1. Introduction
There are a great variety of peach trees (Prunus persica L.), not only in terms of the length of ripening period, but also in terms of the pomological characteristics of the fruit, where we can distinguish yellow-fleshed, white-fleshed, red-fleshed, fully separable from the stone or clings, flat-shaped varieties, referred to as Peento, that are very popular in southern Italy and Asia. There are also well-known selections of varieties without any anthocyanin content, originating in Italy (the ‘ice peach’), and the Californian ‘Royal’ series of varieties, which are characterised by their very hard flesh and very low acid content, giving the fruit a sweet taste.
From a nutritional point of view, peaches contain a number of beneficial substances, making them an interesting addition to the human diet. Peaches are a rich source of dietary fibre (1.5 g.100 g
−1) and provitamin A [
1]. This fruit is considerably rich in antioxidants and is an important source of vitamins A, B, and C, carotenoids and phenolic compounds. Among the most important phenolic acids are chlorogenic and neochlorogenic acids, catechin, epicatechin, 3-glucoside of cyanidin (chrysanthemin), and quercetin derivatives [
2,
3,
4,
5]. Polyphenols represent the majority of antioxidants present in the diet and their daily intake should exceed 1 g/day, which is much higher than that of all other classes of phytochemicals and known dietary antioxidants [
6]. They are low in fat and contain a lot of water, approximately 89 g per 100 g of fruit [
7,
8]. Peaches are very low in sugars (9–20 °Rf), with the main sugars present being sucrose, fructose, sorbitol, and glucose. The proportions of these sugars undergo changes during fruit ripening, with glucose and fructose being present in greater amounts in immature fruit and increasing as ripening progresses. At full maturity, sucrose content dominates [
9,
10,
11]. Carbohydrates are an important source of energy in the human diet and also play an important role in the regulation of the gut microbiota [
12]. They also have low levels of organic acids (0.13–1.16%) such as malic, citric, and folic acids. The content of L-ascorbic acid (vitamin C) in peaches is relatively low compared to other fruits such as kiwifruit or oranges, in which it is the most important antioxidant. Quinic, fumaric, and shikimic acids are present in smaller concentrations [
13,
14]. Amino acids (arginine, asparagine, isoleucine, lysine, serine, threonine, valine, leucine, phenylalanine, tryptophan, tyrosine, proline, and alanine) also contribute to the flavour of fruit and are found in peaches in different concentrations depending on the cultivar [
15,
16]. Among the mineral elements, they contain nitrogen, phosphorus, potassium, calcium, magnesium, iron, manganese, zinc, cooper, chromium, nickel, cobalt, lead, selenium, and fluoride [
17,
18]. Similar to apricots, the glycoside amygdalin (26%), protein amandine (3.8%), enzymes, lactase, and oleic acids are present in peach kernels. The leaves contain about 1% prunasin and are used against rheumatism, gastritis, headaches, and as a diuretic; when used externally, they are effective against eczema, ulcers, and other dermatoses [
19].
The potential of peaches, especially those rich in phenolics, lies in delaying or even preventing the onset of neurogenerative diseases such as Alzheimer’s and Parkinson’s. They also help in the prevention of inflammation, atherosclerosis, diabetes, obesity, and cardiovascular disease. Due to their low sugar content, they can easily be included in nutritional therapy. They are easily digestible, have a strong alkaline effect on the body, and stimulate the secretion of digestive juices. They have both a laxative and a diuretic effect. Peach phenolics have been shown to display several biological activities such as antioxidant activity [
20,
21], anti-allergic and anti-inflammatory activities [
22], antibacterial activity [
23], hepatoprotective activity [
24], nephroprotective activity [
25], antiproliferative [
26], chemopreventive, and anticancer activities [
27,
28].
The aim of this study was to compare varieties from different pomological groups as well as different geographical origins and thus get an overview of the differences in content composition from the point of view of titratable acidity, soluble solid content, sugars, phenolic compounds, flavonoids, antioxidant activity, carotenoids, and total anthocyanin content.
3. Results
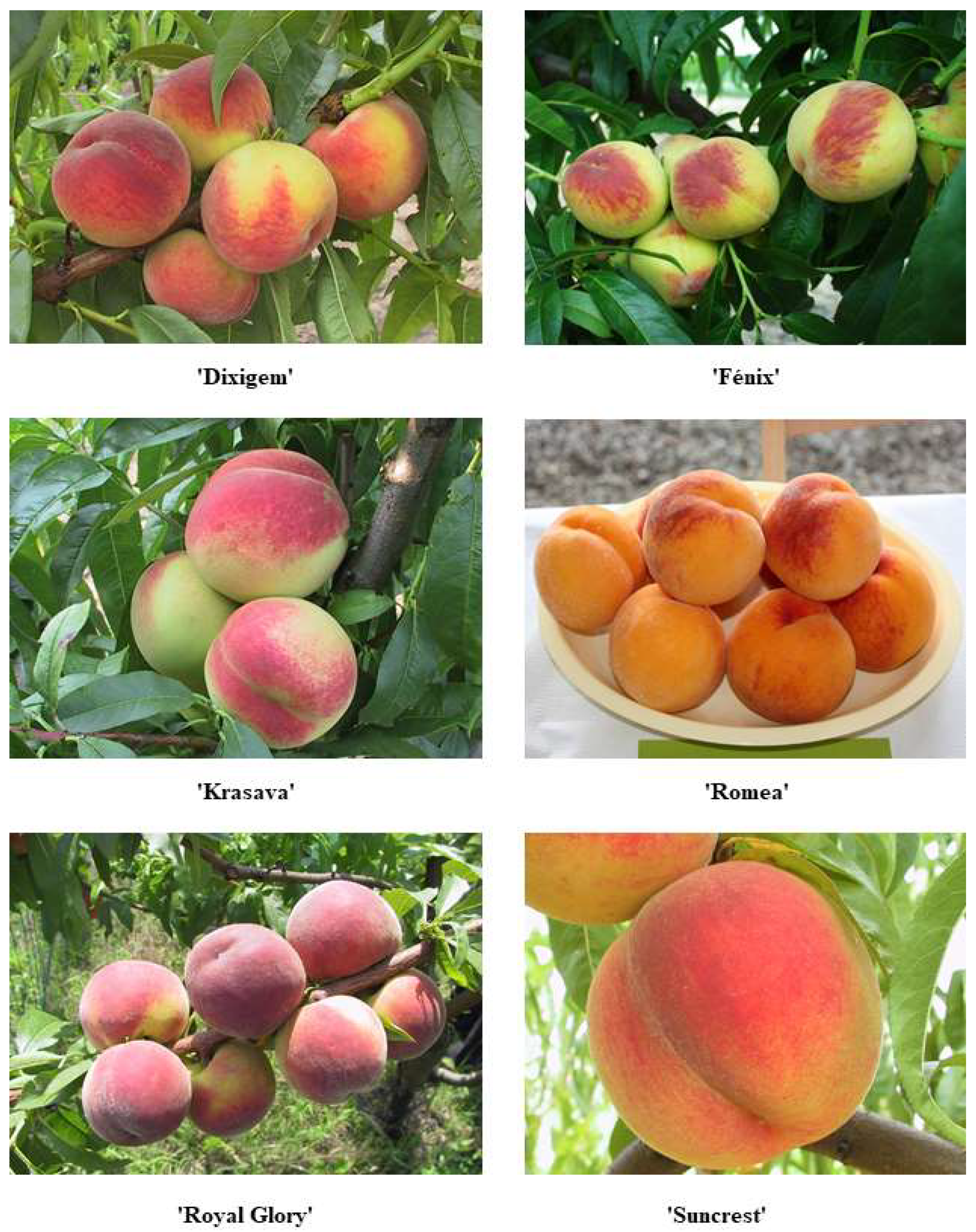
The highest acid content was recorded in the fruit of the varieties ‘Benedicte’ (1.32% malic acid), ‘Helene’ (0.91% malic acid), and ‘Royal Majestic’ (0.85% malic acid). The varieties with the lowest acid content were ‘UFO 3’ (0.25% malic acid), ‘Fidelia’ (0.26% malic acid) and ‘Royal Glory’ (0.26% malic acid,
Figure 1). The average value of the test set was 0.59% malic acid. The differences between the varieties were confirmed as statistically highly significant (
Table 2).
Significantly, the highest representation of total phenolic compounds was found in fruits of the variety ‘Carolina Belle’ (577.72 mg GAE.100 g
−1 FW), then in the variety ‘Krasava’ (334.02 mg GAE.100 g
−1 FW,
Figure 1), ‘Dixigem’ (285.24 mg GAE.100g
−1 FW,
Figure 1), and in the variety ‘Benedicte’ (238.09 mg GAE.100 g
−1 FW). On the other hand, the lowest values of phenolic compounds content were observed in fruits of ‘Favorita Morettini’ (9.43 mg GAE.100 g
−1 FW), ‘Early Redhaven’ (12.90 mg GAE.100 g
−1 FW), and ‘Strelec’ (17.39 mg GAE.100 g
−1 FW). In the studied set of cultivars, the total phenolic content in fruits ranged from 9.43 to 577 mg GAE.100 g
−1 FW. The differences between the values were highly statistically significant (
Table 3).
The highest concentration of flavonoids was measured in the fruits of ‘Carolina Belle’ (95.1 mg CAE.100 g
−1 FW), ‘Benedicte’ (53.2 mg CAE.100 g
−1 FW), and ‘Admiral de Wey’ (50.8 mg CAE.100 g
−1 FW). The lowest values were observed in ‘UFO 3’, ‘Favorita Morettini’, ‘Alexandra’ and ‘Candor’ (1.12; 3.37; 4.09 and 5.16 mg CAE.100 g
−1 FW). The average flavonoid value in the test set was 22.3 mg CAE.100 g
−1 FW. The differences between the varieties were confirmed as statistically highly significant (
Table 4).
Using the DPPH (2,2-diphenyl-1-picrylhydrazyl) method, values of antioxidant activity in peach fruits ranging from 136 to 462 mg TE.100 g
−1 FW were determined. Specifically, the cultivar ‘Carolina Belle’ (249.08 mg TE.100 g
−1 FW) had the highest value. All other varieties analysed showed relatively high values. The results varied within a few units. High values were also found in the fruit of the variety ‘Admiral de Wey’ (280.46 mg TE.100 g
−1 FW) and in the variety ‘Dixigem’ (255.61 mg TE.100 g
−1 FW). The Czech variety ‘Krasava’ also had high antioxidant capacity (250.07 mg TE.100 g
−1 FW). The lowest total antioxidant capacity was measured in the fruits of ‘Favorita Morettini’ (136.15 mg TE.100 g
−1 FW) and ‘Candor’ (150.72 mg TE.100 g
−1 FW). The differences in the values were highly statistically significant (
Table 5).
The average carotenoids content in the fruits of the studied varieties reached 1.67g.100 g
−1 DW. The varieties with the highest carotenoids (4.77 mg.100 g
−1 DW) include fruits of the variety ‘Romea’ (3.50 mg.100 g
−1 DW,
Figure 1), followed by fruits of the variety ‘Royal Majestic’ (3.14 mg.100 g
−1 DW), ‘Favorita Morettini’ (3.12 mg.100 g
−1 DW), and ‘Early Redhaven’ (3.12 mg.100 g
−1 DW). On the other hand, the lowest total carotenoids content was determined in the fruits of ‘Krasava’, ‘Fidelia’, and ‘Anita’ (0.05; 0.24 and 0.24 mg.100 g
−1 DW). Total carotenoids content was not detected in the cultivars ‘Benedicte’ and ‘Royal Glory’. The differences between the varieties were confirmed as statistically highly significant (
Table 6).
High levels of anthocyanins were measured in the fruits of ‘Helene’ (3.74 mg.100 g
−1 FW), ‘Royal Majestic’ (2.64 mg.100 g
−1 FW), and ‘Favorita Morettini’ (2.13 mg.100 g
−1 FW). On the other hand, low values were recorded in fruits of ‘Early Redhaven’, ‘UFO 3’, ‘Dostojnyj’, ‘Strelec’ and ‘Admiral de Wey’ (0.05; 0.05 0.14; 0.18 mg.100 g
−1 FW). The average value of total anthocyanins of the tested set of varieties reached 0.70 mg.100 g
−1 FW. The differences between the varieties were confirmed as statistically highly significant (
Table 7).
In the set of varieties studied, the total soluble solids content of the fruit ranged from 8.3 to 14.7 °Rf. The varieties with the highest content were ‘Royal Majestic’ (14.7 °Rf), followed by ‘Helene’ (13.8 °Rf) and ‘Nerine’ (13.7 °Rf). The lowest values of the evaluated set of varieties were measured for the fruits of the ‘Fénix’ variety (8.3 °Rf,
Figure 1), ‘Krasava’ and ‘Romea’, which had the same soluble solids value for both varieties (9.2 °Rf). The differences in the values found were highly statistically significant (
Table 8).
The average sucrose, glucose, fructose, and sorbitol contents of the fruit were determined for each variety. The average sucrose content was 9.62 g.100 g
−1 FW. The highest sucrose content was measured in the varieties ‘Narjadnyj Nikitskiy’ (16.57 g.100 g
−1) and ‘Sonet’ (16.44 g.100 g
−1). The lowest contents were observed in the cultivars ‘Alexandra’, ‘Suncrest’(
Figure 1), and ‘Iris Rosso’ (4.89, 4.69 and 4.66 g.100 g
−1, respectively). The glucose content ranged from 0.74 to 3.67 g.100 g
−1. The highest contents were determined in the varieties ‘Sunshine’ (3.67 g.100 g
−1) and ‘Admiral de Wey’ (3.50 g.100 g
−1). The lowest content was measured in the varieties ‘UFO 3’ (0.82 g.100 g
−1) and ‘Nerine’ (0.74 g.100 g
−1). The average value of glucose content was 1.94 g.100 g
−1. In the studied set of varieties, the total fructose content ranged from 0.48 to 2.39 g.100 g
−1, with an average value of 1.37 g.100 g
−1. The highest content was measured in the cultivars ‘Sunshine’ and ‘Dixigem’ (2.39 and 2.36 g.100 g
−1). The lowest fructose content was observed in the variety ‘UFO 3’ (0.48 g.100 g
−1). The average value of alcoholic sugar sorbitol in our study was 0.23 g.100 g
−1. The variety ‘Benedicte’ greatly exceeded all other varieties in sorbitol content, with its content being determined at 1.57 g.100 g
−1. Very low amounts were measured in the cultivars ‘Lakomyj’, ‘Nerine’, ‘Iris Rosso’, and ‘Alexandra’ (0.09; 0.09; 0.08 and 0.06 g.100 g
−1). The differences in the values found were highly statistically significant (
Table 9).
Colorimetric parameters
L*,
a*,
b* for the basic skin colour of the fruit were measured for all varieties. In the varieties ‘Alexandra’, ‘Anita’, ‘Helene’, ‘Iris Rosso’, ‘Royal Glory’ and ‘Royal Majestic’, the skin was completely covered by the blush. The average values of
L*,
a*,
b* are summarised in
Table 10. The highest values of
L* were found for the basic colour in the varieties ‘Krasava’, ‘Aurelia’, ‘Sunshine’ and for the cheek in the varieties ‘Romea’, ‘Dostojnyj’, ‘Carolina Belle’. In our study the highest value of
a* were found for ‘Nerine’, ‘Admiral de Wey’, ‘Avalon Pride’, the lowest value were found for ‘Krasava’, ‘Otličnik’, ‘Carolina Belle’ and ‘Queen Lady’. For chromatic parameter
b* the highest values were measured for ‘Romea’, ‘Otličnik’, ‘Lakomyj’ and the lowest values were found for ‘Fidelia’, ‘UFO 3’ and ‘Red Robin’. Colour intensity is represented by the chromatic parameter
C*ab, which was determined using the chromatic parameters
a* and
b*, and its highest values were found for the basic colour of ‘Romea’, ‘Otličnik’, ‘Lakomyj’ and for the cheek colour of ‘Romea’, ‘Sunshine’, ‘Admiral de Wey’. From the measured values for base colour and cheek colour, the greatest colour difference
ΔE*ab (
Table 11) was found for the cultivars ‘Otličnik’, ‘Lakomyj’, ‘Queen Lady’. These varieties had the richest cheeks when compared to the base colour. On the other hand, the lowest
ΔE*ab were found for the varieties ‘Red Robin’, ‘Romea’, ‘UFO 3’, where the cheek almost merged with the base colour.
Figure 2 captures the exact colour found in the
L*,
a*,
b* coordinates.
4. Discussion
The acid content of fruit is a key quality parameter and is an important factor in determining the taste of the fruit. Titratable acidity indicates the concentration of organic acids present in the fruit. Peaches have a very low level of organic acids. The total titratable acid content found in our set of varieties ranged from 0.26 to 1.32% malic acid on fresh weight. These values are similar to the results found in many other publications. Scordino et al. (2012) [
38] reported TA contents ranging from 0.52–0.86% malic acid in Sicilian yellow flesh peaches on fresh weight. Similar values were also found in the work by Tomás-Barberán et al. (2010) [
39], where the contents ranged from 0.53–0.97% malic acid in yellow flesh peaches on fresh weight, and 0.15–0.34% malic acid in white flesh peaches on fresh weight. Gil et al. (2002) [
40] investigated the differences between white- and yellow-fleshed peach cultivars grown in California. The average TAC content found in the white-fleshed varieties was 0.22%, and in the yellow-fleshed varieties it was 0.69%.
In a publication by Cantin et al. (2009) [
41], the total phenolic content ranged from 12.7 to 71.3 mg GAE.100 g
−1 FW, with an average of 36.4 mg GAE.100 g
−1 FW. In our selected set of cultivars, the average content reached 122.4 mg GAE.100 g
−1 FW. Marinova et al. (2005) [
42] investigated the determination of all phenolic compounds in fruit grown in Bulgaria. The total phenolic content in peach fruits was 50.9 mg GAE.100 g
−1 FW, and similar values were reached by figs—
Ficus carica (59.0 mg GAE.100 g
−1 FW). Another publication by Saidani et al. (2017) [
43] dealt with the determination of phenolic compounds separately in the peel and in the pulp. In the peel, contents ranging from 88.9 to 277 mg GAE.100 g
−1 FW were determined, while in the pulp, contents ranging from 25.1 to 139 mg GAE.100 g
−1 FW were determined. Previously, Zhao et al. (2015) [
44] monitored the content of total phenolics in selected Chinese peach cultivars, ranging from 4.58 to 12.68 mg gallic acid equivalent (GAE).100 g
−1 DW in the peel and from 0.82 to 6.52 mg GAE.100 g
−1 DW in the pulp.
The obtained results of total flavonoids content in the tested set of varieties ranged from 1.1 to 95.1 mg CAE.100 g
−1 FW. Di Vaio et al. (2015) [
45] determined the total flavonoid content, and it ranged from 35.05-58.85 g CAE.kg
−1 FW within the test set. In another publication by Cantin et al. (2009) [
40], total flavonoid content ranged from 1.8 to 30.9 mg CAE.100 g
−1 FW, with an average of 8.8 mg CAE.100 g
−1 FW. Marinova et al. (2005) [
42] investigated the determination of all phenolic compounds, as well as flavonoids in crops grown in Bulgaria. The total flavonoid content in peach fruits was 15.0 mg CAE.100 g
−1 FW; similar values are seen in figs—
Ficus carica (20.2 mg CAE.100 g
−1 FW) and sweet cherries (19.6 mg CAE.100 g
−1 FW). The highest representation of flavonoids was found in this work in blueberries (190.3 mg CAE.100 g
−1 FW). Saidani et al. (2017) [
43] determined the flavonoid content in the skin of peach fruits to be between 39 and 245 mg CAE.100 g
−1 FW, and in the flesh between 8.18 and 112 mg CAE.100 g
−1 FW.
Analyses of antioxidant components in products are fast becoming a recognized profile, primarily emphasizing antioxidant capacity as a quality index for many fruits and vegetables. The high phenolic content showed an increased antioxidant capacity in the studied varieties. The average value of antioxidant capacity determined by the DPPH (1-diphenyl-2,2-picrylhydrazyl) method showed values of 205.7 mg TE.100 g
−1 FW. The authors of Di Vaio et al. (2015) [
45] determined average antioxidant capacity values of 111.1 mg TE.100 g
−1 FW in four peach cultivars. Saidani et al. (2017) [
43], in a tested set of peach cultivars, determined the antioxidant capacity value in the skin of the fruit ranging from 133 to 401 mg TE.100 g
−1 FW, and in the flesh ranging from 22.7 to 194 mg TE.100 g
−1 FW. Zhao et al. (2015) [
44] found antioxidant capacity contents in Chinese peach cultivars from 6.35 to 19.84 mg trolox equivalent antioxidant capacity (TE).100 g
−1 DW in the peel and from 1.05 to 15.01 mg TE.100 g
−1 DW in the pulp.
The content of carotenoids (especially β-carotene, zeaxanthin, lutein, neoxanthin) and anthocyanins increases with fruit maturity, largely due to the colouring of the fruit (formation of the cheek). The results obtained for total anthocyanin content in the tested set of cultivars ranged from 0.04 to 3.74 mg.100 g
−1 FW. Cantín et al. (2009) [
41] monitored the content of total anthocyanins in selected cultivars and found contents ranging between 0.1 and 26.7 mg of C3GE.kg
−1 FW (0.1-26.7 mg of cyanidin-3-glucoside equiv. (C3GE) per kg of FW). In another publication by Saidani et al. (2017) [
43], they discussed the determination of total anthocyanins separately in the peel and in the pulp. The average anthocyanin content in the peel was 5.53 mg C3GE.100 g
−1 FW, while in the pulp the average content was 0.37 mg C3GE.100 g
−1 FW. In other research on total anthocyanin content in apricot fruits, Rababah et al. (2011) [
46] reported an average anthocyanin content of 2.54 mg.100 g
−1 FW, whereas Contessa et al. (2013) [
47] reported an anthocyanin content of 0.99 mg C3GE.100 g
−1 FW.
The carotenoids content found in the set of cultivars ranged from 0.00 to 4.77 mg.100 g
−1 DW. Gil et al. (2002) [
40] observed differences in carotenoids content between white- and yellow-skinned peach cultivars. The average carotenoids content found in white-fleshed cultivars was 11.6 μg.100 g
−1, while in yellow-fleshed cultivars it was 131.6 μg.100 g
−1. Vizzotto et al. (2007) [
48] also found higher carotenoids content in genotypes with yellow flesh (0.8 to 3.7 milligrams β-carotene per 100 g tissue) than in peaches with white flesh (0.0 to 0.1 milligrams β-carotene per 100 g tissue).
Soluble solid content (SSC) is an important characteristic of fruit, as it is closely related to consumer satisfaction and how well the fruit is liked. Zhao et al. (2015) [
44] evaluated the soluble solid content of different Chinese peach cultivars; their findings ranged from 8.34 to 15.48 °Rf. These results are similar to ours, with values ranging from 8.30 to 14.70 °Rf in our set of cultivars. In another work, Gil et al. (2002) [
40] investigated the differences between white- and yellow-fleshed peach cultivars grown in California. The average SSC content found in the white-fleshed varieties was 11.22 °Rf, while in the yellow-fleshed varieties it was 11.90 °Rf. For the Spanish varieties, Legua et al. (2011) [
49] found SSC contents between 9.98 and 18.36 °Rf. Tavarini et al. (2008) [
50] determined an average SSC value of 12.42 °Rf for Italian varieties.
In this study, sucrose, glucose, fructose, and sorbitol were determined as the basic sugars of peaches and there were differences found among the cultivars (
Table 10). The mean values of sucrose, glucose, fructose, and sorbitol were 9.62 g.100 g
−1, 1.94 g.100 g
−1, 1.37 g.100 g
−1 and 0.23 g.100 g
−1, respectively. These values are very similar to those determined by Forcada et al. (2014) [
9]. The values found ranged from 3.5–9.8 g.100 g
−1 sucrose, 0.4–1.5 g.100 g
−1 glucose, 0.2–1.4 g.100 g
−1 fructose, and 0.2–3.5 g.100 g
−1 sorbitol. Nowicka et al. (2019) [
51] investigated the sugar content of 20 peach cultivars. They determined sucrose content ranging from 3.4–5.4 g.100 g
−1, glucose 0.27–0.84 g.100 g
−1, fructose 0.41–1.03 g.100 g
−1, and sorbitol content ranging from 0.15–0.74 g.100 g
−1. Colaric et al. (2005) [
13] determined sucrose levels between 46.14-66.92 g.kg
−1 in some nectarine and peach cultivars. Cantin et al. (2009) [
41] determined a similar sucrose content (47.10–64.00 g.kg
−1), and further investigated the determination of glucose (5.60–8.00 g.kg
−1) and fructose (6.9–10.3 g.kg
−1) in peach and nectarine fruits. Gecer (2020) [
52] measured sucrose (5216.3–9122.4 mg.100 g
−1), glucose (721.7–1902.1 mg.100 g
−1), and fructose (325.7–1048.1 mg.100 g
−1) in some peach and nectarine cultivars. Robertson et al. (1990) [
53] determined the average sorbitol content in yellow-fleshed cultivars, 0.46% and in white-fleshed cultivars, 0.37%. The colour of the fruit is an important parameter that influences the attractiveness of the fruit to consumers. A colorimetric analysis can also provide information on the degree of ripeness of the fruit. The colour of peaches using CIELAB was measured in studies before [
54,
55,
56,
57]. The value of
a* has been suggested as colour index maturity [
54]. The study was associated with changes of
a* with chlorophyl degradation and an increase of anthocyanin content. Because of low values of anthocyanin in most cultivars of peaches, Ferrer et al. (2010) [
55] found that changes of chromatic parameter
b* can be a good indicator of ripeness of peach fruit. These changes correlated to an increase of carotenoids pigments. In our study the correlation relationship between carotenoids and chromatic parameter
b* with a correlation coefficient R = 0.7951 and
C* with R = 0.8051 were found (
Figure 3). The cultivars ‘Otličnik’ and ‘Aurelia’ were accomplished as outliers and they are not included in the correlations; this can be attributed to insufficient maturity of these two cultivars.