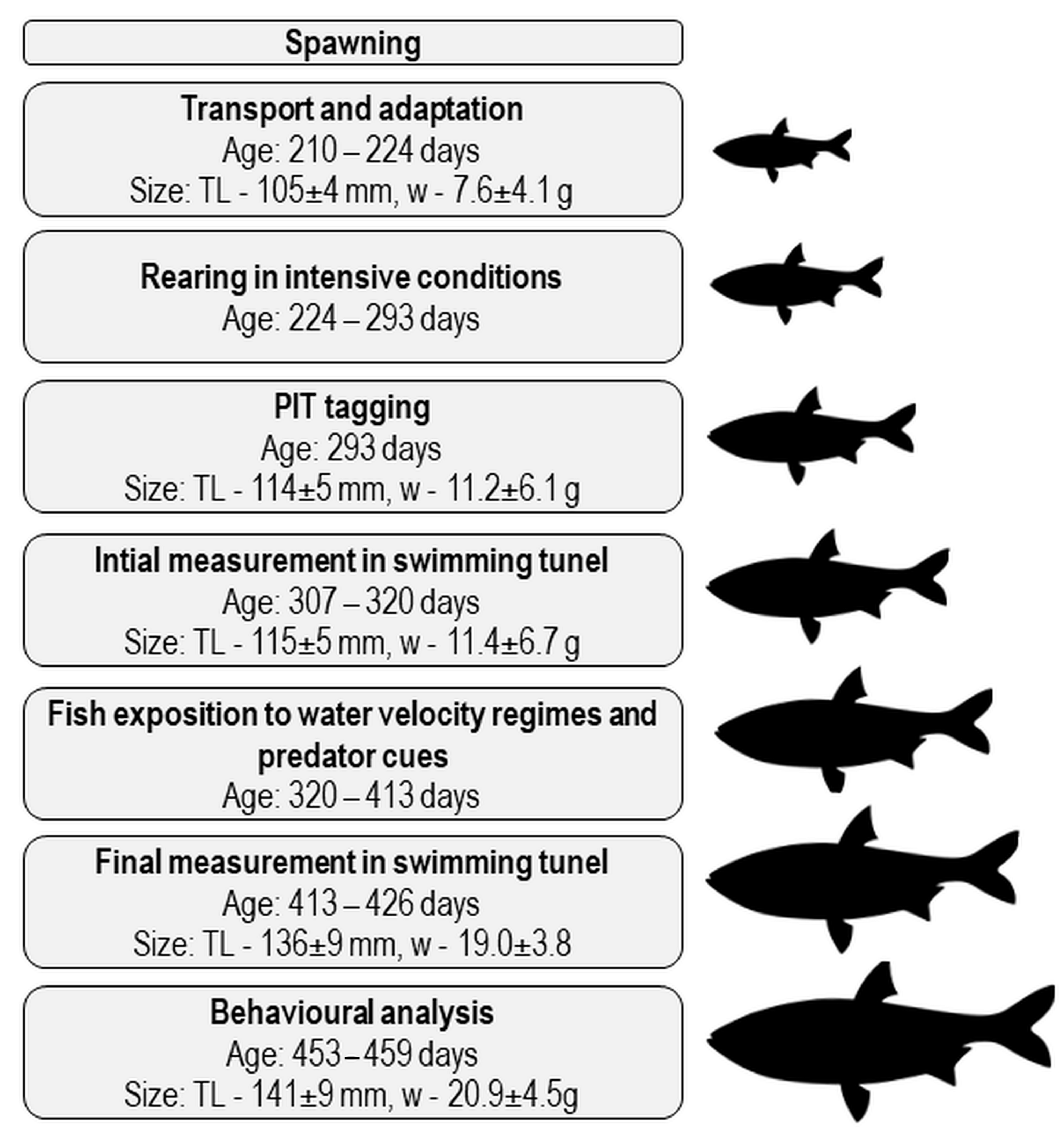
2. Materials and Methods
2.1. Species Description
2.2. Fish Origin and Ethical Statement
2.3. Rearing of Asp Juveniles in Intensive Conditions
2.4. Fish Exposure to Increased Water Velocity and Predator Cues
2.5. Pond Mesocosm Experiment
2.6. Reservoir Stocking Experiment
2.7. Laboratory Assays on Conditioned Fish
2.7.1. Measurement of Critical Swimming Speed, Oxygen Consumption, and Cost of Transport
2.7.2. Reaction to Predator Chemical Cues
2.8. Data Preparation and Analysis
2.8.1. Pond Mesocosm Experiment
2.8.2. Reservoir Stocking Experiment
2.8.3. Laboratory Assays on Conditioned Fish
3. Results
3.1. Pond Mesocosm Experiment
3.2. Reservoir Stocking Experiment
3.3. Laboratory Assays on Conditioned Fish
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olden, J.D.; Poff, N.L.R.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef]
- Chong, V.C.; Lee, P.K.Y.; Lau, C.M. Diversity, extinction risk and conservation of Malaysian fishes. J. Fish Biol. 2010, 76, 2009–2066. [Google Scholar] [CrossRef]
- Rahel, F.J. Homogenization of Freshwater Faunas. Annu. Rev. Ecol. Syst. 2002, 33, 291–315. [Google Scholar] [CrossRef] [Green Version]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef]
- Fitzgerald, D.B.; Tobler, M.; Winemiller, K.O. From richer to poorer: Successful invasion by freshwater fishes depends on species richness of donor and recipient basins. Glob. Chang. Biol. 2016, 22, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.K.; Worm, B. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 2009, 78, 699–714. [Google Scholar] [CrossRef]
- Bartoň, D.; Blabolil, P.; Sajdlová, Z.; Vejřík, L.; Souza, A.T.; Kubečka, J.; Šmejkal, M. Effects of hydropeaking on the attached eggs of a rheophilic cyprinid species. Ecohydrology 2021, 14, e2280. [Google Scholar] [CrossRef]
- Liu, X.; Qin, J.; Xu, Y.; Ouyang, S.; Wu, X. Biodiversity decline of fish assemblages after the impoundment of the Three Gorges Dam in the Yangtze River Basin, China. Rev. Fish Biol. Fish. 2019, 29, 177–195. [Google Scholar] [CrossRef]
- Moyle, P.B.; Williams, J.E. Biodiversity Loss in the Temperate Zone: Decline of the Native Fish Fauna of California. Conserv. Biol. 1990, 4, 275–284. [Google Scholar] [CrossRef]
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish conservation in freshwater and marine realms: Status, threats and management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 838–857. [Google Scholar] [CrossRef] [Green Version]
- Sayer, C.D.; Copp, G.H.; Emson, D.; Godard, M.J.; Zięba, G.; Wesley, K.J. Towards the conservation of crucian carp Carassius carassius: Understanding the extent and causes of decline within part of its native English range. J. Fish Biol. 2011, 79, 1608–1624. [Google Scholar] [CrossRef]
- Souza, A.T.; Soukalová, K.; Děd, V.; Šmejkal, M.; Moraes, K.; Říha, M.; Muška, M.; Frouzová, J.; Kubečka, J. Otolith shape variations between artificially stocked and autochthonous pikeperch (Sander lucioperca). Fish. Res. 2020, 231. [Google Scholar] [CrossRef]
- Eby, L.A.; Roach, W.J.; Crowder, L.B.; Stanford, J.A. Effects of stocking-up freshwater food webs. Trends Ecol. Evol. 2006, 21, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, J.I.; Brockmark, S.; Näslund, J. Environmental effects on behavioural development consequences for fitness of captive-reared fishes in the wild. J. Fish Biol. 2014, 85, 1946–1971. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.M.; Palmer, M.; Grau, A.; Deudero, S.; Alconchel, J.I.; Catalán, I.A. Adapting to the wild: The case of aquaculture-produced and released meagres Argyrosomus regius. J. Fish Biol. 2014, 84, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Araki, H.; Schmid, C. Is hatchery stocking a help or harm?: Evidence, limitations and future directions in ecological and genetic surveys. Aquaculture 2010, 308, S2–S11. [Google Scholar] [CrossRef]
- Saloniemi, I.; Jokikokko, E.; Kallio-Nyberg, I.; Jutila, E.; Pasanen, P. Survival of reared and wild Atlantic salmon smolts: Size matters more in bad years. ICES J. Mar. Sci. 2004, 61, 782–787. [Google Scholar] [CrossRef]
- D’Anna, G.; Giacalone, V.M.; Vega Fernández, T.; Vaccaro, A.M.; Pipitone, C.; Mirto, S.; Mazzola, S.; Badalamenti, F. Effects of predator and shelter conditioning on hatchery-reared white seabream Diplodus sargus (L., 1758) released at sea. Aquaculture 2012, 356–357, 91–97. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Wisenden, B.D.; Chivers, D.P. Chemical ecology of predator–prey interactions in aquatic ecosystems: A review and prospectus. Can. J. Zool. 2010, 88, 698–724. [Google Scholar] [CrossRef]
- Rahman, M.M. Role of common carp (Cyprinus carpio) in aquaculture production systems. Front. Life Sci. 2015, 8, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Diana, J.S. Aquaculture Production and Biodiversity Conservation. Bioscience 2009, 59, 27–38. [Google Scholar] [CrossRef]
- Baras, E.; Kestemont, P.; Mélard, C. Effect of stocking density on the dynamics of cannibalism in sibling larvae of Perca fluviatilis under controlled conditions. Aquaculture 2003, 219. [Google Scholar] [CrossRef]
- Buckmeier, D.L.; Betsill, R.K.; Schlechte, J.W. Initial Predation of Stocked Fingerling Largemouth Bass in a Texas Reservoir and Implications for Improving Stocking Efficiency. N. Am. J. Fish. Manag. 2011, 25, 652–659. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Uglem, I.; Finstad, B.; Chittenden, C.M.; Nilsen, R.; Økland, F.; Bjørn, P.A. Stocking location and predation by marine fishes affect survival of hatchery-reared Atlantic salmon smolts. Fish. Manag. Ecol. 2012, 19, 400–409. [Google Scholar] [CrossRef]
- Wisenden, B.D. Olfactory assessment of predation risk in the aquatic environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1205–1208. [Google Scholar] [CrossRef] [Green Version]
- Šmejkal, M.; Ricard, D.; Sajdlová, Z.; Čech, M.; Vejřík, L.; Blabolil, P.; Vejříková, I.; Prchalová, M.; Vašek, M.; Souza, A.T.; et al. Can species-specific prey responses to chemical cues explain prey susceptibility to predation? Ecol. Evol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mathis, A.; Smith, R.J.F. Chemical alarm signals increase the survival time of fathead minnows (Pimephales promelas) during encounters with northern pike (Esox lucius). Behav. Ecol. 1993, 4, 260–265. [Google Scholar] [CrossRef]
- Chivers, D.P.; Smith, R.J.F. Fathead minnows, Pimephales promelas, acquire predator recognition when alarm substance is associated with the sight of unfamiliar fish. Anim. Behav. 1994, 48, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.E.; Ferrari, M.C.O.; Chivers, D.P. Adaptive Forgetting: Why Predator Recognition Training Might Not Enhance Poststocking Survival. Fisheries 2013, 38, 16–25. [Google Scholar] [CrossRef]
- Greggor, A.L.; Price, C.J.; Shier, D.M. Examining the efficacy of anti-predator training for increasing survival in conservation translocations: A systematic review protocol. Environ. Evid. 2019, 8, 11. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, D.; Lin, K.; Sun, C.; Yang, X. Intelligent feeding control methods in aquaculture with an emphasis on fish: A review. Rev. Aquac. 2018, 10, 975–993. [Google Scholar] [CrossRef]
- Říha, M.; Gjelland, K.Ø.; Děd, V.; Eloranta, A.P.; Rabaneda-Bueno, R.; Baktoft, H.; Vejřík, L.; Vejříková, I.; Draštík, V.; Šmejkal, M.; et al. Contrasting structural complexity differentiate hunting strategy in an ambush apex predator. Sci. Rep. 2021, 11, 17472. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.V.; Johnson, T.B.; Metcalfe, B.; Fisk, A.T. Spatial distribution of lake trout (Salvelinus namaycush) across seasonal thermal cycles in a large lake. Freshw. Biol. 2021, 66, 615–627. [Google Scholar] [CrossRef]
- Stejskal, V.; Matousek, J.; Sebesta, R.; Prokesova, M.; Vanina, T.; Podhorec, P. Prevalence of deformities in intensively reared peled Coregonus peled and comparative morphometry with pond-reared fish. J. Fish Dis. 2018, 41, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Bosakowski, T.; Wagner, E.J. Assessment of Fin Erosion by Comparison of Relative Fin Length in Hatchery and Wild Trout in Utah. Can. J. Fish. Aquat. Sci. 2011, 51, 636–641. [Google Scholar] [CrossRef]
- Latremouille, D.N. Fin Erosion in Aquaculture and Natural Environments. Rev. Fish. Sci. 2010, 11, 315–335. [Google Scholar] [CrossRef]
- Stejskal, V.; Policar, T.; Křišťan, J.; Kouřil, J.; Hamáčková, J. Fin condition in intensively cultured Eurasian perch (Perca fluviatilis). J. Vertebr. Biol. 2011, 60, 122–128. [Google Scholar] [CrossRef]
- Plaut, I. Effects of fin size on swimming performance, swimming behaviour and routine activity of zebrafish Danio rerio. J. Exp. Biol. 2000, 203, 813–820. [Google Scholar] [CrossRef]
- Fu, C.; Cao, Z.-D.; Fu, S.-J. The effects of caudal fin loss and regeneration on the swimming performance of three cyprinid fish species with different swimming capacities. J. Exp. Biol. 2013, 216, 3164–3174. [Google Scholar] [CrossRef] [Green Version]
- Härkönen, L.; Hyvärinen, P.; Paappanen, J.; Vainikka, A. Explorative behavior increases vulnerability to angling in hatchery-reared brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 2014, 71, 1900–1909. [Google Scholar] [CrossRef]
- Malavasi, S.; Georgalas, V.; Lugli, M.; Torricelli, P.; Mainardi, D. Differences in the pattern of antipredator behaviour between hatchery-reared and wild European sea bass juveniles. J. Fish Biol. 2004, 65, 143–155. [Google Scholar] [CrossRef]
- Ersbak, K.; Haase, B.L. Nutritional Deprivation after Stocking as a Possible Mechanism Leading to Mortality in Stream-Stocked Brook Trout. N. Am. J. Fish. Manag. 1983, 3, 142–151. [Google Scholar] [CrossRef]
- Hulthén, K.; Chapman, B.B.; Nilsson, P.A.; Hansson, L.-A.; Skov, C.; Brodersen, J.; Vinterstare, J.; Brönmark, C. A predation cost to bold fish in the wild. Sci. Rep. 2017, 7, 1239. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.M.; McMurtry, M.J.; Bowlby, J.; Casselman, J.M.; Liimatainen, V.A. Survival and Growth of Stocked Lake Trout in Relation to Body Size, Stocking Season, Lake Acidity, and Biomass of Competitors. Trans. Am. Fish. Soc. 1987, 116, 618–627. [Google Scholar] [CrossRef]
- Tatara, C.P.; Riley, S.C.; Scheurer, J.A. Growth, Survival, and Habitat Use of Naturally Reared and Hatchery Steelhead Fry in Streams: Effects of an Enriched Hatchery Rearing Environment. Trans. Am. Fish. Soc. 2011, 138, 441–457. [Google Scholar] [CrossRef]
- Vašek, M.; Eloranta, A.P.; Vejříková, I.; Blabolil, P.; Říha, M.; Jůza, T.; Šmejkal, M.; Matěna, J.; Kubečka, J.; Peterka, J. Stable isotopes and gut contents indicate differential resource use by coexisting asp (Leuciscus aspius) and pikeperch (Sander lucioperca). Ecol. Freshw. Fish 2018, 27, 1054–1065. [Google Scholar] [CrossRef]
- Vašek, M.; Vejřík, L.; Vejříková, I.; Šmejkal, M.; Baran, R.; Muška, M.; Kubečka, J.; Peterka, J. Development of non-lethal monitoring of stable isotopes in asp (Leuciscus aspius): A comparison of muscle, fin and scale tissues. Hydrobiologia 2016, 785, 327–335. [Google Scholar] [CrossRef]
- Šmejkal, M.; Bartoň, D.; Brabec, M.; Sajdlová, Z.; Souza, A.T.; Moraes, K.R.; Soukalová, K.; Blabolil, P.; Vejřík, L.; Kubečka, J. Climbing up the ladder: Male reproductive behaviour changes with age in a long-lived fish. Behav. Ecol. Sociobiol. 2021, 75, 22. [Google Scholar] [CrossRef]
- Šmejkal, M.; Ricard, D.; Vejřík, L.; Mrkvička, T.; Vebrová, L.; Baran, R.; Blabolil, P.; Sajdlová, Z.; Vejříková, I.; Prchalová, M.; et al. Seasonal and daily protandry in a cyprinid fish. Sci. Rep. 2017, 7, 4737. [Google Scholar] [CrossRef] [Green Version]
- Blabolil, P.; Bartoň, D.; Halačka, K.; Kočvara, L.; Kolařík, T.; Kubečka, J.; Šmejkal, M.; Peterka, J. The fate of 0+ asp (Leuciscus aspius) after being stocked in a reservoir. Biologia 2020, 75, 989–996. [Google Scholar] [CrossRef]
- Skov, C.; Brodersen, J.; Bronmark, C.; Hansson, L.-A.; Hertonsson, P.; Nilsson, P.A. Evaluation of PIT-tagging in cyprinids. J. Fish Biol. 2005, 67, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Hulthén, K.; Chapman, B.B.; Nilsson, P.A.; Hansson, L.A.; Skov, C.; Baktoft, H.; Brodersen, J.; Brönmark, C. Sex identification and PIT-tagging: Tools and prospects for studying intersexual differences in freshwater fishes. J. Fish Biol. 2014, 84, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollset, K.W.; Lennox, R.J.; Thorstad, E.B.; Auer, S.; Bär, K.; Larsen, M.H.; Mahlum, S.; Näslund, J.; Stryhn, H.; Dohoo, I. Systematic review and meta-analysis of PIT tagging effects on mortality and growth of juvenile salmonids. Rev. Fish Biol. Fish. 2020, 30, 553–568. [Google Scholar] [CrossRef]
- Brett, J.R. The Respiratory Metabolism and Swimming Performance of Young Sockeye Salmon. J. Fish. Res. Board Canada 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Dowle, M.; Srinivasan, A. Data.Table: Extension of ‘Data.Frame’. R Package Version 1.14.2. 2021. Available online: https://CRAN.R-project.org/package=data.table (accessed on 15 December 2021).
- Grolemund, G.; Wickham, H. Dates and Times Made Easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.7. 2021. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 15 December 2021).
- Wood, S.N. mgcv: GAMs and generalized ridge regression for R. R News 2001. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hawkins, L.A.; Armstrong, J.D.; Magurran, A.E. A test of how predator conditioning influences survival of hatchery-reared Atlantic salmon, Salmo salar, in restocking programmes. Fish. Manag. Ecol. 2007, 14, 291–293. [Google Scholar] [CrossRef]
- Berejikian, B.A.; Smith, R.J.F.; Tezak, E.P.; Schroder, S.L.; Knudsen, C.M. Chemical alarm signals and complex hatchery rearing habitats affect antipredator behavior and survival of chinook salmon (Oncorhynchus tshawytscha) juveniles. Can. J. Fish. Aquat. Sci. 2011, 56, 830–838. [Google Scholar] [CrossRef]
- Brown, G.E.; Smith, R.J.F. Acquired predator recognition in juvenili rainbow trot (Oncorhynchus mykiss): Conditioning Hatchery-Reared Fish To Recognize Chemical Cues of a Predator. Can. J. Fish. Aquat. Sci. 1998, 55, 611–617. [Google Scholar] [CrossRef]
- Vejřík, L.; Vejříková, I.; Blabolil, P.; Eloranta, A.P.; Kočvara, L.; Peterka, J.; Sajdlová, Z.; Chung, S.H.T.; Šmejkal, M.; Kiljunen, M.; et al. European catfish (Silurus glanis) as a freshwater apex predator drives ecosystem via its diet adaptability. Sci. Rep. 2017, 7, 15970. [Google Scholar] [CrossRef]
- Rask, M.; Arvola, L. The biomass and production of pike, perch and whitefish in two small lakes in southern Finland. Ann. Zool. Fennici 1985, 22, 129–136. [Google Scholar]
- Šmejkal, M.; Bartoň, D.; Děd, V.; Souza, A.T.; Blabolil, P.; Vejřík, L.; Sajdlová, Z.; Říha, M.; Kubečka, J. Negative feedback concept in tagging: Ghost tags imperil the long-term monitoring of fishes. PLoS One 2020, 15, e0229350. [Google Scholar] [CrossRef] [Green Version]
- Šmejkal, M.; Souza, A.T.; Blabolil, P.; Bartoň, D.; Sajdlová, Z.; Vejřík, L.; Kubečka, J. Nocturnal spawning as a way to avoid egg exposure to diurnal predators. Sci. Rep. 2018, 8, 15377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidabadi, F.; Abdoli, A.; Tajbakhsh, F.; Nejat, F.; Avigliano, E. Unravelling the stock structure of the Persian brown trout by otolith and scale shape. J. Fish Biol. 2019, 96, 307–315. [Google Scholar] [CrossRef]
- Bergendahl, I.A.; Miller, S.; Depasquale, C.; Giralico, L.; Braithwaite, V.A. Becoming a better swimmer: Structural complexity enhances agility in a captive-reared fish. J. Fish Biol. 2017, 90, 1112–1117. [Google Scholar] [CrossRef]
- Näslund, J.; Johnsson, J.I. Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. Fish Fish. 2016, 17, 1–30. [Google Scholar] [CrossRef]
- Anttila, K.; Jokikokko, E.; Erkinaro, J.; Järvilehto, M.; Mänttäri, S. Effects of training on functional variables of muscles in reared Atlantic salmon Salmo salar smolts: Connection to downstream migration pattern. J. Fish Biol. 2011, 78, 552–566. [Google Scholar] [CrossRef]
- He, W.; Xia, W.; Cao, Z.D.; Fu, S.J. The effect of prolonged exercise training on swimming performance and the underlying biochemical mechanisms in juvenile common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 166, 308–315. [Google Scholar] [CrossRef]
- Biro, P.A.; Post, J.R. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl. Acad. Sci. USA 2008, 105, 2919–2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.-Y.; Yan, X.-Y.; Li, X.-M.; Fu, X.; Xia, J.-G.; Fu, S.-J. Effect of exercise training on swimming performance, survival under predation and hypoxia tolerance in an endangered fish species in China. Mar. Freshw. Behav. Physiol. 2019, 52, 67–82. [Google Scholar] [CrossRef]
- Lennox, R.J.; Westrelin, S.; Souza, A.T.; Šmejkal, M.; Říha, M.; Prchalová, M.; Nathan, R.; Koeck, B.; Killen, S.; Jari, I.; et al. A role for lakes in revealing the nature of animal movement using high dimensional telemetry systems. Mov. Ecol. 2021, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.L. Nonlethal effects in the ecology of predator-prey interactions. Bioscience 1998, 48, 25. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, B.R.S.; Mormul, R.P.; Chapman, B.B.; Lolis, L.A.; Fiori, L.F.; Benedito, E. Turbidity amplifies the non-lethal effects of predation and affects the foraging success of characid fish shoals. Freshw. Biol. 2016, 61, 293–300. [Google Scholar] [CrossRef]
- Fredrich, F. Long-term investigations of migratory behaviour of asp (Aspius aspius L.) in the middle part of the Elbe River, Germany. J. Appl. Ichthyol. 2003, 19, 294–302. [Google Scholar] [CrossRef]
- Šmejkal, M.; Prchalová, M.; Čech, M.; Vašek, M.; Říha, M.; Jůza, T.; Blabolil, P.; Kubečka, J. Associations of fish with various types of littoral habitats in reservoirs. Ecol. Freshw. Fish 2014, 23, 405–413. [Google Scholar] [CrossRef]






| Estimate | Standard Error | Z Value | p Value | |
|---|---|---|---|---|
| Intercept | 2.553 | 0.855 | 2.986 | 0.003 |
| Condition | −0.865 | 0.648 | −1.334 | 0.182 |
| SWNC | 0.010 | 0.175 | 0.059 | 0.953 |
| SWC | 0.145 | 0.179 | 0.812 | 0.417 |
| FWNC | −0.163 | 0.168 | −0.968 | 0.333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šmejkal, M.; Bartoň, D.; Blabolil, P.; Podhorec, P.; Souza, A.T.; Stejskal, V.; Stepanyshyna, Y.; Tapkir, S. Does Fish Conditioning in Aquaculture Increase Survival Success in the Wild? A Case Study on a Cyprinid Fish. Sustainability 2021, 13, 13936. https://doi.org/10.3390/su132413936
Šmejkal M, Bartoň D, Blabolil P, Podhorec P, Souza AT, Stejskal V, Stepanyshyna Y, Tapkir S. Does Fish Conditioning in Aquaculture Increase Survival Success in the Wild? A Case Study on a Cyprinid Fish. Sustainability. 2021; 13(24):13936. https://doi.org/10.3390/su132413936
Chicago/Turabian StyleŠmejkal, Marek, Daniel Bartoň, Petr Blabolil, Peter Podhorec, Allan T. Souza, Vlastimil Stejskal, Yevdokiia Stepanyshyna, and Sandip Tapkir. 2021. "Does Fish Conditioning in Aquaculture Increase Survival Success in the Wild? A Case Study on a Cyprinid Fish" Sustainability 13, no. 24: 13936. https://doi.org/10.3390/su132413936
APA StyleŠmejkal, M., Bartoň, D., Blabolil, P., Podhorec, P., Souza, A. T., Stejskal, V., Stepanyshyna, Y., & Tapkir, S. (2021). Does Fish Conditioning in Aquaculture Increase Survival Success in the Wild? A Case Study on a Cyprinid Fish. Sustainability, 13(24), 13936. https://doi.org/10.3390/su132413936








