Valorization of the Olive Oil Production Residue: Healthy Ingredient for Developing High Value-Added Spread
Abstract
:1. Introduction
2. Materials and Methods
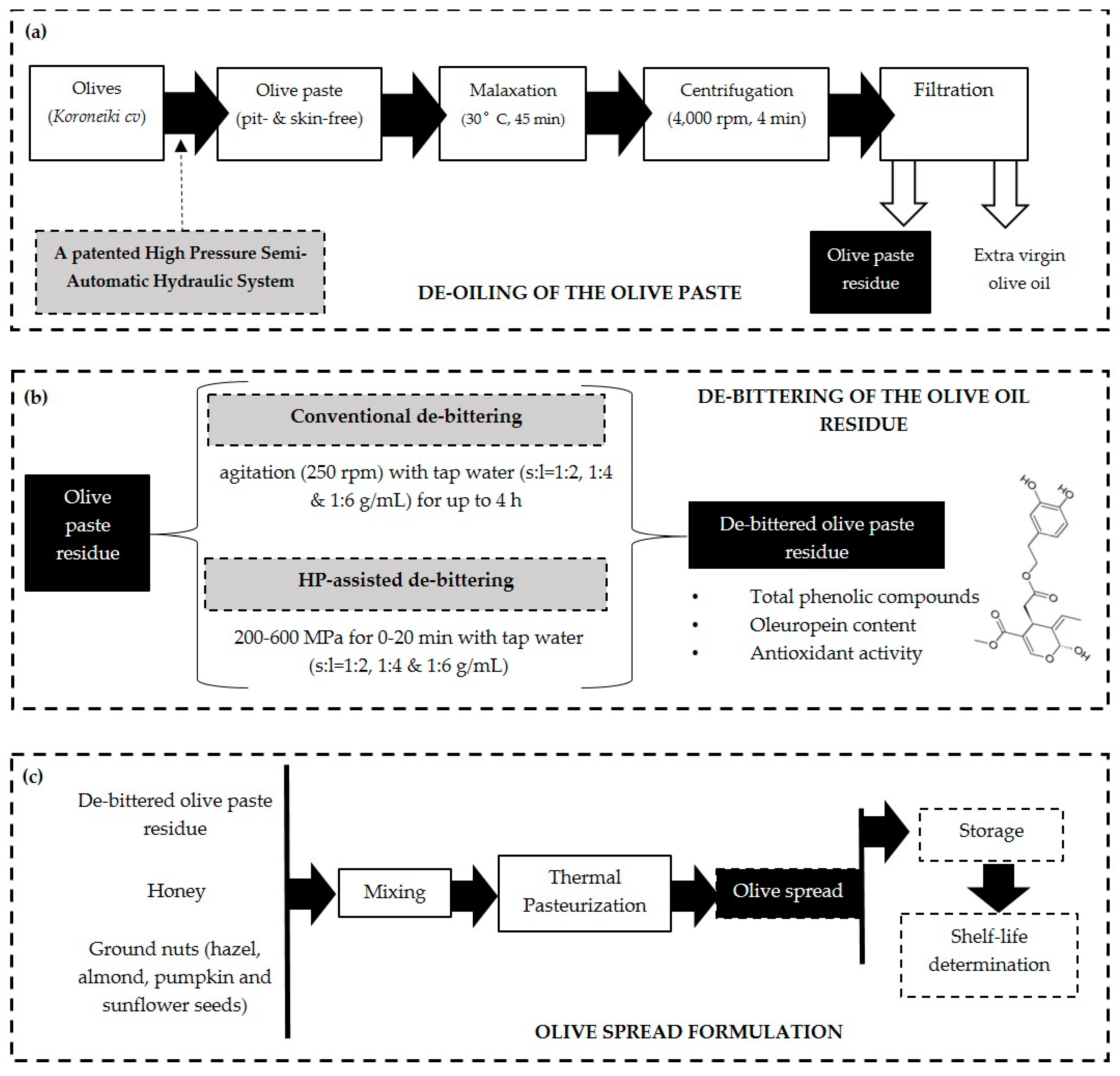
2.1. Raw Material
2.2. Experimental Design
2.3. Olive Oil Cold Extraction
Determination of Olive Oil Extraction Yield
2.4. De-Bittering of Olive Oil Residue
2.4.1. Conventional De-Bittering
2.4.2. High-Pressure-Assisted De-Bittering
2.5. New Product Formulation
2.6. Quality Assessment and Microbiological Stability of the Olive Spread
2.6.1. Physicochemical Parameters of the Olive Spread
2.6.2. Texture Analysis of the OLIVE Spread
2.6.3. Nutritional Profile of the Olive Spread
2.6.4. Fatty Acids Profile
2.6.5. Bioactive Compounds of the Olive Spread
2.6.6. Microbiological Analysis
2.6.7. Determination of Peroxide Value (PV)
2.6.8. Sensory Evaluation
2.7. Accelerated Shelf-Life Determination
2.8. Data Analysis
Mathematical Modelling of the Shelf-Life Determination of the Olive Spreads
2.9. Statistical Analysis
3. Results and Discussion
3.1. Olive Oil Production
3.2. Composition of Olive Paste Residue
3.3. De-Bittering Process of Olive Residue
3.4. Quality Characteristics of OLIVE Spread
3.5. Shelf-Life Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsarouhas, P.; Achillas, C.; Aidonis, D.; Folinas, D.; Maslis, V. Life Cycle Assessment of olive oil production in Greece. J. Clean. Prod. 2015, 93, 75–83. [Google Scholar] [CrossRef]
- Anania, G.; Pulpo, M.R. Olive oil in the Mediterranean area production. Consumption and trade. Obs. CIHEAM 2011, 16, 1–26. [Google Scholar]
- Bedbabis, S.; Trigui, D.; Ben Ahmed, C.; Clodoveo, M.L.; Camposeo, S.; Vivaldi, G.A.; Ben Rouina, B. Long-terms effects of irrigation with treated municipal wastewater on soil, yield and olive oil quality. Agric. Water Manag. 2015, 160, 14–21. [Google Scholar] [CrossRef]
- Turkekul, B.; Gunden, C.; Abay, C.; Miran, B. A Market Share Analysis of Virgin Olive Oil Producer Countries with Special Respect to Competitiveness; European Association of Agricultural Economists: Barcelona, Spain, 2007; No. 688-2016-47166. [Google Scholar]
- Servili, M.; Taticchi, A.; Esposto, S.; Sordini, B.; Urbani, B.S.A.S. Technological Aspects of Olive Oil Production. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; InTech: Rijeka, Croatia, 2012; pp. 151–172. [Google Scholar]
- Ranalli, A.; Costantini, N.; De Mattia, G.; Ferrante, M.L. Evaluating two kinds of centrifuged virgin oils arising from continuous olive processing. J. Sci. Food Agric. 2000, 80, 673–683. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Malaxation: Influence on virgin olive oil quality. Past, present and future—An overview. Trends Food Sci. Technol. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Vlyssides, A.; Loizides, M.; Karlis, P. Integrated strategic approach for reusing olive oil extraction by-products. J. Clean. Prod. 2004, 12, 603–611. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Gonzálvez, J.; Garcıa, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Ranalli, A.; Lucera, L.; Contento, S. Antioxidizing Potency of Phenol Compounds in Olive Oil Mill Wastewater. J. Agric. Food Chem. 2003, 51, 7636–7641. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Heredia, A.; Guillén, R.; Jiménez, A. Production in large quan-tities of highly purified hydroxytyrosol from liquid− solid waste of two-phase olive oil processing or “Alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Andreou, V.; Psarianos, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Effect of pulsed electric fields and high pressure on improved recovery of high-added-value compounds from olive pomace. J. Food Sci. 2020, 85, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging oppor-tunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Moral, P.S.; Ruiz-Méndez, M.V. Production of pomace olive oil. Grasas y Aceites 2006, 57, 47–55. [Google Scholar] [CrossRef]
- Lanza, B.; Di Serio, M.G.; Iannucci, E.; Russi, F.; Marfisi, P. Nutritional, textural and sensorial characterisation of Italian table olives (Olea europaea L. cv. ‘Intosso d’Abruzzo’). Int. J. Food Sci. Technol. 2009, 45, 67–74. [Google Scholar] [CrossRef]
- Bianchi, G. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003, 105, 229–242. [Google Scholar] [CrossRef]
- Ramirez, E.; García-García, P.; de Castro, A.; Romero, C.; Brenes, M. Debittering of black dry-salted olives. Eur. J. Lipid Sci. Technol. 2013, 115, 1319–1324. [Google Scholar] [CrossRef]
- Tamer, C.E.; Incedayı, B.; Yıldız, B.; Çopur, Ö.U. The Use of Vacuum Impregnation for Debittering Green Olives. Food Bioprocess Technol. 2013, 6, 3604–3612. [Google Scholar] [CrossRef]
- Owen, R.; Haubner, R.; Hull, W.; Erben, G.; Spiegelhalder, B.; Bartsch, H.; Haber, B. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem. Toxicol. 2003, 41, 1727–1738. [Google Scholar] [CrossRef]
- Shouqin, Z.; Jun, X.; Changzheng, W. High hydrostatic pressure extraction of flavonoids from propolis. J. Chem. Technol. Biotechnol. 2005, 80, 50–54. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, J.; Wang, C. Novel high pressure extraction technology. Int. J. Pharm. 2004, 78, 471–474. [Google Scholar]
- Chanioti, S.; Andreou, V.; Stergiou, P.; Katsaros, G. Development of a high added-value healthy spread by valorizing olive oil production residue. In Proceedings of the RETASTE Conference Abstracts Vol. 1 RETASTE-VAL-117, Athens, Greece, 6–8 May 2021. [Google Scholar]
- Andreou, V.; Dimopoulos, G.; Alexandrakis, Z.; Katsaros, G.; Oikonomou, D.; Toepfl, S.; Heinz, V.; Taoukis, P. Shelf-life evaluation of virgin olive oil extracted from olives subjected to nonthermal pretreatments for yield increase. Innov. Food Sci. Emerg. Technol. 2017, 40, 52–57. [Google Scholar] [CrossRef]
- Henneberg, W.; Stohmann, F. Fiber das Erhaltungsfutter volljahrigen Rindviehs.1. Landwirtsch 1859, 3, 485–551. [Google Scholar]
- Khanizadeh, S.; Buszard, D.; Zarkadas, C.G. Misuse of the Kjeldahl Method for Estimating Protein Content in Plant Tissue. HortScience 1995, 30, 1341–1342. [Google Scholar] [CrossRef] [Green Version]
- Andreou, V.; Dimopoulos, G.; Tsonas, T.; Katsimichas, A.; Limnaios, A.; Katsaros, G.; Taoukis, P. Pulsed Electric Fields-Assisted Drying and Frying of Fresh Zucchini. Food Bioprocess Technol. 2021, 14, 2091–2106. [Google Scholar] [CrossRef]
- Segura, M.E.M.; Rosell, C.M. Chemical Composition and Starch Digestibility of Different Gluten-free Breads. Plant Foods Hum. Nutr. 2011, 66, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. Environ. Boil. Fishes 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Official Methods of Analysis, 16th ed.; AOAC INTERNATIONAL: Arlington, VA, USA, 1995.
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Aka-Kayguluoglu, A.; Akpinar-Bayizit, A.; Sahin-Cebeci, O.I. Evaluation of physicochemical and sensory properties of green olive pastes. Res. J. 2014, 13, 654–658. [Google Scholar]
- Marx, I.; Rodrigues, N.; Dias, L.; Veloso, A.C.A.; Pereira, J.A.; Drunkler, D.A.; Peres, A.M. Sensory classification of table olives using an electronic tongue: Analysis of aqueous pastes and brines. Talanta 2017, 162, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendini, A.; Valli, E.; Barbieri, S.; Toschi, T.G. Sensory analysis of virgin olive oil. In Olive Oil—Constituents, Quality, Health Properties and Bioconversions; InTech: Rijeka, Croatia, 2012; pp. 109–130. [Google Scholar]
- Conte, P.; Fadda, C.; Del Caro, A.; Urgeghe, P.P.; Piga, A. Table Olives: An Overview on Effects of Processing on Nutritional and Sensory Quality. Foods 2020, 9, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhoosh, R.; Hoseini-Yazdi, S.-Z. Shelf-life prediction of olive oils using empirical models developed at low and high temperatures. Food Chem. 2013, 141, 557–565. [Google Scholar] [CrossRef]
- Okpala, C.O.R.; Bono, G.; Geraci, M.L.; Sardo, G.; Vitale, S.; Schaschke, C.J. Lipid oxidation kinetics of ozone-processed shrimp during iced storage using peroxide value measurements. Food Biosci. 2016, 16, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Gotoh, N.; Wada, S. The importance of peroxide value in assessing food quality and food safety. J. Am. Oil Chem. Soc. 2006, 83, 473–474. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Gómez-Rico, A.; Salvador, M.D.; Fregapane, G. Influence of malaxation conditions on virgin olive oil yield, overall quality and composition. Eur. Food Res. Technol. 2009, 228, 671–677. [Google Scholar] [CrossRef]
- Ranalli, A. Aspects and problems connected with the storage and the preservation of the olive-oil. Inf. Agrar. 1988, 44, 79–85. [Google Scholar]
- Ranalli, A.; Pollastri, L.; Contento, S.; Iannucci, E.; Lucera, L. Effect of olive paste kneading process time on the overall quality of virgin olive oil. Eur. J. Lipid Sci. Technol. 2003, 105, 57–67. [Google Scholar] [CrossRef]
- Di Giovacchino, L.; Costantini, N.; Serraiocco, A.; Surricchio, G.; Basti, C. Natural antioxidants and volatile com-pounds of virgin olive oils obtained by two or three-phases centrifugal decanters. Eur. J. Lipid Sci. Technol. 2001, 103, 279–285. [Google Scholar] [CrossRef]
- Caponio, F.; Alloggio, V.; Gomes, T. Phenolic compounds of virgin olive oil: Influence of paste preparation tech-niques. Food Chem. 1999, 64, 203–209. [Google Scholar] [CrossRef]
- Di Giovacchino, L.; Sestili, S.; Di Vincenzo, D. Influence of olive processing on virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 587–601. [Google Scholar] [CrossRef]
- Catalano, P.; Caponio, F. Machines for olive paste preparation producing quality virgin olive oil. Lipid Fett 1996, 98, 408–412. [Google Scholar] [CrossRef]
- Caponio, F.; Catalano, P. Hammer crushers vs disk crushers: The influence of working temperature on the quality and preservation of virgin olive oil. Eur. Food Res. Technol. 2001, 213, 219–224. [Google Scholar] [CrossRef]
- Amirante, P.; Clodoveo, M.L.; Tamborrino, A.; Leone, A.; Paice, A.G. Influence of the crushing system: Phenol content in virgin olive oil produced from whole and de-stoned pastes. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2010; pp. 69–76. [Google Scholar]
- Nunes, M.A.; Costa, A.S.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid-and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zabaniotou, A.; Kalogiannis, G.; Kappas, E.; Karabelas, A. Olive residues (cuttings and kernels) rapid pyrolysis product yields and kinetics. Biomass Bioenergy 2000, 18, 411–420. [Google Scholar] [CrossRef]
- Cocolin, L.; Alessandria, V.; Botta, C.; Gorra, R.; De Filippis, F.; Ercolini, D.; Rantsiou, K. NaOH debittering induces changes in bacterial ecology during table olives fermentation. PLoS ONE 2013, 8, e69074. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; de Castro, A. Transformation of oleuropein and its hydrolysis products during Spanish-style green olive processing. J. Sci. Food Agric. 1998, 77, 353–358. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors Influencing Phenolic Compounds in Table Olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Golmakani, M.T.; Mesbahi, G.; Majzoobi, M.; Farahnaky, A. Ultrasound-accelerated debittering of olive fruits. Innov. Food Sci. Emerg. Technol. 2015, 31, 105–115. [Google Scholar] [CrossRef]
- Habibi, M.; Golmakani, M.T.; Farahnaky, A.; Mesbahi, G.; Majzoobi, M. NaOH-free debittering of table olives using power ultrasound. Food Chem. 2016, 192, 775–781. [Google Scholar] [CrossRef]
- García, A.; Romero, C.; Medina, E.; García, P.; De Castro, A.; Brenes, M. Debittering of olives by polyphenol oxidation. J. Agric. Food Chem. 2008, 56, 11862–11867. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Settanni, L.; Veneziani, G.; Esposto, S.; Massitti, O.; Taticchi, A.; Urbani, S.; Montedoro, G.F.; Corsetti, A. The Use of Lactobacillus pentosus 1MO To Shorten the Debittering Process Time of Black Table Olives (Cv. Itrana and Leccino): A Pilot-Scale Application. J. Agric. Food Chem. 2006, 54, 3869–3875. [Google Scholar] [CrossRef]
- Muego, K.F.; Resurreccion, A.V.A.; Hung, Y. Characterization of the textural properties of spreadable peanut based products. J. Texture Stud. 1990, 21, 61–74. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Ali, T. Textural Quality of Peanut Butter as Influenced by Peanut Seed and Oil Contents1. Peanut Sci. 1986, 13, 18–20. [Google Scholar] [CrossRef]
- King, J.C.; Blumberg, J.; Ingwersen, L.; Jenab, M.; Tucker, K.L. Tree Nuts and Peanuts as Components of a Healthy Diet. J. Nutr. 2008, 138, 1736S–1740S. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Seven, S. Physıcal and chemıcal analysıs and fatty acıd composıtıon of peanut, peanut oıl and peanut butter from ÇOM and NC-7 cultıvars. Grasas y Aceites 2003, 54, 12–18. [Google Scholar]
- Labuza, T.P. Shelf-Life Dating of Foods; Food & Nutrition Press, Inc.: Westport, CT, USA, 1982. [Google Scholar]
- Chu, Y.-H.; Hsu, H.-F. Effects of antioxidants on peanut oil stability. Food Chem. 1999, 66, 29–34. [Google Scholar] [CrossRef]
- García-Pascual, P.; Mateos, M.; Carbonell, V.; Salazar, D. Influence of Storage Conditions on the Quality of Shelled and Roasted Almonds. Biosyst. Eng. 2003, 84, 201–209. [Google Scholar] [CrossRef]
- Cert, A.; Alba, J.; León-Camacho, M.; Moreda, W.; Pérez-Camino, M.C. Effects of Talc Addition and Operating Mode on the Quality and Oxidative Stability of Virgin Olive Oils Obtained by Centrifugation. J. Agric. Food Chem. 1996, 44, 3930–3934. [Google Scholar] [CrossRef]
- Argyri, E.A.; Piromalis, S.P.; Koutelidakis, A.; Kafetzopoulos, D.; Petsas, A.S.; Skalkos, D.; Karantonis, H.C. Ol-ive Paste-Enriched Cookies Exert Increased Antioxidant Activities. Appl. Sci. 2021, 11, 5515. [Google Scholar] [CrossRef]
- Oey, I.; Lille, M.; Van Loey, A.; Hendrickx, M. Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: A review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- Food Code. Recommendations of the United States Public Health Service; National Technical Information Service Publication, PB2005-102200; Food and Drug Administration: New Orleans, LA, USA, 2005. [Google Scholar]


| Oleuropein Content (mg/g d.m.) | Bitterness Intensity (9 is the Max) | ||
|---|---|---|---|
| Untreated paste | 2.14 ± 0.22 a | 8.9 ± 0.1 a | |
| HP-assisted de-bittering | 200 MPa/10 min | 1.09 ± 0.06 c | 6.5 ± 0.2 c |
| 200 MPa/20 min | 0.96 ± 0.03 cd | 6.4 ± 0.2 c | |
| 600 MPa/10 min | 0.95 ± 0.09 cd | 6.6 ± 0.1 c | |
| 600 MPa/20 min | 0.78 ± 0.06 d | 5.5 ± 0.3 d | |
| Conventional de-bittering | 1 h | 1.55 ± 0.22 b | 7.0 ± 0.1 b |
| F-value | 41.5 *** | 116.6 *** | |
| Nutritional and Bioactive Compounds | Untreated Olive Paste Residue | HP-Assisted De-bittered Olive Paste Residue | Conventionally de-Bittered Olive Paste Residue | F-Value |
|---|---|---|---|---|
| Moisture (g H2O/100 g fresh product) | 72.12 ± 0.65 b | 85.57 ± 1.20 a | 86.79 ± 1.55 a | 139.84 *** |
| Ash (g/100 g d.m) | 11.43 ± 0.15 a | 1.62 ± 0.04 b | 1.75± 0.12 b | 7400 *** |
| Proteins (g/100 g d.m) | 10.01 ± 1.22 | 10.59 ± 1.01 a | 10.20 ± 0.83 a | 0.24 ns |
| Total fat (g/100 g d.m) | 33.58 ± 1.05 a | 34.32 ± 0.99 a | 34.57 ± 0.78 a | 1.45 ns |
| Total carbohydrates (g/100 g d.m) | 14.45 ± 2.12 a | 14.51 ± 1.45 a | 15.98 ± 1.01 a | 0.83 ns |
| Crude fibers (g/100 g d.m) | 30.53 ± 2.12 b | 38.96 ± 1.15 a | 37.50 ± 0.85 a | 27.93 *** |
| Antioxidant capacity (mg Trolox/100 g d.m.) | 40.41 ± 1.65 a | 20.81 ± 1.05 b | 17.98 ± 1.22 c | 252.74 *** |
| Quality Parameters | Spread Produced by HP-Assisted de-Bittered Olive Residue | Spread Produced by Conventionally de-Bittered Olive Residue | F-Value |
|---|---|---|---|
| pH | 4.22 ± 0.01 a | 4.23 ± 0.01 a | 5.00 ns |
| aw | 0.936 ± 0.004 a | 0.941 ± 0.010 a | 3.80 ns |
| Moisture (g H2O/100 g) | 47.98 ± 2.84 a | 49.15 ± 1.04 a | 0.30 ns |
| Ash (g/100 g) | 0.88 ± 0.03 b | 0.93 ± 0.04 a | 10.24 ns |
| L* | 47.79 ± 0.39 b | 49.59 ± 0.67 a | 53.70 ** |
| a* | 0.63 ± 0.14 a | 0.78 ± 0.11 a | 5.56 ns |
| b* | 21.33 ± 0.51 a | 18.74 ± 0.36 b | 160.96 *** |
| Firmness (N) | 8.38 ± 1.69 a | 9.12 ± 0.89 a | 0.45 ns |
| Spreadability (kg s) | 1.19 ± 0.34 a | 1.45 ± 0.21 a | 1.26 ns |
| Adhesiveness (kg s) | 0.42 ± 0.09 a | 0.65 ± 0.15 a | 5.18 ns |
| Nutritional Parameters | Spread Produced by HP-Assisted de-Bittered Olive Residue | Spread Produced by Conventionally de-Bittered Olive Residue | F-Value |
|---|---|---|---|
| Total Fat (g/100 g) | 17.88 ± 1.22 a | 16.48 ± 1.15 a | 2.09 ns |
| Proteins (g/100 g) | 8.57 ± 0.82 a | 9.42 ± 0.95 a | 1.37 ns |
| Total Carbohydrates (g/100 g) | 17.72 ± 0.15 a | 16.25 ± 0.88 b | 9.45 ns |
| Sugars (g/100 g) | 1.43 ± 0.12 a | 1.25 ± 0.06 a | 5.40 ns |
| Crude fibers (g/100 g) | 6.97 ± 0.56 a | 7.77 ± 0.35 a | 4.40 ns |
| Total phenolic content (mg CAE/g d.m.) | 10.08 ± 0.15 a | 5.71 ± 0.08 b | 1982.38 *** |
| Antioxidant capacity (mg Trolox/g d.m.) | 8.67 ± 0.09 a | 4.65 ± 0.05 b | 4573.70 *** |
| Fatty Acids Composition (%) | Untreated Olive Paste Residue | Spread Produced by HP-Assisted de-Bittered Olive Residue | Spread Produced by Conventionally de-Bittered Olive Residue | F-Value |
|---|---|---|---|---|
| Myristic acid (C14:0) | 0.094 ± 0.005 a | 0.056 ± 0.002 c | 0.071 ± 0.003 b | 86.76 *** |
| Palmitic acid (C16:0) | 11.50 ± 0.09 a | 7.48 ± 0.05 c | 7.85 ± 0.04 b | 3641.80 *** |
| Palmitoleic acid (C16:1, cis-9) | 0.623 ± 0.019 a | 0.268 ± 0.011 b | 0.273 ± 0.018 b | 473.82 *** |
| Stearic acid (C18:0) | 2.89 ± 0.01 c | 3.00 ± 0.02 b | 3.14 ± 0.05 a | 47.10 *** |
| Elaidic acid (C18:1, trans-9) | 0.058 ± 0.019 b | 0.390 ± 0.010 a | 0.390 ± 0.015 a | 482.03 *** |
| Oleic acid (C18:1, cis-9) | 75.10 ± 0.05 a | 67.10 ± 0.08 b | 64.70 ± 0.09 c | 15699.00 *** |
| Linoleic acid (C18:2 cis-9,12), | 4.77 ± 0.03 c | 17.90 ± 0.19 a | 17.50 ± 0.28 b | 4419.67 *** |
| α-Linolenic acid (C18:3, cis-9,12,15) | 0.491 ± 0.045 a | 0.175 ± 0.010 b | 0.172 ± 0.009 b | 163.61 *** |
| Arachidic acid (C20:0) | 0.491 ± 0.032 a | 0.236 ± 0.022 b | 0.212 ± 0.017 b | 140.23 *** |
| cis-11-Eicosenoic acid (C20:1) | 0.265 ± 0.017 a | 0.180 ± 0.032 b | 0.186 ± 0.014 b | 8.65 ns |
| cis-11,14-Eicosadienoic acid (C20:2) | 0.548 ± 0.012 a | 0.550 ± 0.010 a | 0.546 ± 0.017 a | 0.07 ns |
| Behenic acid (C22:0) | 0.105 ± 0.027 b | 0.160 ± 0.008 a | 0.161 ± 0.011 a | 10.66 ** |
| Lignoceric acid (C24:0) | 0.063 ± 0.009 a | 0.065 ± 0.012 a | 0.073 ± 0.015 a | 0.57 ns |
| Storage Temperature (°C) | k (d−1) | |
|---|---|---|
| Spread Produced by HP-Assisted de-Bittered Olive Residue | Spread Produced by Conventionally de-Bittered Olive Residue | |
| 20 | 0.1234 ± 0.0012 aA | 0.1377 ± 0.0022 aB |
| 30 | 0.1406 ± 0.0026 bA | 0.1499 ± 0.0037 bB |
| 40 | 0.1588 ± 0.0036 cA | 0.1761 ± 0.0096 bB |
| Shelf-life determination (days) | ||
| 4 °C | 293 ± 6 A | 275 ± 12 B |
| 20 °C | 211 ± 5 A | 182 ± 9 B |
| Fatty Acids Composition (%) | Spread Produced by HP-Assisted de-Bittered Olive Residue | Spread Produced by Conventionally de-Bittered Olive Residue | ||||
|---|---|---|---|---|---|---|
| 0 Days | 4 Months at 40 °C | F-Value | 0 Days | 4 Months at 40 °C | F-Value | |
| SFA (%) | 10.99 ± 0.25 bA | 12.91 ± 0.12 aB | 143.81 *** | 11.51 ± 0.45 bA | 13.80 ± 0.22 aA | 62.70 *** |
| MUFA (%) | 67.93 ± 1.12 aA | 64.75 ± 0.99 bA | 13.58 * | 65.55 ± 0.82 aA | 62.25 ± 0.35 bB | 41.10 ** |
| PUFA (%) | 18.62 ± 0.12 aA | 18.60 ± 0.08 aA | 0.1 ns | 18.22 ± 0.17 aB | 17.21 ± 0.33 bB | 22.21 ** |
| OS (%) | 890.93 ± 2.35 aA | 887.45 ± 3.21 aA | 2.3 ns | 870.25 ± 1.89 aB | 821.75 ± 2.05 bB | 908.10 *** |
| F-value for 0 days | SFA (%) | 3.06 ns | F-value for 4 months at 40 °C | SFA (%) | 37.84 ** | |
| MUFA (%) | 8.82 ns | MUFA (%) | 17.01 * | |||
| PUFA (%) | 11.09 * | PUFA (%) | 50.27 ** | |||
| OS (%) | 141.00 *** | OS (%) | 892.70 *** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreou, V.; Chanioti, S.; Stergiou, P.; Katsaros, G. Valorization of the Olive Oil Production Residue: Healthy Ingredient for Developing High Value-Added Spread. Sustainability 2021, 13, 13984. https://doi.org/10.3390/su132413984
Andreou V, Chanioti S, Stergiou P, Katsaros G. Valorization of the Olive Oil Production Residue: Healthy Ingredient for Developing High Value-Added Spread. Sustainability. 2021; 13(24):13984. https://doi.org/10.3390/su132413984
Chicago/Turabian StyleAndreou, Varvara, Sofia Chanioti, Panagiota Stergiou, and George Katsaros. 2021. "Valorization of the Olive Oil Production Residue: Healthy Ingredient for Developing High Value-Added Spread" Sustainability 13, no. 24: 13984. https://doi.org/10.3390/su132413984






