Bioaerosol Contribution to Atmospheric Particulate Matter in Indoor University Environments
Abstract
1. Introduction
2. Experimental
2.1. Study Design
2.2. Equipment
2.3. Analysis
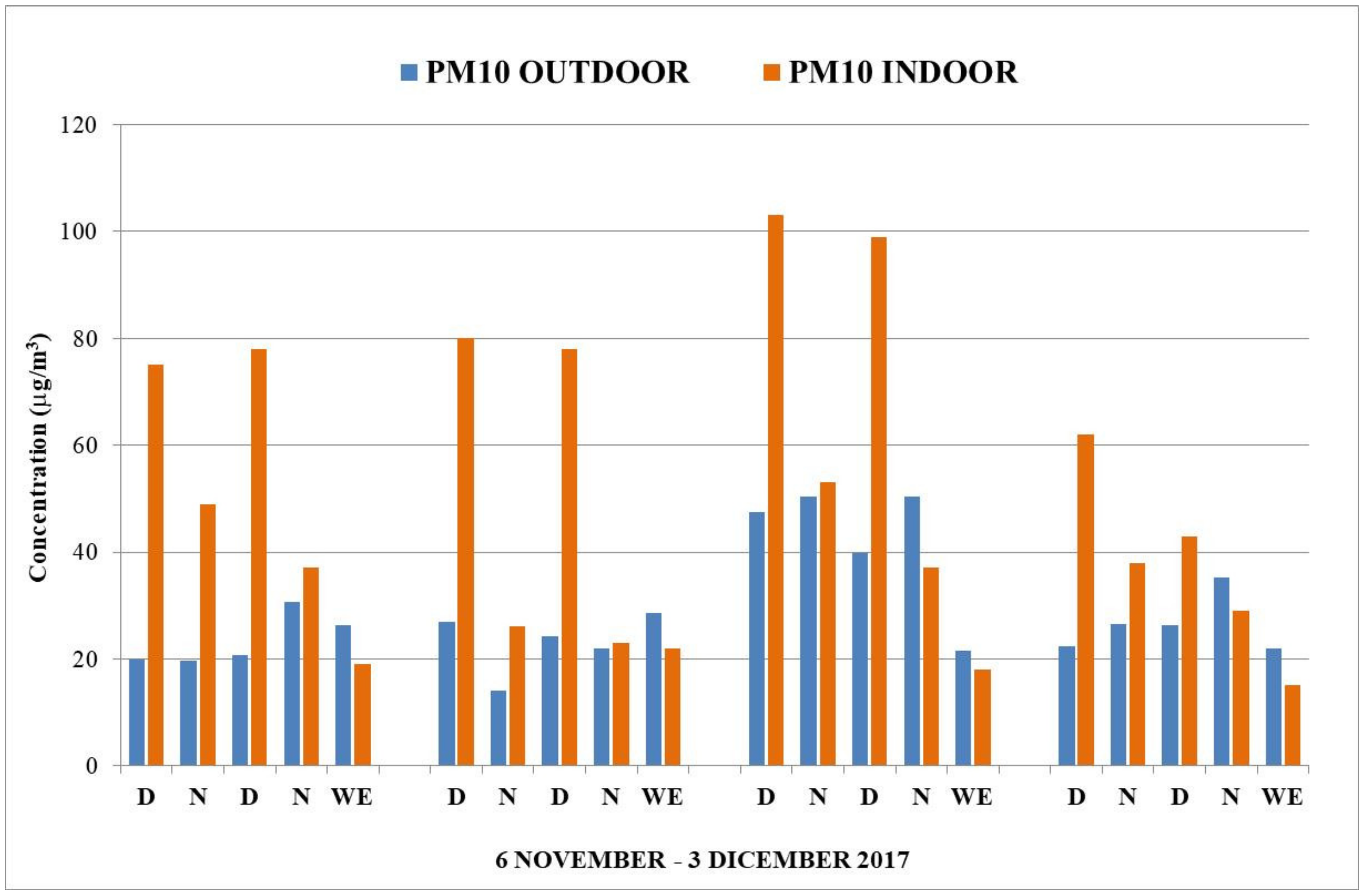
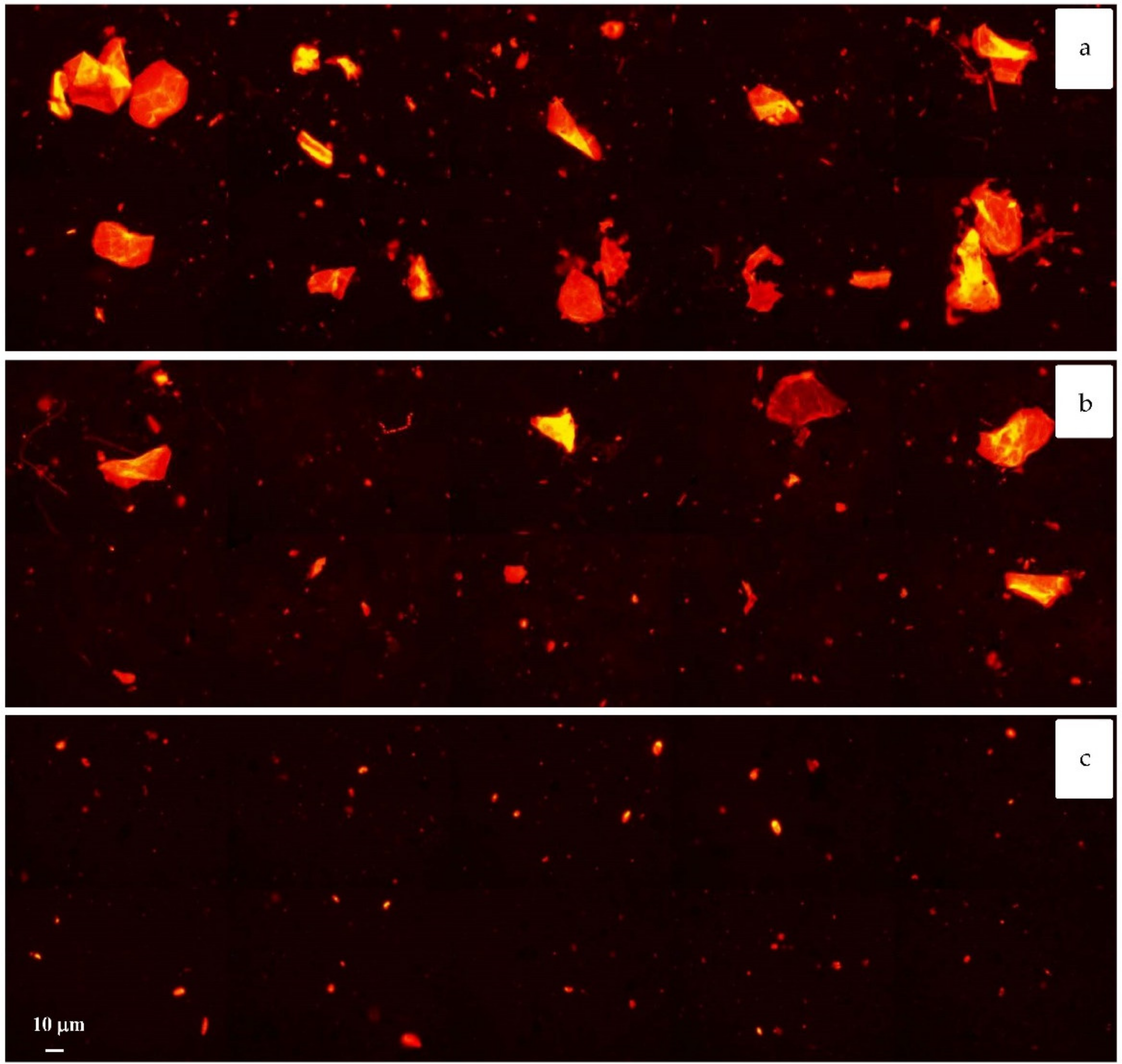
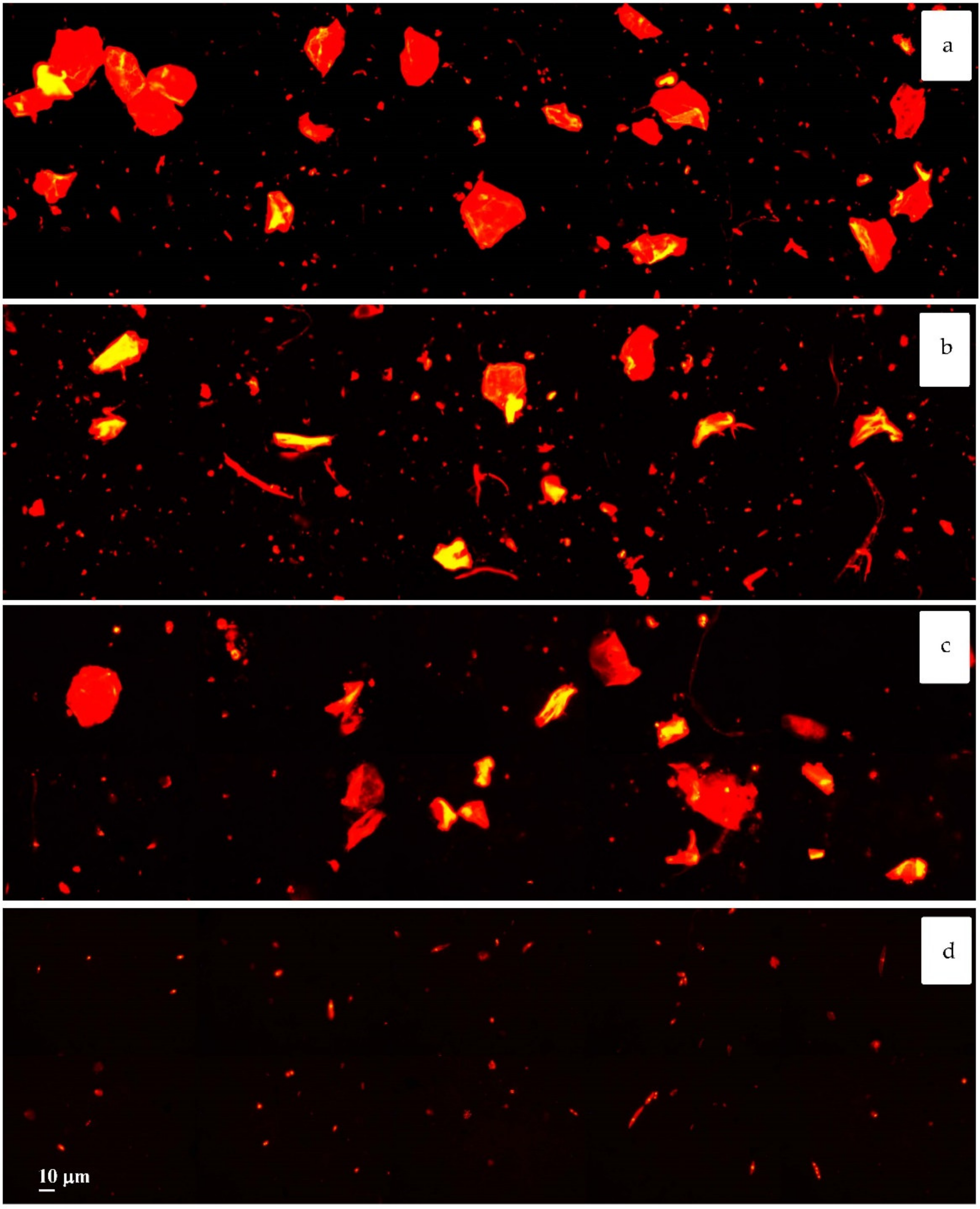
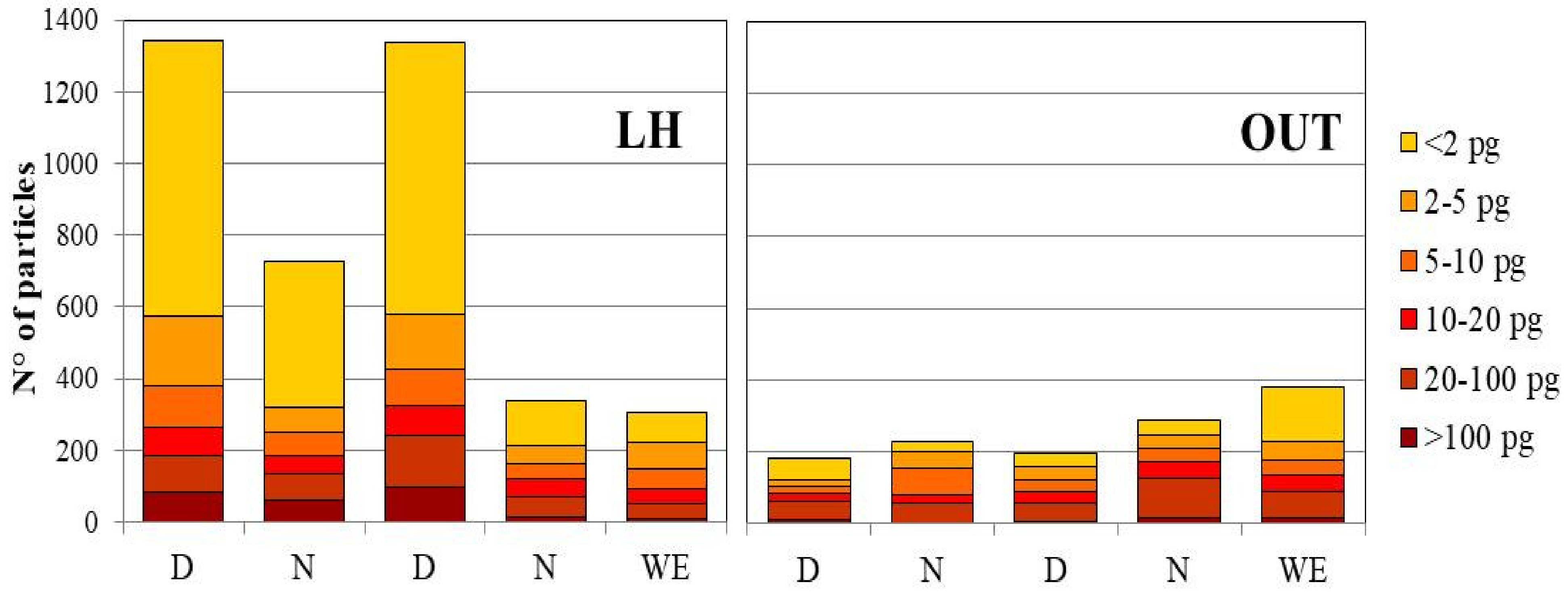
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pelliccioni, A.; Monti, P.; Cattani, G.; Boccuni, F.; Cacciani, M.; Canepari, S.; Capone, P.; Catrambone, M.; Cusano, M.; D’Ovidio, M.C.; et al. Integrated evaluation of indoor particulate exposure: The VIEPI project. Sustainability 2020, 12, 9758. [Google Scholar] [CrossRef]
- Merriam-Webster.com Medical Dictionary, Merriam-Webster. Available online: https://www.merriam-webster.com/medical/bioaerosol (accessed on 5 January 2021).
- Womiloju, T.O.; Miller, J.D.; Mayer, P.M.; Brook, J.R. Methods to determine the biological composition of particulate matter collected from outdoor air. Atmos. Environ. 2003, 37, 4335–4344. [Google Scholar] [CrossRef]
- Li, Q.; Dasgupta, P.K.; Temkin, H.; Crawford, M.H.; Fischer, A.J.; Allerman, A.A.; Bogart, K.H.A.; Lee, S.R. Mid-ultraviolet light-emitting diode detects dipicolinic acid. Appl. Spectrosc. 2004, 58, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Buiarelli, F.; Canepari, S.; Di Filippo, P.; Perrino, C.; Pomata, D.; Riccardi, C.; Speziale, R. Extraction and analysis of fungal spore biomarkers in atmospheric bioaerosol by HPLC–MS–MS and GC–MS. Talanta 2013, 105, 142–151. [Google Scholar] [CrossRef]
- Buiarelli, F.; Riccardi, C.; Uccelletti, D.; Pomata, D.; Sonego, E.; Bruni, E.; Marcovecchio, F.; Simonetti, G.; Di Filippo, P.; Perrino, C. Determination of the main bioaerosol components using chemical markers by liquid chromatography–tandem mass spectrometry. Microchem. J. 2019, 149, 103974. [Google Scholar] [CrossRef]
- Di Filippo, P.; Pomata, D.; Riccardi, C.; Buiarelli, F.; Uccelletti, D.; Zanni, E. Muramic and dipicolinic acids in atmospheric particulate matter as biomarkers of bacteria and bacterial spores. Anal. Bioanal. Chem. 2017, 409, 1657–1666. [Google Scholar] [CrossRef]
- An, H.R.; Mainelis, G.; While, L. Development and calibration of real-time PCR for quantification of airborne microorganisms in air samples. Atmos. Environ. 2006, 40, 7924–7939. [Google Scholar] [CrossRef]
- Blais-Lecours, P.; Perrott, P.; Cuchaine, C. Non-culturable bioaerosols in indoor settings: Impact on health and molecular approach for detection. Atmos. Environ. 2015, 110, 45–53. [Google Scholar] [CrossRef]
- Huffman, J.A.; Sinha, B.; Garland, R.M.; Snee-Pollmann, A.; Gunthe, S.S.; Artaxo, P.; Martin, S.T.; Andreae, M.; Poschl, U. Size distribution and temporal variations of biological aerosol particles in the Amazon rainforest characterised by microscopy and real-time UV-APS fluorescence techniques during AMAZE-08. Atmos. Chem. Phys. 2012, 12, 11997–12019. [Google Scholar] [CrossRef]
- Toprak, E.; Schnaiter, M. Fluorescent biological aerosol particels measured with the waveband integrated bioaerosol sensor WIBS-4: Laboratory tests combined with a one year field study. Atmos. Chem. Phys. 2013, 13, 225–243. [Google Scholar] [CrossRef]
- Fennelly, M.J.; Sewell, G.; Prentice, M.B.; O’Connor, D.J.; Sodeau, J.R. The use of real-time fluorescence instrumentation to monitor ambient primary biological aerosol particles (PBAP). Atmosphere 2018, 9, 1. [Google Scholar] [CrossRef]
- Perrino, C.; Marcovecchio, F. A new method for assessing the contribution of Primary Biological Atmospheric Particles to the mass concentration of the atmospheric aerosol. Environ. Int. 2016, 87, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.A.; Zhang, M.; Liu, T.; Fortier, K.; Chow, J.C.; Alonzo, F.; Kolberg, R.; Cao, J.; Lin, G.; Tanviben, Y.P.; et al. Evaluation of epifluorescence methods for quantifying bioaerosols in fine and coarse particulate air pollution. Atmos. Environ. 2019, 213, 620–628. [Google Scholar] [CrossRef]
- Dechow, M.; Sohn, H.; Steinhanses, J. Concentrations of selected contaminants in cabin air of airbus aircrafts. Chemosphere 1997, 35, 21–31. [Google Scholar] [CrossRef]
- Chen, Q.; Hildemann, L.M. The effects of human activities on exposure to particulate matter and bioaerosols in residential homes. Environ. Sci. Technol. 2009, 43, 4641–4646. [Google Scholar] [CrossRef]
- Pereira, M.L.; Knibbs, L.D.; He, C.; Grzybowski, P.; Johnson, G.R.; Huffman, J.A.; Bell, S.C.; Wainwright, C.E.; Matte, D.L.; Dominski, F.H.; et al. Sources and dynamics of fluorescent particles in hospitals. Indoor Air 2017, 27, 988–1000. [Google Scholar] [CrossRef]
- Brągoszewska, E. Exposure to Bacterial and Fungal Aerosols: Microorganism Indices in A Waste-Sorting Plant in Poland. Int. J. Environ. Res. Public Health 2019, 16, 3308. [Google Scholar] [CrossRef]
- Gaidajis, G.; Angelakoglou, K. Indoor air quality in university classrooms and relative environment in terms of mass concentrations of particulate matter. J. Environ. Sci. Health Part A 2009, 44, 1227–1232. [Google Scholar] [CrossRef]
- Stabile, L.; Buonanno, G.; Avino, P.; Fuoco, F.C. Dimensional and chemical characterisation of airborne particles in schools: Respiratory effects in children. Aerosol Air Qual. Res. 2013, 13, 887–900. [Google Scholar] [CrossRef]
- Viana, M.; Rivas, I.; Querol, X.; Alastuey, A.; Sunyer, J.; Álvarez-Pedrerol, M.; Bouso, L.; Sioutas, C. Indoor/outdoor relationships and mass closure of quasi-ultrafine, accumulation and coarse particles in Barcelona schools. Atmos. Chem. Phys. 2014, 14, 4459–4472. [Google Scholar] [CrossRef]
- Fuoco, F.C.; Stabile, L.; Buonanno, G.; Trassiera, C.V.; Massimo, A.; Russi, A.; Mazaheri, M.; Morawska, L.; Andrade, A. Indoor air quality in naturally ventilated Italian classrooms. Atmosphere 2015, 6, 1652–1675. [Google Scholar] [CrossRef]
- Tofful, L.; Perrino, C. Chemical composition of indoor and outdoor PM2.5 in three schools in the city of Rome. Atmosphere 2015, 6, 1422–1443. [Google Scholar] [CrossRef]
- Pallarés, S.; Gómez, E.; Martínez, A.; Jordán, M.M. The relationship between indoor and outdoor levels of PM10 and its chemical composition at schools in a coastal region in Spain. Heliyon 2019, 5, e02270. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, S.; Longo, V.; Perrino, C.; Canepari, S.; Drago, G.; L’Abbate, L.; Balzan, M.; Cuttitta, G.; Scaccianoce, G.; Minardi, R.; et al. Indoor air quality in schools of a highly polluted South Mediterranean area. Indoor Air 2019, 29, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Soberón, F.; Rovira, J.; Sierra, J.; Mari, M.; Domingo, J.L.; Schuhmacher, M. Seasonal characterisation and dosimetry-assisted risk assessment of indoor particulate matter (PM10–2.5, PM2.5–0.25, and PM0.25) collected in different schools. Environ. Res. 2019, 175, 287–296. [Google Scholar]
- Brągoszewska, E.; Biedroń, I.; Mainka, A. Microbiological Air Quality in a Highschool Gym Located in an Urban Area of Southern Poland—Preliminary Research. Atmosphere 2020, 11, 797. [Google Scholar] [CrossRef]
- Martins, V.; Faria, T.; Diapouli, E.; Manousakas, M.I.; Eleftheriadis, K.; Viana, M.; Almeida, S.M. Relationship between indoor and outdoor size-fractionated particulate matter in urban microenvironments: Levels, chemical composition and sources. Environ. Res. 2020, 183, 109203. [Google Scholar] [CrossRef]
- Toivola, M.; Alm, S.; Reponen, T.; Kolari, S.; Nevalainen, A. Personal exposures and microenvironmental concentrations of particles and bioaerosols. J. Environ. Monit. 2002, 4, 166–174. [Google Scholar] [CrossRef]
- Bartlett, K.H.; Kennedy, S.M.; Brauer, M.; van Netten, C.; Dill, B. Evaluation and determinants of airborne bacterial concentrations in school classrooms. J. Occup. Environ. Hyg. 2004, 1, 639–647. [Google Scholar] [CrossRef]
- Fox, A.; Harley, W.; Feigley, C.; Salzberg, D.; Toole, C.; Sebastian, A.; Larsson, L. Large particles are responsible for elevated bacterial marker levels in school air upon occupation. J. Environ. Monitor. 2005, 7, 450–456. [Google Scholar] [CrossRef]
- Brandl, H.; von Däniken, A.; Hitz, C.; Krebs, W. Short-term dynamic patterns of bioaerosol generation and displacement in an indoor environment. Aerobiologia 2008, 24, 203–209. [Google Scholar] [CrossRef]
- Mandal, J.; Brandl, H. Bioaerosols in indoor environment-a review with special reference to residential and occupational locations. Open Environ. Biol. Monit. J. 2011, 4, 83–96. [Google Scholar]
- Grisoli, P.; Rodolfi, M.; Chiara, T.; Zonta, L.A.; Dacarro, C. Evaluation of microbiological air quality and of microclimate in university classrooms. Environ. Monit. Assess. 2012, 184, 4171–4180. [Google Scholar] [CrossRef] [PubMed]
- Canha, N.; Almeida, S.M.; do Carmo Freitas, M.; Wolterbeek, H.T. Assessment of bioaerosols in urban and rural primary schools using passive and active sampling methodologies. Arch. Environ. Prot. 2015, 41, 11–22. [Google Scholar] [CrossRef]
- Madureira, J.; Paciência, I.; Pereira, C.; Teixeira, J.P.; Fernandes, E.D.O. Indoor air quality in Portuguese schools: Levels and sources of pollutants. Indoor Air 2016, 26, 526–537. [Google Scholar] [CrossRef]
- Bragoszewska, E.; Mainka, A.; Pastuszka, J.S.; Lizończyk, K.; Desta, Y.G. Assessment of bacterial aerosol in a preschool, primary school and high school in Poland. Atmosphere 2018, 9, 87. [Google Scholar] [CrossRef]
- Madureira, J.; Aguiar, L.; Pereira, C.; Mendes, A.; Querido, M.M.; Neves, P.; Teixeira, J.P. Indoor exposure to bioaerosol particles: Levels and implications for inhalation dose rates in schoolchildren. Air Qual. Atmos. Health 2018, 11, 955–964. [Google Scholar] [CrossRef]
- Hospodsky, D.; Qian, J.; Nazaroff, W.W.; Yamamoto, N.; Bibby, K.; Rismani-Yazdi, H.; Peccia, J. Human occupancy as a source of indoor airborne bacteria. PLoS ONE 2012, 7, e34867. [Google Scholar] [CrossRef]
- Marcovecchio, F.; Perrino, C. Contribution of Primary Biological Aerosol Particles to airborne particulate matter in indoor and outdoor environments. Chemosphere 2021, 264, 128510. [Google Scholar] [CrossRef]
- Pongracic, J.A.; O’Connor, G.T.; Muilenberg, M.L.; Vaughn, B.; Gold, D.R.; Kattan, M.; Morgan, W.J.; Gruchalla, R.S.; Smartt, E.; Mitchell, H.E. Differential effects of outdoor versus indoor fungal spores on asthma morbidity in inner-city children. J. Allergy Clin. Immun. 2010, 125, 593–599. [Google Scholar] [CrossRef]
- Laumbach, R.J.; Kipen, H.M. Bioaerosols and sick building syndrome: Particles, inflammation, and allergy. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, C.A.; Lidwell, O.M.; Towers, A.G.; Marples, R.R. The dimensions of skin fragments dispersed into the air during activity. Epidemiol. Infect. 1978, 81, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.E.; Waring, M.S. Predicting the importance of oxidative aging on indoor organic aerosol concentrations using the two-dimensional volatility basis set (2D-VBS). Indoor Air 2019, 29, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Turpin, B.J.; Lim, H.J. Species contributions to PM2.5 mass concentrations: Revisiting common assumptions for estimating organic mass. Aerosol Sci. Technol. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Reff, A.; Turpin, B.J.; Offenberg, J.H.; Weisel, C.P.; Zhang, J.; Morandi, M.; Stock, T.; Colome, S.; Winer, A. A functional group characterisation of organic PM2.5 exposure: Results from the RIOPA study. Atmos. Environ. 2007, 41, 4585–4598. [Google Scholar] [CrossRef]
- Bhangar, S.; Adams, R.I.; Pasut, W.; Huffman, J.A.; Arens, E.A.; Taylor, J.W.; Bruns, T.D.; Nazaroff, W.W. Chamber bioaerosol study: Human emissions of size-resolved fluorescent biological aerosol particles. Indoor Air 2016, 26, 193–206. [Google Scholar] [CrossRef]




| Bioaerosol | PM10 | OM | ||
|---|---|---|---|---|
| 13–14/11 day | LH | 14 | 54 | 23 |
| 13–14/11 night | 4.5 | 15 | 11 | |
| 15–16–17/11 day | 9.9 | 51 | 14 | |
| 15–16–17/11 night | 0.94 | 16 | 7.8 | |
| 18–19/11 weekend | 0.46 | 17 | 8.6 | |
| 13–14/11 day | A4 | 5.2 | 80 | 27 |
| 13–14/11 night | 4.2 | 26 | 12 | |
| 15–16–17/11 day | 9.0 | 78 | 16 | |
| 15–16–17/11 night | 3.3 | 23 | 9.6 | |
| 18–19/11 weekend | 1.6 | 22 | 9.9 | |
| 13–14/11 day | CR | 6.8 | 43 | 16 |
| 13–14/11 night | 0.66 | 8.5 | 6.7 | |
| 15–16–17/11 day | 2.3 | 72 | 15 | |
| 15–16–17/11 night | 1.8 | 14 | 8.3 | |
| 18–19/11 weekend | 0.46 | 15 | 9.8 | |
| 13–14/11 day | OUT | 2.7 | 27 | 15 |
| 13–14/11 night | 0.59 | 14 | 8.0 | |
| 15–16–17/11 day | 0.8 | 24 | 14 | |
| 15–16–17/11 night | 1.1 | 22 | 12 | |
| 18–19/11 weekend | 0.70 | 29 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcovecchio, F.; Perrino, C. Bioaerosol Contribution to Atmospheric Particulate Matter in Indoor University Environments. Sustainability 2021, 13, 1149. https://doi.org/10.3390/su13031149
Marcovecchio F, Perrino C. Bioaerosol Contribution to Atmospheric Particulate Matter in Indoor University Environments. Sustainability. 2021; 13(3):1149. https://doi.org/10.3390/su13031149
Chicago/Turabian StyleMarcovecchio, Francesca, and Cinzia Perrino. 2021. "Bioaerosol Contribution to Atmospheric Particulate Matter in Indoor University Environments" Sustainability 13, no. 3: 1149. https://doi.org/10.3390/su13031149
APA StyleMarcovecchio, F., & Perrino, C. (2021). Bioaerosol Contribution to Atmospheric Particulate Matter in Indoor University Environments. Sustainability, 13(3), 1149. https://doi.org/10.3390/su13031149







