Abstract
Fuel cells as clean power sources are very attractive for the maritime sector, which is committed to sustainability and reducing greenhouse gas and atmospheric pollutant emissions from ships. This paper presents a technological review on fuel cell power systems for maritime applications from the past two decades. The available fuels including hydrogen, ammonia, renewable methane and methanol for fuel cells under the context of sustainable maritime transportation and their pre-processing technologies are analyzed. Proton exchange membrane, molten carbonate and solid oxide fuel cells are found to be the most promising options for maritime applications, once energy efficiency, power capacity and sensitivity to fuel impurities are considered. The types, layouts and characteristics of fuel cell modules are summarized based on the existing applications in particular industrial or residential sectors. The various research and demonstration projects of fuel cell power systems in the maritime industry are reviewed and the challenges with regard to power capacity, safety, reliability, durability, operability and costs are analyzed. Currently, power capacity, costs and lifetime of the fuel cell stack are the primary barriers. Coupling with batteries, modularization, mass production and optimized operating and control strategies are all important pathways to improve the performance of fuel cell power systems.
1. Introduction
Marine diesel engines have driven the shipping industry for over a century. Owing to the use of fossil fuels and particularly marine residual oils, greenhouse gases (GHG) and air pollutants from ships, such as carbon dioxide (CO2), nitrogen oxides (NOx), sulfur oxides (SOx) and particulate matters (PM), have become the key regulatory targets in the maritime sector [1]. The International Maritime Organization (IMO) has adopted a variety of regulations under Annex VI (titled Regulations for the Prevention of Air Pollution from Ships) of the International Convention for the Prevention of Pollution from Ships (MARPOL) [2,3,4]. As well as this, the phasing out of GHG emissions from ships as soon as possible in this century has been set as a target [5]. Correspondingly, a series of technological and operational measures have been employed to mitigate shipping emissions and improve ship energy efficiency [6,7]. However, current measures are not sufficient to make the shipping industry consistent with the global response to the threat of climate change [8]. Hence, alternative fuels and energy sources are expected to play a vital role as a synergistic solution for reductions of SOx, NOx, PM and CO2 emissions. Apart from innovative technologies and systems for traditional engines, fuel cell power systems are proposed to be an important option to improve the use of alternative marine fuels. High energy efficiencies make fuel cells very attractive compared to marine combustion engines and gas turbines (GTs), though the power capacities of fuel cells cannot cover all maritime applications. However, the efficiencies and power capacities of fuel cells continue to be a focal point of research and development, leading to constant improvements that bring the technology closer to widespread adoption with every passing year.
Maritime fuel cells used onboard underwater vehicles can be traced back to the 1960s [9]. Possible applications of fuel cells onboard merchant ships include: low power demand main propulsion; auxiliary power for hybrid propulsion; electricity generation; and emergency power supply [10]. Several demonstration projects for fuel cell applications in the merchant marine sector have been carried out since 2000 [11]. Through a literature review of existing studies on fuel cells for maritime applications, three key aspects were identified:
(i) Economic and environmental analysis. To determine the feasibility of fuel cells for maritime applications, an exergy analysis was carried out for both a methanol reforming proton exchange membrane fuel cell (PEMFC) system and a direct methanol fuel cell (DMFC) system, where the two systems exhibited similar power capacity and energy efficiency figures [12]. However, weight, volume and unit cost should be considered for further thermo-economic analysis. A hybrid solar photovoltaic (PV)-PEMFC-diesel generator power system was modelled and optimized for cruise ship trading in Stockholm, Sweden [13]. The renewable energy system contributed 13.8% of the overall energy requirement and achieved 9.8% emissions reduction compared to the conventional diesel engine power system. A life cycle assessment of a molten carbonate fuel cell (MCFC) plant for marine applications was conducted and compared to a conventional diesel engine power plant [14]. The results showed that the operational phase is the major contributor to climate change for both systems when hydrocarbons are used as fuels. The production of raw materials and the manufacturing of MCFC components were shown to have higher environmental impact compared to that of diesel engines. Hence, the recycling and re-use of MCFC components are important to improve the overall environmental performance of such systems. A testbed of a hybrid power source composed of a MCFC, a lead-acid battery and a diesel generator was developed to simulate the fuel consumption of five types of ships based on respective operating profiles [15]. Average CO2 savings of 70–74% based on different ship types and load scenarios were reported, compared to the standalone operation of the diesel generator. An integrated energy system was developed and evaluated, which was composed of a hydrogen-fueled solid oxide fuel cell gas turbine (SOFC-GT) hybrid system, a solar PV system, wind turbines and an absorption refrigeration plant. The ship consumed renewable energy only and the overall energy efficiency was 41.5% [16]. A SOFC power system used for propulsion, electricity and heat generation onboard ships was investigated and optimized in terms of energy, cost and emission savings [17]. The abatement of GHG emissions was claimed to be up to 34% and SOFCs fed with liquefied natural gas (LNG) were deemed to be the most cost-optimal solution for reducing GHG emissions. A four-scheme energy management strategy for a hybrid fuel cell-battery driven passenger ship was proposed with the aim of minimizing energy consumption [18]; the simulation results showed that maximum energy savings of 8% could be achieved.
(ii) Safety and reliability analysis. A PEMFC stack operated under marine environmental conditions was experimentally analyzed [19], from which it was concluded that sea salt (sodium chloride) vapor was the major contaminant and caused a significant performance decrement for the fuel cells. Based on SF-BREEZE projects, safety-related physical and combustion properties of liquefied hydrogen (LH2) and LNG were evaluated and compared [20]. The results showed that LH2 and LNG pose similar safety risks, and several countermeasures such as avoiding fuel leaks, providing adequate ventilation, monitoring confined spaces, etc., are required to minimize the risks. A numerical calculation was carried out to simulate the leakage and diffusion of hydrogen in a fuel cell ship to better understand hydrogen safety issues [21]. The hydrogen concentration distributions and the effects of different ventilation conditions were determined, providing guidance for the optimal positions for hydrogen sensors and ventilation. Risk assessment of a hydrogen driven high speed passenger ferry was performed [22], with the results illustrating that the estimated risk related to hydrogen systems is relatively low and within acceptable limits. Based on a MCFC power system for a LH2 tanker, the safety integrity levels for an electric propulsion system were investigated [23]. Fire and explosion caused by fuel overflows or a control failure in the stack were identified as the most severe potential incidents. A failure mode and effects analysis (FMEA) approach was proposed to evaluate the safety and reliability of a hybrid MCFC-GT system for LH2 tankers [24]. A similar approach was verified by successfully applying it to a hybrid power system composed of a MCFC, a battery system and a diesel engine [25].
(iii) Power system development. A PEMFC-battery hybrid propulsion system was developed for a tourist boat [26], the reliable operation of which was successfully demonstrated in the coastal waters of South Korea. A hybrid propulsion system coupling an LNG-fueled combustion engine with a hydrogen-fueled PEMFC was proposed for an LNG carrier [27]. To satisfy the required energy efficiency design index, the energy fractions from hydrogen were determined. This allowed the cost competitiveness of the hydrogen system to be evaluated against the conventional LNG propulsion system. A MCFC-based marine auxiliary power unit (APU) fed with diesel oil was developed and modelled [28], allowing the system efficiency under different reforming strategies and process configurations to be assessed. A hybrid propulsion system coupling a MCFC with a bottoming cycle was developed for a LH2 tanker [29]. System efficiency, economic feasibility and exhaust emissions were evaluated. Currently, the fuel cell systems are less economical than other propulsion systems, but their environmental performance is brilliant. It was found that the MCFC-GT system was preferable with regard to overall system efficiency. A SOFC-GT tri-generation system was developed for marine applications [30]. An absorption chiller could be employed to drive the heating, ventilation and air conditioning (HVAC), and thus the overall system efficiency could be significantly improved considering different system configurations. A hybrid diesel electric propulsion system coupled with two methanol-fed 250-kW SOFC systems was designed for an offshore platform supply vessel, where notable reductions of pollutant emissions were observed [31]. A propulsion system composed of a dual-fuel diesel generator and a SOFC-GT hybrid system was developed and optimized for a 90,000 m3 ethane carrier [32]. The optimal system configuration was determined and the energy efficiency design index complied with all requirements set by the IMO regulations.
Very few research papers focus on the specific application of fuel cells for the maritime sector. While there are a number of parallels to be drawn in terms of transferable knowledge with stationary power, automotive and other land-based applications, the unique challenges posed by the maritime sector (particularly for international deep sea shipping) create a number of additional barriers to entry for fuel cell technology. These will be explored in greater detail in the coming sections, but can broadly be attributed to a harsh working environment and limitations relating to onboard energy storage (including any fuel pre/post processing systems) that would ultimately encroach upon the payload (and hence profitability) of a vessel.
The possibility of using fuel cells onboard ships was analyzed in ref. [33], which reviewed some existing research and demonstration projects of fuel cells for maritime applications. The costs and expected service lifetime of potential fuel cells were highlighted. In addition, marine fuel cell systems were reviewed in terms of fuel cell types, potential fuels and system characteristics in ref. [34]. The authors presented information on the potential of fuel cell systems fueled by LH2 and LNG. However, a summary of the specific layouts and characteristics of different types of fuel cell modules is absent in the published literature. Moreover, the topic of design and development of fuel cell hybrid power systems for maritime applications is lacking a comprehensive review. Therefore, this paper aims to address these shortcomings in the literature by conducting a comprehensive review on the development of the fuel cell modules and systems, culminating in a summary of the most promising pathways for future maritime applications.
2. Fuel Cells and Available Fuels for Sustainable Shipping
2.1. Types of Fuel Cells
A fuel cell consists of an anode, a cathode and an electrolyte, and converts the chemical energy from a fuel into electricity through an electrochemical reaction. A basic schematic diagram of a hydrogen fuel cell is shown in Figure 1, where the two illustrations depict the different possible ion transfer characteristics across the electrolyte depending on the type of fuel cell. Only a very small electric potential, about 0.7 volts (V), is produced by an individual fuel cell. Hence, cells are placed in series to create sufficient voltage to meet the requirement of an application, resulting in a “fuel cell stack”. Fuel cells are usually classified by the type of electrolyte they use. Typical fuel cells and their electrochemical reactions are summarized in Table 1.
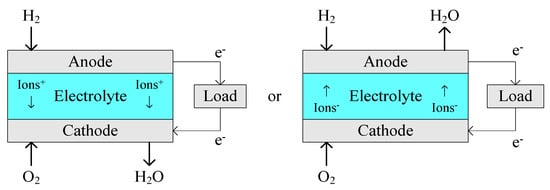
Figure 1.
Basic schematic diagram of a hydrogen fuel cell.

Table 1.
Typical materials of electrodes and electrolyte, and the electrochemical reactions on electrodes. [11].
(i) AFC (alkaline fuel cell). The AFC is relatively low cost. The only product of the reaction is water and there are no other emissions. However, CO2 poisoning is a major concern as CO2 could react with the alkaline electrolyte. Hence, pure hydrogen and pure oxygen (O2) are required, meaning that other fuels and air are not recommended owing to the significant purification measures required [11].
(ii) PEMFC. The low operating temperature of PEMFCs allows flexible and safe operation, less stringent material requirements and quick start-up. However, low temperature also leads to a lack of waste heat recovery options and a complex system for water management [35]. The complexity of the latter issue is not to be underestimated, with humidification of the air supply and removal of excess water from the cathode both posing challenges. In addition, the platinum catalysts add to system cost and can be poisoned by carbon monoxide (CO) and sulphur (S) with a medium sensitivity. Therefore, a reforming and purification unit is necessary to obtain the required purity of hydrogen if hydrocarbons rather than pure hydrogen are to be used as fuels.
(iii) HT-PEMFC. Apart from continuous development of PEMFC technology to improve operational flexibility, extend lifetime and reduce cost, the development of high temperature PEMFC systems is also an area of research interest [36]. Compared to PEMFC, HT-PEMFC uses a mineral acid electrolyte instead of a water based one. Thus, it can work at a temperature up to 200 °C. Due to this higher temperature, HT-PEMFC is less sensitive to CO and S poisoning, and there is no need for water management. In addition, a waste heat recovery (WHR) system could be employed by using a bottoming cycle to enhance overall system efficiency.
(iv) PAFC (phosphoric acid fuel cell). The PAFC has a moderate cost and works at temperatures up to 200 °C. Due to the higher temperatures, fuels other than pure hydrogen (hydrocarbons such as LNG and methanol) can be used, whilst both a reforming unit and a WHR system (typically a steam turbine) might be included [11]. Consequently, while the product of the electrochemical reaction is water, the reforming process generates CO2. The higher operating temperature makes the platinum catalyst less sensitive to CO poisoning and other contaminants.
(v) DMFC. Compared to PEMFC, DMFC is slightly higher cost and lower efficiency, but it has the benefit that liquid methanol is appreciably easier to handle than hydrogen. Using methanol as fuel leads to CO2 emissions but no NOx emissions. The major challenge with DMFC is that methanol crosses over the membrane to the cathode and reacts directly with oxygen, leading to low efficiency [37]. Thus, the prerequisite of DMFC application is to enhance membrane performance, which would facilitate increased efficiency and power capacity of the resulting fuel cell stack.
(vi) MCFC. MCFC is relatively high cost and operates at temperatures in the range 600–700 °C. Due to the higher temperature, LNG, methanol and hydrocarbons other than pure hydrogen can be used as fuels. There are no CO2 emissions if hydrogen is used as fuel, CO2 only circulates in fuel cells to regenerate carbonate in the electrolyte. In addition, high operating temperatures dictate that a WHR system is also suitable for MCFC. Using hydrocarbons as fuel leads to CO2 emissions, but no NOx emissions exist as no air is present when the reforming takes place at the anode. However, potential NOx emissions might exist from the subsequent WHR systems. MCFC is a highly efficient fuel cell, with low cost catalyst and electrolytes, and high flexibility towards fuels and contaminants [38]. While the high operating temperature makes it suitable for energy recovery systems, it also makes MCFC systems vulnerable to negative cycling effects like corrosion and cracking of components. MCFC has a slow start-up, and is less flexible towards changing power demands than low temperature fuel cells [11]. Combining MCFCs with batteries/supercapacitors or an electrolyser to allow for more steady operation of the fuel cell could significantly reduce the strain from thermal cycling. This could also allow for more flexible operation with a faster start-up and the ability to cater to changing power demands. MCFCs are commercially available, but still struggle with high cost, limited lifetime and low power density [34,39].
(vii) SOFC. Unlike a MCFC, no CO2 is required to be circulated to the cathode of a SOFC. SOFCs are relatively high cost and work at temperatures ranging between 500–1000 °C. Due to the higher temperature, direct internal reforming of hydrocarbon fuels, such as LNG and methanol, and direct thermal cracking of ammonia (NH3) in a SOFC stack are possible. Meanwhile, a WHR system is also suitable for SOFC, and like MCFC, the high temperature makes SOFC vulnerable to negative cycling effects. In spite of the stability of tubular SOFC in terms of thermal cycling effects, the planar SOFC is more favorable due to higher energy density and easier manufacture [11]. Combining a SOFC with a battery will reduce thermal strain and ensure a more flexible operation as well.
Regarding NH3-fed SOFCs, both oxygen ion-conducting and proton-conducting electrolytes have been reported [40]. Where an oxygen ion-conducting electrolyte (namely SOFC-O) is employed, the main reactions are same as reactions for either hydrogen or hydrocarbon fuels. But if a proton-conducting electrolyte (namely SOFC-H) is employed, the main reactions are as follows:
Anode reaction:
2H2 → 4H+ + 4e
Cathode reaction:
4H+ + O2 + 4e− → 2H2O
In the case of SOFC-O, water vapor is produced at the anode and the exhaust gases at the anode include N2, H2O, and the remaining NH3 and H2. As for SOFC-H, water vapor is produced at the cathode and the exhaust gases at the anode include N2 and the remaining NH3 and H2. Thus, one of the major advantages of SOFC-H is that hydrogen is not diluted by the water vapor generated in the electrochemical reaction [41].
Key characteristics of different types of fuel cells are summarized in Table 2. Among the seven types of fuel cell, differences in performance in terms of technical, environmental and economic issues, as well as the applicability of the technology onboard ships, are usually considered. However, it is difficult to choose the promising pathways based on an individual indicator. Therefore, a multi-criterion decision-making approach, or the analytic hierarchy process, are usually employed [42], whereby PEMFC/HT-PEMFC, MCFC and SOFC are thought to be the most promising types of fuel cells for maritime applications [11,43].

Table 2.
Key characteristics of fuel cells. [11,17,34,35,36,37,38,39,44].
2.2. Potential Marine Fuels
Although most of the fuel cell technologies have higher energy efficiency than traditional marine diesel engines or dual-fuel engines, the advantages are not overwhelming when costs and technical maturity are taken into account [34]. Considering low carbon or zero carbon future shipping, the scenario of fuel cell applications in the maritime industry is assumed to utilize zero carbon or carbon-neutral fuels. That is to say, there is a basic assumption in this paper that carbon capture and storage (CCS) is regarded as being unavailable onboard ships. Therefore, conventional marine fossil fuels are excluded due to limited long-term prospects, but hydrogen, ammonia and synthetic natural gas (SNG, predominantly methane) and methanol from renewable sources are regarded as marine fuels with long-term prospects and will be investigated in this section. As a transition, short-term applications of fossil raw materials being used as feedstocks for hydrogen, ammonia, SNG and methanol are assumed to be acceptable.
2.2.1. Hydrogen
Hydrogen is the most abundant element on earth, but due to its high reactivity, it is only found in usable quantities within chemical compounds. Consequently, in order to obtain hydrogen in its pure form, energy must be expended for the purposes of extraction. The feedstocks of hydrogen include fossil fuels, biomass and water. However, natural gas (NG) and coal are currently the primary feedstocks. The typical production processes of hydrogen include thermochemical conversion and electrolysis at present, as well as photoelectrochemical and biological conversion in the future [45,46]. Currently, thermochemical conversion is the primary process of hydrogen production from fossil and biomass feedstocks, and it can be classified into steam reforming, partial oxidation, autothermal reforming and coal/biomass gasification [34,46]. The product of thermochemical conversion of hydrocarbon fuels is known as syngas, a mixture of H2 and CO.
The endothermic reaction of steam reforming can be expressed as follows:
CnHm + n H2O ⇌ (n + m/2) H2 + n CO
The exothermic reaction of partial oxidation can be expressed as follows:
CnHm + n/2 O2 → m/2 H2 + n CO
Autothermal reforming is a combination of steam reforming and partial oxidation, and the chemical reaction can be expressed as follows:
2CnHm + n/2 O2 + n H2O → (n + m) H2 + 2n CO
The endothermic reaction of coal/biomass gasification can be expressed as follows:
C + H2O → H2 + CO
The purification of hydrogen from syngas is usually achieved by water gas shift. Thus, the CO produced during the above thermochemical conversions reacts further with steam, resulting in the production of hydrogen and CO2:
where higher hydrogen yields and lower CO concentrations are obtained. Meanwhile, combining CO2 separation technologies and CCS technologies, high purity hydrogen is obtained with no CO2 emissions.
CO + H2O ⇌ H2 + CO2
The electrolysis process splits water into hydrogen and oxygen in an electrolyser. Alkaline electrolysers are the most technically mature, but polymer electrolyte membrane electrolysers and solid oxide electrolysers show potential for future applications due to offering higher efficiency values [47]. During the electrolysis process, electricity is consumed and pure hydrogen is obtained, which is particularly ideal for low temperature fuel cells. With the increasing proportion of renewable electricity in the world energy mix, sustainable hydrogen production is an increasingly viable prospect, and would also be beneficial as an energy storage medium to deal with the fluctuations and demand mismatch inherent to most renewable technologies. In addition, photoelectrochemical conversion, or the photolysis/photolytic process, splits water into hydrogen and oxygen by using solar energy, and biological processes can convert biomass to hydrogen. These two conversion routes are also regarded as having great potential in the future. In the near to mid-term future, the deployment of sustainable hydrogen depends greatly on the costs of CCS and renewable electricity.
Regarding the transportation and storage of hydrogen, low volumetric energy density is a big challenge. Even discounting the energy requirement for liquification at −253 °C or compression at either 350 bar or 700 bar, it is notable that a larger storage space is still required compared to conventional marine fuels. Therefore, hydrogen as a marine fuel is not ideal for long distance shipping. However, hydrogen’s excellent environmental performance always attracts industrial attention. Accordingly, further development for better hydrogen carriers is necessary. Ammonia, SNG and liquid organic hydrogen carriers (LOHCs, e.g., methanol) [48] are potential options.
2.2.2. Ammonia
Ammonia is one of the most abundant synthetic chemicals in the world. The Haber–Bosch process is the most typical method of ammonia production. At 300–500 °C and 200–350 bar over a Fe-, Ni- or Ru-based catalyst, the chemical reaction could be expressed as follows [49,50,51]:
N2 + 3H2 ⇌ 2NH3
The air is usually used for nitrogen production by the pressure swing absorption or membrane filtration method, whilst hydrogen production is as discussed in Section 2.2.1. The ammonia is stored at ambient temperature and 8 bar vapour pressure. Due to the toxicity of liquid ammonia, ammonia storage in solid form such as metal amine salts, ammonium carbonates or urea has been proposed [49,50]. However, the slightly increased storage mass and additional energy consumption for ammonia release would result in extra costs. In spite of this, ammonia is easier and less expensive to transport and store than hydrogen, and it is feasible to use ammonia as a hydrogen carrier [51]. As a hydrogen carrier, ammonia could be decomposed or cracked to release the products of hydrogen and nitrogen. Since no carbon and sulphur are contained, there is no the risk of CO or S poisoning [34]. Ammonia could be used as direct fuel for fuel cells, where ammonia-fuelled SOFC arouses significant research interest due to the decomposition of ammonia under high operating temperature and over catalysts [40,41,52]. Direct ammonia alkaline/alkaline membrane fuel cells and direct hydrazine/ammonia borane fuel cells are also possible options [40,50].
2.2.3. Synthetic Natural Gas
NG has already seen use as an alternative marine fuel to reduce SOx and NOx emissions. In parallel with this, NG also has the potential to reduce CO2 emissions owing to its minimum carbon emissions per unit of energy release among hydrocarbon and alcoholic fuels. NG can be synthesised from syngas using the thermochemical conversion of fossil raw materials. The exothermic reactions can be expressed as follows:
3H2 + CO ⇌ CH4 + H2O
Apart from fossil-based NG, SNG from renewable sources could have a more favourable climate impact. Carbon-neutral SNG can be synthesised from biomass or power-to-gas systems [53]. Anaerobic digestion is the predominant process for SNG production from biomass compared to thermal gasification of organic biomass or the Sabatier reaction [54]. Power-to-gas systems produce SNG through a catalytic or biological methanation reaction, where hydrogen produced by water electrolysis from renewable energy and CO2 captured from industrial processes are combined together [55]. The SNG production from power-to-gas systems can be expressed as follows [56]:
4H2 + CO2 ⇌ CH4 + 2H2O
SNG is stored below −163 °C in liquefied state or above 200 bar in compressed state. Hence, transportation and storage of SNG in a cryogenic or pressurized state are costly and relatively inefficient, which are the main challenges for the widespread applications of SNG. Currently, NG is an important source of hydrogen and methanol. Although the volumetric energy density of NG is twice that of hydrogen, the synthesis of SNG on land and then reforming for hydrogen onboard requires more capital for equipment, as well as the corresponding increased energy consumption. Hence, except for high temperature fuel cell power systems, there are no significant advantages for SNG compared to hydrogen.
2.2.4. Renewable Methanol
Methanol is traditionally produced from NG and coal, but oil, biomass, wastes and even CO2 can also be taken as feedstocks [57]. The chemical reactions of fossil methanol synthesis from syngas can be expressed as follows:
2H2 + CO ⇌ CH3OH
3H2 + CO2 ⇌ CH3OH+ H2O
Renewable methanol is mainly produced from second generation biomass, such as forest residues, agriculture residues, municipal solid waste and black liquor produced from pulp and the paper industry. The production process is the same as fossil methanol production, where syngas production, methanol synthesis and processing of crude methanol are covered. Renewable methanol could be regarded as carbon-neutral if renewable energy is used for the production processes [57]. Methanol can be produced by catalytic synthesis of CO2 captured from industrial processes and hydrogen electrolysed by renewable electricity, so called power-to-liquid (PtL) [58]. Methanol is liquid at ambient temperatures, making it easier to transport and store than NG, hydrogen and ammonia. The methanol industry is global and fuel methanol could be available in major port terminals globally with minimal infrastructure changes. Hence, as an important hydrogen carrier, renewable methanol has several advantages with regard to transportation, storage and energy density.
2.3. Onboard Pre-Processing of Marine Fuels
The electrochemical reaction of fuel cells happens between hydrogen and oxidizing agents. Hence, pre-processing is required for marine fuels other than hydrogen. Although several marine fuels could be converted into hydrogen, a complex pre-processing system installed onboard a ship means complicated operation and probably expensive operational costs. Moreover, fossil fuels supplied onboard ships mean that an onboard CCS system is required for low carbon or zero carbon shipping. Therefore, only hydrogen, ammonia, SNG and renewable methanol are suggested to be supplied onboard ships directly in this paper. However, large-scale fuel conversions from fossil raw materials, biomass or renewable energy sources are suggested to be conducted on land. Meanwhile, sulphur would poison the catalysts used for steam reforming, water gas shift and the electrochemical reaction of fuel cells. Hence, a desulphurization process is suggested to be conducted on land as much as possible, before the fuels are supplied onboard ships. However, onboard pre-processing for converting ammonia, SNG and renewable methanol into hydrogen is still required. Apart from conversion to a hydrogen-rich mixture, there are requirements for hydrogen purity, especially for low temperature fuel cells.
2.3.1. Hydrogen Pre-Processing for Low Temperature Fuel Cells
Low temperature fuel cells are very sensitive to CO. High purity hydrogen from land industry is required to be supplied onboard ships. Otherwise, CO clean-up processes are required. Commonly, if the hydrogen is from the conversions of hydrocarbon fuels, the CO concentrations in hydrogen after the water gas shift reaction probably exceed the allowable limits (e.g., 0.2 ppm) of low temperature fuel cells [59]. At that point, selective oxidation, selective methanation, membrane separation or pressure swing adsorption could be employed to remove CO or purify H2 [34].
Selective oxidation:
2CO + O2 → 2CO2
Selective methanation:
CO + 3H2 ⇌ CH4 + H2O
2.3.2. Ammonia Pre-Processing for SOFC
Ammonia fuel cells include direct ammonia alkaline and alkaline membrane fuel cells, direct hydrazine and ammonia borane fuel cells and direct ammonia SOFC [40]. However, an ammonia-fed SOFC has better performance and direct catalytic thermal decomposition of ammonia over catalysts at the anode is possible. Direct thermal cracking of ammonia occurs at 400–1000 °C and the equilibrium reaction can be expressed as follows:
2NH3 ⇌ N2 + 3H2
The rate of NH3 decomposition is influenced by different catalysts and different operating conditions. High partial pressure of NH3 and high operating temperatures could increase the decomposition of NH3 [41]. However, both the SOFC-H and SOFC-O might achieve a lower efficiency and change of external voltage as the temperature increases [40]. Therefore, there is an optimal operating temperature relating to NH3 decomposition, theoretical efficiency and external voltage of a SOFC.
2.3.3. NG Pre-Processing for High Temperature Fuel Cells
When NG is fed to fuel cells, steam reforming is commonly used to convert it into hydrogen. The reforming reactions of NG are as follows:
Steam reforming:
CH4 + H2O ⇌ CO + 3H2
Water gas shift:
CO + H2O ⇌ CO2 + H2
Total reaction from reforming:
CH4 + 2H2O ⇌ CO2 + 4H2
Steam reforming usually takes place at 500–1000 °C in the presence of a catalyst, e.g., nickel. Following the reforming reaction, a water gas shift reaction is usually conducted to improve hydrogen yield and lower CO concentration. An external reforming system is viable by using a suitable catalyst, external heat and steam. However, due to the availability of waste heat from the electrochemical reaction in a high temperature fuel cell, NG can be reformed internally by an independent reforming unit, known as indirect internal reforming. By mixing a part of the anode tail gas with the fresh fuel, heat and steam are supplied to sustain the reforming reaction. However, decreased fuel utilization reduces the overall system efficiency. To increase the fuel-to-electricity conversion efficiency, NG is reformed directly on the anode, known as direct internal reforming. The high temperature released and water vapor produced in the anode promote the reforming reactions, and the overall system efficiency is improved by enhanced system integration [60]. However, a series of problems, such as thermal stress and inhomogeneous current distributions, limit the extent of direct internal reforming [40].
2.3.4. Methanol Pre-Processing
When methanol is fed to fuel cells, steam reforming is also used to convert it into hydrogen. The reforming reactions of methanol are as follows:
Steam reforming:
CH3OH ⇌ CO + 2H2
Water gas shift:
CO + H2O ⇌ CO2 + H2
Total reaction from reforming:
CH3OH + H2O ⇌ CO2 + 3H2
As for methanol, steam reforming can take place at temperatures as low as 200 °C in the presence of a catalyst, e.g., nickel. Similarly, external reforming, indirect internal reforming or direct internal reforming could be employed for medium or high temperature fuel cells.
3. Fuel Cell Modules
A fuel cell power system consists of a hydrogen storage subsystem, a fuel cell module subsystem, a control subsystem and an energy management subsystem. Considering the energy efficiency, power capacity and sensitivity to fuel/oxidant impurities, AFC, PAFC and DMFC are excluded when discussing fuel cell modules in maritime applications below. For low temperature applications, PEMFC/HT-PEMFC is recommended as the power source and hydrogen is recommended as fuel. For high temperature applications, MCFC or SOFC are recommended as the power source and all kinds of hydrogen carriers or renewable fuels are recommended as fuels, where a reforming unit and a WHR system might be employed simultaneously to enhance system efficiency.
3.1. PEMFC Modules
A PEMFC module subsystem consists of a stack, a hydrogen delivery unit, an air delivery unit and a cooling unit [26,61]. A schematic diagram of a PEMFC module is shown in Figure 2. The hydrogen is stored in the storage tank in a cryogenic liquefied state, or in a compressed state with pressure of 350–700 bar. The pressure of hydrogen is regulated by the pressure regulator. After the fuel cell stack, a purge valve at the outlet of the anode chamber is periodically opened to prevent the cell voltage from decreasing below a certain limit [26]. A humidifier and a water separator may be installed before the pressure regulator and after the purge valve, to humidify the hydrogen and to remove the water from the purge gas respectively [19,26]. In addition, auxiliary components between the fuel inlet and outlet of the stack might be employed for hydrogen recirculation [61]. The filtered air is pressurized by an air blower and then humidified to maintain the performance of the polymer membrane within the fuel cells. For maritime applications, degradation of the polymer membrane may occur due to the cathode being exposed to the sea-air conditions [19]. Therefore, appropriate pretreatment of the inlet air to remove sodium chloride vapor might be required. The air blower not only maintains sufficient air flow to the stack, but also facilitates reduced stack size by increasing the inlet air density and eases the humidification process by increasing intake air temperature. Water in the residual air is separated by a condenser before being discharged to the outside of the module. The heat generated by the stack is removed by a cooling module, which comprises a water tank, a water pump and a heat exchanger. The heat exchanger transfers the heat to chilled water or sea water outboard [62]. The temperature of the cooling water at the stack inlet is controlled by adjusting the flow of the chilled water and at the stack outlet is regulated by adjusting the flow of the cooling water through the stack [26].
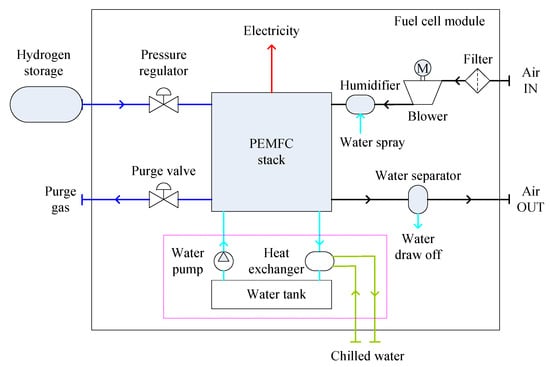
Figure 2.
Schematic diagram of a PEMFC (proton exchange membrane fuel cell) module.
As for HT-PEMFC, there is no need for strict water management, but thermal management is required for the fuel cell stack. Coolant oil is commonly used for the coolant fluid instead of water since the operating temperature is 140–200 °C. This higher operating temperature opens up the possibility of incorporating a WHR system to enhance energy efficiency. For instance, thermally activated absorption chillers directly utilize the heat of the hot coolant exiting the fuel cell stack, and the low temperature coolant exiting the absorption chiller is fed back to the fuel cell to repeat the process. The components of the absorption chiller refrigeration cycle include a generator, absorber, condenser and evaporator [63]. The total system efficiency is claimed as being up to 87% depending on the operational mode.
3.2. MCFC Modules
3.2.1. Overview
MCFC is a potential alternative to conventional marine power plants due to its high efficiency and power capacity. As discussed in Section 2.3.3, when NG, methanol and liquid hydrocarbons are used as fuels for a MCFC, an external reforming or internal reforming unit is required. Moreover, a WHR system is commonly employed and the overall system efficiency could be more than 80% [64]. The layout of a MCFC module can exhibit a number of different configurations depending on the required design parameters [65].
(i) An atmospheric system or a pressurized system. An atmospheric MCFC system operates at a near ambient pressure. However, a pressurized MCFC system is designed to operate at an elevated pressure, where the pressurized oxidant is delivered to the MCFC and the exiting gas is at a pressure of 3–4 bar. The pressurized MCFC system is more competitive due to higher efficiency and increased power density.
(ii) A standalone system or a hybrid system. A standalone MCFC system means that the MCFC stack is the only component outputting the electrical power. However, the electrical or mechanical work of a hybrid MCFC system is output by both the MCFC stack and the expander used for WHR purposes. The standalone MCFC system is simple and reliable. However, the hybrid MCFC system has significant advantages for large-scale applications because the highly exothermic electrochemical reaction and high operating temperature make it suitable for driving a bottoming cycle (Rankine cycle, Brayton cycle or a tri-generation system for the cooling, heat and power demands of a ship). Consequently, higher overall efficiency and larger power outputs can be obtained in the hybrid configuration.
(iii) An indirect hybrid system or a direct hybrid system. Regarding hybrid MCFC systems, either a ST or GT can be combined with the MCFC module. Indirect hybrid systems use the fuel cell exhaust gas to heat the working fluid of a bottoming cycle, where the power turbine is driven by the steam or the air heated by the energy from the combustion of the unreacted anode gas and the cathode gas, probably along with the auxiliary fuel. However, a direct hybrid system uses the energy from the combustion of the exiting gas of the MCFC stack to drive a GT directly. The indirect hybrid system is simple and reliable, and the operation of the MCFC is independent from the GT, which allows a safe and robust operation for both the fuel cell and the GT. However, the direct hybrid system is more attractive for large-scale applications.
3.2.2. Standalone MCFC System
The first marine MCFC system was installed onboard the offshore supply ship “Viking Lady” as an APU. The MCFC module consisted of a MCFC stack, an LNG supply unit, an air supply unit, a water supply unit, a catalytic combustion burner (CCB), a reforming unit and a fuel/steam heater for fuel humidification and water evaporation, as well as several auxiliary elements to fulfil regulation, measurement, safety and control functions [66,67]. A schematic illustration of the configuration is shown in Figure 3. Pre-heated fuel and water vapor flowed into the reforming unit, which was in thermal contact with the CCB to obtain the reforming heat. The reformed fuel products including H2, CO2, CO and H2O entered the anode chamber. The residual fuels were mixed with the air from the mechanical blower in the CCB, along with part of the residual air exiting from the cathode. Hot CO2-enriched air was fed to the cathode inlet for the electrochemical reactions of the fuel cell stack. The rest of the hot exhaust gas exiting from the cathode was used to pre-heat the mixtures of the fuel and water vapor, and then discharged to the atmosphere after evaporating the water supply.
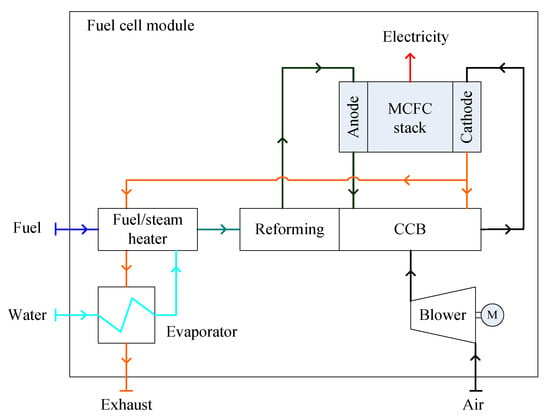
Figure 3.
Schematic diagram of a standalone MCFC (molten carbonate fuel cell) module.
3.2.3. Indirect Hybrid MCFC System
In Figure 3, the exhaust gas could be used to heat the working fluid of a bottoming cycle (either ST or GT are possible). The hybrid system coupling a MCFC with a ST or GT is called an indirect hybrid system [65,68,69]. Both the standalone MCFC system and the indirect hybrid MCFC system operate at atmospheric pressure.
Combined MCFC-ST System
A ST may be used in a bottoming cycle for WHR [29,67]. The water supply is changed into superheated steam by the exhaust gas from the cathode of the fuel cell stack through a cascade of pre-heater, evaporator and superheater after the fuel/steam heater. The superheated steam operates a ST for power generation. The residual steam along with the fuel is then pre-heated in the fuel/steam heater. Other components and processes are similar to those of the standalone MCFC system.
Combined MCFC-GT System
In some indirect hybrid systems, a power turbine is driven by air heated by energy from the combustion of the unreacted anode gas and the cathode gas, possibly along with auxiliary fuel [29]. This kind of indirect system might be preferred for a small-scale system. In some configurations [69], the exiting gas from the combustor heats the inlet air of the power turbine and then flows into the cathode; the exhaust gas from the cathode heats the pressurized air, the fuel and the water one by one before leaving the module. The fuel and water are reformed in an internal reformer and then enter into the anode. The residual fuels from the anode react with the exiting air from the power turbine in the combustor. The applications of these configurations are limited due to the relatively low efficiencies and the incompatibility of current commercial GT units.
3.2.4. Direct Hybrid MCFC System
If the exhaust gas of the MCFC stack is used to drive a GT directly, this kind of WHR system is called a direct hybrid system, where the operating pressure is usually 3–4 bar [65,70,71,72]. In the direct hybrid system, the power turbine is driven by the exhaust gas from the combustion of the unreacted anode gas and the cathode gas, probably along with the auxiliary fuel. The significant feature of the direct system is that without the heat exchanger present in the indirect system, it produces turbine inlet gas with higher temperature and pressure. This offers a greater variety of options for system layouts compared to the indirect system and increases the potential of the system to more efficiently exploit the heat released from the MCFC [65]. The direct hybrid system is suitable for large-scale systems.
A basic configuration of a combined system of MCFC and WHR is shown in Figure 4. A MCFC module consists of a stack, a fuel delivery and reforming unit, two CCBs (CCB1 and CCB2) and a GT unit. The main fuel (renewable NG or methanol) and water vapor are heated in the recuperator and then enter into the indirect internal reforming unit [70]. The reformed fuel compositions, including H2, CO2, CO and H2O, enter into the anode chamber. The residual fuels (all combustible components) exiting from the anode react with the residual air exiting from the cathode in the CCB1, providing heat to sustain the reactions in the reforming unit and CO2 required by the cathode. The air pressurized by the compressor and the exiting gas from the CCB1 enters the cathode to sustain the electrochemical reactions of the stack. The temperature of the exiting gas from the MCFC cathode is not enough to sustain the work of the GT. Typically 900–1050 °C of the turbine inlet temperature is required, so auxiliary fuel and a combustion burner are required. Thus, CCB2 is arranged to sustain the operation of the GT and to separate the operations of the FC stack and the GT as well. The supply of auxiliary fuel is dependent on the turbine inlet temperature required. The bypass air from the outlet of the compressor is controlled according to the load of the stack. The residual heat of the exhaust gas from the turbine is recovered by an evaporator to heat the water supply.
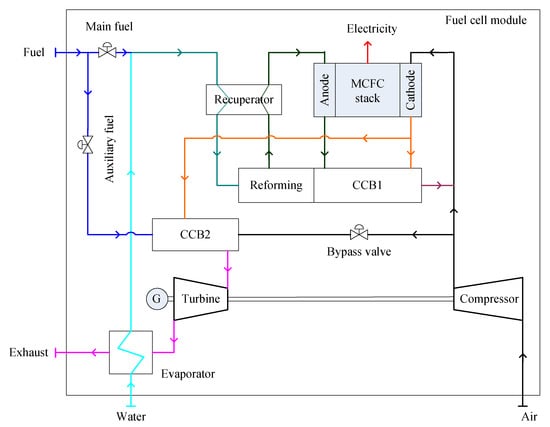
Figure 4.
Schematic diagram of the basic configuration of a MCFC-GT (molten carbonate fuel cell gas turbine) system.
If no auxiliary fuels are fed into the CCB2, a turbocharger, such as those commonly used in the application of internal combustion engines, could be employed to pressurize the air [70]. In such circumstances, the total power output and the efficiency of the MCFC system is limited. Accordingly, hybrid MCFC systems using GT are relatively common and several configurations have been investigated. The residual fuels exiting from the anode and the residual air from the cathode were recycled to either CCB1 or CCB2, or both of them. In addition, auxiliary fuels were fed to either CCB1 or CCB2, and a heat exchanger (regenerator) was used to pre-heat the pressurized air from the compressor by the exhaust gas from the turbine [70]. In some configurations, in addition to generating mechanical power for the air compressor, the turbine might generate extra electrical power through a generator [70]. Thus, both electrical power and efficiency increase. CCB2 could be a direct combustor and part of the reformed fuel products might be used for hydrogen separation in a pressure swing absorption system. The extracted hydrogen could be stored or mixed with the residual fuels exiting from the anode and the residual air exiting from the cathode [73]. In addition, the main fuel might be reformed by an external reformer using the waste heat from the system; the pressurized air is heated by the exhaust gas from the turbine, and reacts with part of the residual fuels from the anode in the CCB1, then entering into the cathode. Meanwhile, the exiting gas from the cathode reacts with the auxiliary fuel and the remaining part of the residual fuel from the anode in the CCB2 to drive the turbine [71]. It is clear therefore that the hybrid MCFC system efficiency, power output, complexity of the layout, power ratio of the stack to WHR system, power ratio of electricity to heat and plant costs are heavily influenced by system configuration factors such as post-combustion and operating pressure of the stack. [70].
3.3. SOFC Modules
3.3.1. Overview
The basic components of a SOFC module consist of a SOFC stack, a fuel supply unit, an air supply unit, a reforming unit, an after combustor and a WHR unit, as well as several auxiliary elements serving regulation, measurement, safety and control functions [74,75]. The operating temperature of the SOFC stack affects the layout of a SOFC module, in terms of fuel reforming, fuel preheating, air preheating and WHR system. SOFC modules can also have a number of distinct layouts, e.g., external reforming or internal reforming, atmospheric system or pressurized system, standalone system or hybrid system, indirect hybrid system or direct hybrid system [75,76,77]. Internal reforming is more attractive than external reforming due to higher efficiency and lower capital cost. However, the endothermic reaction of the fuel reforming process may lead to significant temperature gradients and inhomogeneous current distributions inside the cells [40,74,76,77], so indirect internal reforming is preferable due to reduced thermal stress. In addition, depending on the source of steam for fuel reforming, there may be recirculation of water from the anode or external water supply. Anode recirculation provides the steam for the fuel reforming process through recirculating part of the exiting gas from the anode. Anode recirculation is normally performed by a blower or an ejector. However, if there is no anode recirculation arrangement, an external water supply is heated by a heat recovery steam generator to provide the steam for the fuel reforming process [75]. Anode recirculation seems to be more attractive for marine applications since it is typically less expensive and more efficient. In addition, the limited fresh water storage available onboard a ship is another consideration. However, it is difficult to control the steam-to-carbon ratio inside the stack accurately [75,76,77].
3.3.2. Standalone SOFC System
A standalone SOFC system consists of a SOFC stack, a fuel supply unit, an air supply unit, a reforming unit, a catalytic burner and a WHR system [75], as shown in Figure 5. Anode recirculation and indirect internal reforming are supposed to be employed for the SOFC modules discussed in Section 3.3. Therefore, water supply and steam generation from the WHR system are not needed. The reformer is thermally coupled with the SOFC stack, i.e., the heat used for the fuel reforming process is from the electrochemical reactions and is transferred to the reforming unit either by heat radiation or by direct physical contact between the reforming unit and the SOFC stack. Part of the exiting gas from the anode is recirculated for fuel reforming. Another part of the anode exiting gas reacts with the residual air from the cathode in an after catalytic combustor. The exhaust gas from the combustor flows through the air preheater, the fuel preheater and the economizer before discharging to the atmosphere. Other options of the layout include: the air being preheated by the residual air from the cathode; the fuel being preheated by the exiting gas from the anode; the catalytic combustor providing heat for an external fuel reforming unit; and the economizer generating steam for the fuel reforming. [74,78,79] Depending upon the operating temperature, varying grades of waste heat can be recovered for distinct applications, which can significantly impact the system economics and environmental issues.
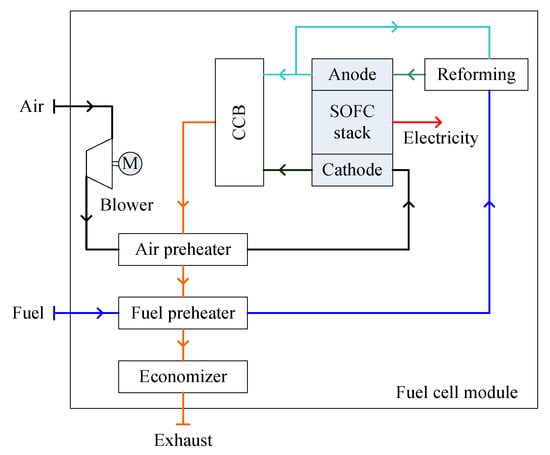
Figure 5.
Schematic diagram of an atmospheric SOFC (solid oxide fuel cell) module.
3.3.3. Indirect Hybrid SOFC System
Combined SOFC-ST System
An indirect hybrid SOFC-ST system consists of a SOFC stack, a fuel supply unit, an air supply unit, a reforming unit, an after catalytic combustor and a WHR system [74,80]. The high temperature exhaust gas from the SOFC stack can be utilized for pre-heating the fuel, the air and for the reforming unit. When the operating temperature of the SOFC stack is lower, Rankine cycles could be considered to generate steam. Thus, additional electrical energy could be generated by a ST and the overall efficiency of the system could be increased to more than 80% [74,80]. The common working fluid of Rankine cycle is water. However, when the temperature of the heat source is lower, organic fluids are typically utilized to substitute water due to their low critical temperature. The combination of a SOFC system and an organic Rankine cycle (ORC) has been investigated and verified by many researchers in recent years [81,82,83,84].
Combined SOFC-GT System
The indirect hybrid SOFC-GT system consists of a SOFC stack, a fuel supply unit, an air supply unit, a reforming unit, an after catalytic combustor, a heat exchanger, turbomachinery (in the form of a GT) and an economizer [74,75,85], as shown in Figure 6. In an indirect hybrid SOFC-GT system, the air from the compressor is heated by the exhaust gas from the combustor through the heat exchanger, which then drives the air turbine before it flows into the cathode. To improve the turbine inlet temperature for a higher pressure ratio, an auxiliary combustor may be arranged before the turbine [74,75,85,86].
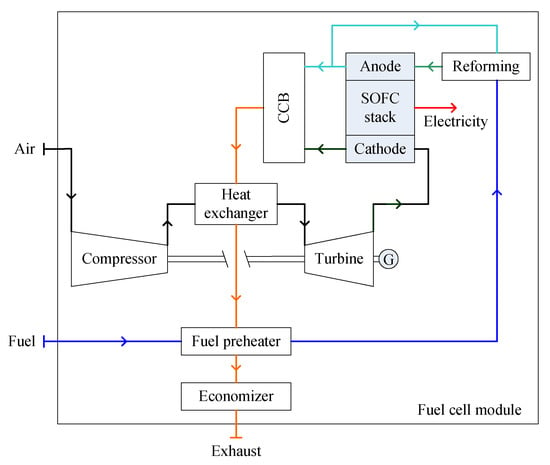
Figure 6.
Schematic diagram of an indirect SOFC-GT (solid oxide fuel cell gas turbine) module.
A more complex indirect hybrid SOFC-GT-ST system has been investigated, where additional fuel is supplied to the combustor to provide the GT cycle with required heat; subsequently, the hot gas exiting from the heat exchanger is used to drive a ST cycle [75,87]. The heat exchanger has to be operated at very high temperatures and pressure differences. Hence, the applications of the indirect hybrid SOFC-GT system are limited by material requirements [74,85].
3.3.4. Direct Hybrid SOFC System
A direct hybrid system uses the energy from the combustion of the exiting gases of the SOFC stack to drive a GT directly, as shown in Figure 7. Consequently, the exhaust gas exiting from the GT is used to preheat the air and the fuel, and then an economizer is used to recover the residual heat. The operating range of a GT, in terms of pressures and mass flow rates, is very restricted due to the intrinsic characteristics of the turbomachinery. Thus, auxiliary fuel is supplied to the combustor to maintain the necessary turbine inlet temperature. Meanwhile, the SOFC and GT work separately through the combustor. A large number of distinct configurations have been investigated. Examples include using the GT to drive both the air compressor and a generator, which means both mechanical and electrical energy are generated from the GT, and also using the GT to drive the air compressor, which is followed by a power turbine driving a generator to output additional electrical energy [30,74,75,88,89].
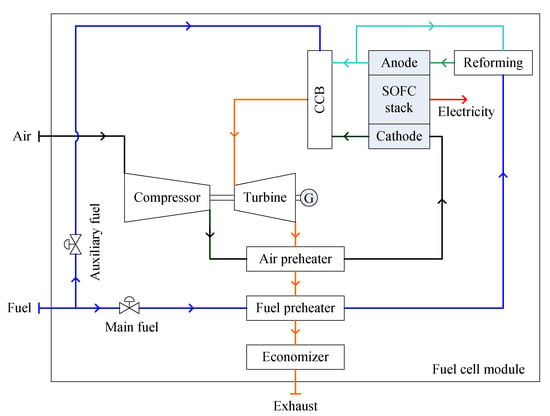
Figure 7.
Schematic diagram of a direct SOFC-GT module.
4. Marine Fuel Cell Power Systems
The basic components of a marine fuel cell power system consist of fuel storage, a fuel cell module and control unit, DC-DC converter, battery banks and charger, DC-AC inverter and DC/AC loads, as shown in Figure 8. Depending upon the operating temperature of the fuel cells, varying grades of waste heat may be recovered for heating, cooling, co-generation or tri-generation application purposes. As discussed in Section 3, WHR systems could be incorporated into the fuel cell module, which can significantly impact the system efficiency, economics and environmental issues [74].
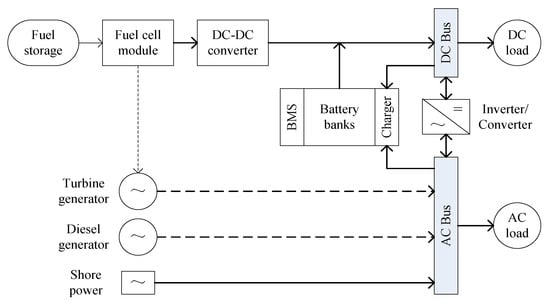
Figure 8.
Schematic diagram of a marine fuel cell power system. (BMS–battery management system).
4.1. Fuel Storage
As discussed in Section 2.3, hydrogen, ammonia, NG and methanol are promising fuels for marine fuel cell power systems under the context of low/zero net carbon maritime transportation. Due to the low volumetric energy density of hydrogen and limited power range of PEMFCs, PEMFC power systems are only available for small-scale ships operating for domestic and short-sea shipping. Correspondingly, hydrogen storage is typically achieved in a compressed state at a pressure of 350 bar or 700 bar rather than in a liquefied state at a temperature of −253 °C. The storage tanks for compressed hydrogen usually comprise a thin aluminum liner and carbon fiber–plastic composite materials [90]. However, 10–15-times larger storage space is required for compressed hydrogen compared to that for conventional marine fuel oils; even for LH2, 4–5-times larger storage space is required. Ammonia as a hydrogen carrier has 3–5-times higher volumetric energy density than compressed hydrogen, and may be stored in stainless steel spheres at ambient temperature and 8 bar vapor pressure.
MCFC and SOFC power systems are expected to use SNG and renewable methanol as fuels for future shipping, which will share similar fuel processing units for these two fuels. SNG is stored either in a compressed state at 200–250 bar or in a liquefied state at −163 °C. For ships trading on short routes, compressed NG may be employed and stored in hard containers, which are usually cylindrical or spherical in shape; for long distance international shipping, LNG may be employed and stored in horizontal or vertical, vacuum-jacketed, pressure vessels. Despite efficient insulation, some heat leakage would result in the production of boil-off gas, which is exported to the fuel cell module or is re-liquefied and returned to LNG storage tank. Methanol is a low flashpoint liquid alcohol fuel, and may be stored in the same way as conventional liquid marine fuels with minor modifications to the storage systems. It is worth noting however that approximately double the storage space is required compared to that for conventional marine fuel oils. For the storage of distinct marine fuels, the pressure and temperature of the fuel storage tank are monitored constantly to ensure safety. Emergency ventilation valves may be fitted to empty the tanks when they are exposed to fire. The fuel exiting the storage tanks is regulated by a pressure-reducing valve or pressurized by a fuel pump before entering the fuel cell module.
4.2. Fuel Cell Module
As discussed in Section 3, PEMFCs, direct hybrid MCFC-GT systems and direct hybrid SOFC-GT systems are the promising options for power modules. Lower costs, longer lifetimes and higher power density and efficiency are the primary requirements for maritime applications.
4.3. Battery Banks
Fuel cells are commonly combined with battery banks to take advantage of the superior energy density of fuel cell systems and the transient response capabilities of batteries [91]. Especially for high temperature fuel cells, battery banks could balance the load change and allow for a more stable operation of the fuel cells, which may significantly reduce the strain due to thermal cycling. The capacities of the battery banks vary depending upon the distinct control strategies. During normal operation, when the external load increases, the battery banks are instantly connected to the power distribution network. Then the power output of the fuel cell module can be increased to the required power level slowly within a short period of time after load increase, followed by the adjustment of the GT. Conversely, when the external load decreases, the excess power generated by the fuel cell module can charge the battery banks.
4.4. Power Electronics and Loads
(i) Battery management system. According to the state of charge (the level of charge of an electric battery relative to its capacity, SoC), the power output of the fuel cell module is adjusted automatically. A battery management system is equipped to monitor the voltage, temperature and state of charge of the battery banks to ensure safe operation. The battery charger is developed to charge the battery banks using either the fuel cell module or an external power source [26].
(ii) DC-DC converter. An individual fuel cell produces a DC voltage of 0.5–1.0 V at rated load, which decreases as current increases. Hence, only a few or as many as hundreds of individual cells could be placed in series (known as stacking) to yield a higher voltage, the maximum limit of which is usually constrained by the manufacturing process. Meanwhile, to output the desired power, a higher current is achieved by increasing the surface area of the cells, or by combining the fuel cells in parallel. The DC-DC converter is used to increase the voltage of the fuel cell module to meet the requirements of industrial applications, e.g., 240–370 V or more [26]. Due to the release of heat from the converter circuits and the use of cooling fans, the efficiency of the converter is less than 100%. As input power increases, the efficiency of the converter also increases because the proportion of the basic power consumed by the cooling fans is decreased. The DC-DC converter normally has an efficiency greater than 90%.
(iii) DC-AC inverter. A DC bus is used to collect power from the DC-DC converter of the fuel cell module and distribute the power to the DC loads. An AC bus is used to collect power from the turbine generators (if fitted) of the fuel cell module, the diesel generators (if fitted) and a shore power supply. A DC-AC inverter is installed to convert the DC power from the fuel cell module to the desired current types and voltage levels, which are supplied to power AC loads.
(iv) Loads. The AC bus distributes the power to the AC loads, such as auxiliary pumps and blowers of fuel cell module, and onboard auxiliary equipment including radiocommunication, navigation, lighting and air conditioners. DC and more often AC electric motors could be used for propulsion directly through driving a propeller or waterjet pump.
5. Marine Applications of Fuel Cell Power Systems
5.1. Fuel Cells for Maritime Demonstrations
Fuel cells have been used for military submarines since the 1960s, but civil applications of the technology did not arise until this century. Up to now, a large number of research and demonstration projects have verified the viability of fuel cells for maritime applications. Some noticeable demonstration projects of marine fuel cells since 2000 are shown in Table 3. Reduced emissions, increased efficiency and quiet operation make fuel cells attractive for future low carbon shipping. In particular, PEMFC, including HT-PEMFC, as well as MCFC and SOFC, are the most promising types of marine fuel cells.

Table 3.
Noticeable demonstration projects of marine fuel cell systems since 2000. [9,11,34,92].
5.2. Marine PEMFC Power Systems
The early applications of PEMFC power systems in the maritime sector include: A 12-m-long yacht with a maximum cruising range of 225 km at a speed of 8 knots, which utilized 6 kg of hydrogen stored in three storage tanks at a pressure of 300 bar, and was powered by four 1.2 kW PEMFC modules combined with nine lead-gel batteries, generating a total power output of up to 20 kW [93]; a canal boat using the hydrogen stored in a five-cylinder metal hydride storage system, powered by a 5 kW PEMFC module together with a lead-acid battery [94]; and a small boat with a maximum operating time of 10 h at a maximum speed of 7 knots, which utilized 5 kg of hydrogen onboard and was powered by an 8 kW PEMFC module [95,96]. However, the installed power of these applications is usually less than 10 kW [9,26].
5.2.1. FCS Alsterwasser
FCS Alsterwasser was the first passenger ship powered by hydrogen fuel cells with a maximum power up to 100 kW. Based on the Zemships project, a PEMFC power system was developed for FCS Alsterwasser, which was about 25 m long and designed to accommodate up to 100 passengers at a maximum speed of 8 knots [97]. The power system consisted of twelve storage tanks with 50 kg of hydrogen at a pressure of 350 bar, two 48 kW PEMFC modules, 7 lead-gel battery packs with total capacities of 234 kWh and total voltage of 560 V, a 100 kW propulsion electric motor and a 20 kW bow thruster. The PEMFC power system was used to power the propulsion motor directly or charge the lead-gel battery packs. The battery packs, used as a back-up option if the fuel cells failed, not only delivered power to the propulsion motor at peak load but also lightened the load on the fuel cells during docking and casting-off procedures, which prolonged the life cycle of the fuel cells. The operation of the battery packs was determined by an energy management system. The hydrogen stored onboard allowed the ship to operate for 2–3 days without refueling, while it took only 12 min to fill up the hydrogen storage tanks.
5.2.2. Nemo H2
A PEMFC power system was developed for Nemo H2, which was about 22 m long and designed to accommodate about 88 passengers at a maximum speed of 8.6 knots [98]. The power system consisted of six storage tanks with 24 kg of hydrogen at a pressure of 350 bar, two 30 kW PEMFC modules, 55 lead-acid battery packs with total capacities of 70 kWh, a 75 kW propulsion electric motor and a 11 kW bow thruster. The PEMFC power system was used to power the propulsion motor directly or charge the lead-acid battery packs. The battery packs were used as a back-up option and to improve the performance of the fuel cells as well. An energy management system was used to determine the operation of the battery packs. The refueling process happened once a day.
5.2.3. SF-BREEZE
SF-BREEZE was a concept hydrogen-powered passenger ferry, which was developed to accommodate 150 passengers at a maximum speed of 35 knots [99]. The power system consisted of a single Type C (pressurized vessel) storage tank on the top deck with 1200 kg of LH2, 41 120 kW PEMFC modules containing four 30 kW PEMFC stacks each, DC-DC converters, DC-AC inverters and two waterjet propulsion systems driven by AC motors with a power of 2000 kW each. In addition, 120 kW of power was used for auxiliary systems such as HVAC and 400 kW was retained for a working margin. No additional battery packs were installed and the endurance was up to 100 nautical miles before refueling. The shoreside refueling facility needed to be able to provide fuel to the vessel twice a day.
5.2.4. A Tourist Boat
A PEMFC power system was developed for a 20-m-long tourist boat, which had a light weight of 20 tons and accommodated a maximum of 50 passengers [26]. Fuel storage consisted of fourteen storage tanks with 74 L hydrogen each at 350 bar, resulting in a total hydrogen capacity of 25 kg onboard. The power system consisted of two 28 kW PEMFC modules, two 25 kW DC-DC converters with input voltages of 82–170 V and output voltages of 240–370 V, three 15.7 kWh Li-ion battery packs, a battery charger and a waterjet propulsion system with a maximum power of 86 kW. The total volume of the system was 7.41 m3 and the total weight was 2190 kg, where the storage tanks including hydrogen were 4.25 m3 and 712 kg respectively. The hydrogen stored onboard allowed the PEMFC modules to operate at the maximum power output of 50 kW for about 8 h. When the boat was propelled by the PEMFC modules only, the speed of the boat was 4.5–5.6 knots at power outputs of 38–51 kW. However, when the PEMFC modules and the Li-ion battery packs delivered power to the boat together, the speed reached 6.6–7.8 knots at a total power output of about 85 kW. For a higher speed, the PEMFC power system would need to be several times the volume and weight of installed version. Therefore, there were some technical and economic limitations for the widespread maritime application of PEMFC power systems due to the relatively low power densities of PEMFCs and the low volumetric energy density of hydrogen storage systems.
5.2.5. Others
Based on the E4Ships Pa-X-ell project, a 60 kW modularized HT-PEMFC power system fueled by methanol was developed for the auxiliary power supply onboard the passenger ship MS Mariella [100,101]. The power system included two 30 kW HT-PEMFC units, each of which comprised six 5 kW modules. The fuel cell stack, the reformer, the afterburner, the in-process heat exchanger, the DC-DC converter and the control units were integrated in one module housing with an exhaust as well as fuel and cooling water piping. In addition, a methanol tank was installed.
Based on the RiverCell project [11], a 250 kW modularized HT-PEMFC power system fueled by methanol was developed as a part of a hybrid power supply for river cruise vessels. Meanwhile, the feasibility of a 192 kW HT-PEMFC power system fueled by hydrogen combining with 1250 kWh fully charged battery packs was conducted. The ship had six storage tanks with 740 kg of hydrogen at a pressure of under 500 bar.
Compared to PEMFC power systems, HT-PEMFC power systems improved the fuel flexibility and avoided complex water management. WHR was also possible with the HT-PEMFC system.
In addition, a hybrid power system was developed to provide the main and auxiliary power of a cruise ship in Stockholm [13]. Solar PV, PEMFC and diesel generators were taken as power sources, and a DC-bus, an AC-bus and a DC-AC inverter were used to collect, distribute and convert power. However, the energy production from the PEMFC was extremely low since the hydrogen production for the PEMFC mainly depended on the surplus electrical power from the solar PV.
5.3. Marine MCFC Power Systems
The offshore supply vessel Viking Lady, which utilized fully electric propulsion powered by dual fuel engines, was the first ship using a MCFC power system onboard as an APU [66]. The newly installed MCFC and existing power plant used the same LNG fuel and supply system. The 320 kW MCFC module was newly developed and comprised of a stack of 500 fuel cells, as well as an internal reforming unit and a WHR system. The MCFC module delivered a DC voltage varying between 380–520 V depending on its load condition and age (expected operational lifetime of 24,000 h). The electrical system had been designed to compensate for the slow response of the fuel cells in order to keep stable conditions to protect against harmful dynamic load changes which could diminish the lifetime of the fuel cells. When the FellowSHIP project entered into the third phase, marine lithium ion battery packs were integrated into the ship power system. When the ship operated at low loads, such as maneuvering or berthing, the fuel cells and its batteries operated alone and relieved the concern of methane slip from the LNG-fueled engines, which would reduce emissions, noise and vibrations significantly.
Apart from the Viking Lady, a 28 MW MCFC-based propulsion system, which consumed both LNG and hydrogen boil-off gas as fuel, was developed for a 140,000 m3 LH2 tanker [24,29,102]. In addition, a 625 kW MCFC power system and a 500 kW (concept design)/150 kW (final design) MCFC power system were developed based on the US SSFC project and the MC-WAP project, respectively [11], both of which were fueled by diesel; no application demonstrations were carried out onboard ships.
5.4. Marine SOFC Power Systems
Based on the METAPHU project, the conceptual study of a 250 kW SOFC APU using methanol was finished, and practical operation of a 20 kW SOFC unit onboard car carrier MV Undine has been carried out [103]. This 20 kW SOFC unit, which was independent of the ship’s propulsion source or main electric system, just aimed at testing the performance and emissions under real-life conditions onboard a ship and at assessing the maturity of methanol-based technology in the shipping sector. The SOFC system comprised a methanol tank, a reformer, the SOFC stack, a catalytic combustion afterburner and in-process heat exchangers [104,105]. The SOFC stack ran on hydrogen and the methanol (or NG in other SOFC applications) was reformed prior to entering the stack. Part of the anode gas was recirculated for methanol reforming and another part was burned with the cathode exit gas in a catalytic burner. The stack operated at the temperature of 600–900 °C. The air was preheated by the cathode exit gas through a heat exchanger. The heat of the exhaust gas from the catalytic burner was absorbed by the methanol prior to entering the reformer through a heat exchanger and was further absorbed by an economizer.
Based on E4Ships SchIBZ project, a hybrid power system combining a 50 kW containerized SOFC unit with lithium-ion battery packs was developed for the auxiliary power supply onboard the general cargo ship MS Forester [101]. The hybrid power system comprised a diesel tank, a water tank, a reformer, the SOFC stack, a catalytic combustion afterburner operating at a temperature of 750 °C, a heat exchanger for WHR, lithium-ion battery packs to compensate the fluctuations of the electrical loads and power electronics. The SOFC module fueled by low-sulphur diesel (maximum 15 ppm sulphur) was expected to provide 25–50% of the onboard power demand. In addition, the power output of the SOFC stack could be scalable up to 500 kW. The module operated at a temperature of around 800 °C, and the heat recovery of exhaust gases and the integrated reforming process made it possible to achieve a higher overall efficiency [11].
6. Challenges and Perspectives
The future prospects of fuel cells can be assessed and analyzed based on a multidimensional framework considering technological indexes, economic costs, environmental performance and social effects. The excellent performance from climate change and local emissions perspectives are the most outstanding advantages of fuel cells, and there are no significant public concerns to speak of. Consequently, environmental and social factors are not the main considerations limiting the widespread uptake of fuel cell technology in the marine sector. As a marketable marine power system, power capacity, safety, reliability, durability, operability and costs are important factors that need more attention.
6.1. Power Capacity
The power demands for marine power systems range from a few kW to tens of MW. Currently, the maximum power output of fuel cells is only several MW. The potential uses within merchant marine applications are therefore limited in terms of power output. As a result, the advantages of fuel cells are being realized through use in APUs, as well as propulsion power plants for inland and short-sea shipping. The maximum power capacity and total performances could be further improved by combining with batteries, according to the existing operational experience [11]. However, through creating hybrid systems by coupling with turbomachinery, high temperature fuel cells, such as MCFC and SOFC, also exhibit the potential to provide propulsive power for larger maritime vessels rather than just contributing auxiliary power. Although a GT itself has no competitiveness for propulsion in a merchant marine setting due to relatively low efficiency, the overall system efficiencies of the co- and tri-generation schemes combining high temperature fuel cells, GT units and HVAC installations are claimed to be up to 70–85%. Optimizing the power distribution between fuel cells and the GT, deriving suitable control strategies and operating condition-dependent power distribution between fuel cell modules and batteries are the feasible pathways to increase the power output without compromising total efficiency. In addition, modularization is an important pathway to increase the total power of the plants, which is similar to battery banks. Several projects have demonstrated the potential of modularized fuel cells, such as the RiverCell project [11], the E4Ships Pa-X-ell project [100], and the E4Ships SchIBZ project [101].
6.2. Safety
Safe operation of power systems onboard ships is of paramount importance. The safety of fuel cell power systems depends primarily on the choice of fuel, key considerations related to which are fuel density, flashpoint, auto-ignition temperature, flammability limits and toxicity, etc. In addition, different working scenarios including bunkering, onboard storage, daily service and emergency response should be covered when managing the risks. Focusing on hydrogen, ammonia, methane and methanol discussed in this paper, gas-tight enclosures of pipelines and fuel cell stacks, redundant monitoring for leakage, emergency shutdown for systems and rapid venting of leaking fuels into the atmosphere are indispensable risk-mitigating measures for these gaseous or low flashpoint fuels. This is especially the case for ammonia and methanol which can be slightly toxic and dangerous for humans to some extent. International maritime regulations and classification rules surrounding fuel cell power systems are currently absent. Although the International Code of Safety for Ships Using Gases or Other Low-flashpoint Fuels (IGF Code) provides some reference, detailed regulations, classification rules and operational guidelines targeting fuel cell power plants are dependent on the accumulation of lots of testing and demonstration experience. Formal safety assessment and risk-based safety management are the important tools and principles for system designers, rule makers and ship operators.
6.3. Reliability
The reliability of fuel cell power systems depends on trouble-free operation on the one hand; on the other hand, it depends on the availability of fuels. An absence of primary mechanical moving parts makes fuel cells relatively reliable, especially for low temperature fuel cells coupled with moderate working conditions. For MCFC and SOFC power systems however, high operating temperatures and cycling effects due to load changes make the fuel cell stack vulnerable, with the probability of failure further increased when introducing integrated fuel reforming units and WHR units. Therefore, the design of redundant systems and components could be employed to avoid a complete loss of power due to single point failures. Apart from improving the reliability of systems and components, battery banks are viable options to buffer the load fluctuations of fuel cells to avoid negative cycling effects. In addition, adequate control strategies are key to ensure reliability of complex systems.
The fuel availability depends on infrastructure, which is one of the primary barriers of low carbon shipping. Purely considering economic interests, there is no market motivation for infrastructure since there are very few ships fueled by eco-friendly fuels in operation; in turn, there is little motivation for fuel-cell-powered ships since there is very little infrastructure for fuel bunkering available. Therefore, legal frameworks and policy incentives in the maritime community, globally, regionally or locally, should be implemented. At the present time, this remains a significant barrier to widespread fuel cell adoption; however, increasingly stringent targets being levied by the IMO and the ultimate goal of net-zero carbon emissions will drive change in the years to come.
6.4. Durability
Durability primarily means the lifetime of a fuel cell stack. The lifetimes of a PEMFC for stationary and transportation applications are expected to be 40,000 and 5000 h, respectively, by the U.S. Department of Energy [44]. Some MCFC and SOFC plants have achieved a lifetime of more than 30,000 h [17,33,106]. The lifetime of a fuel cell stack is mainly dependent on the degradation of electrolyte, electrode and bipolar plate [107,108]. For instance, the degradation mechanisms of PEMFC include loss of catalyst, reduced conductivity of electrolyte, corrosion, poisoning and flooding. [109,110]; the degradation mechanisms of SOFC include loss of catalyst and electrolyte, cracking or corrosion. [111] Based on the degradation mechanisms of a fuel cell stack, novel materials and technologies have been employed to improve performance and durability. However, for large-scale commercialization, the focus also includes maintaining the performance throughout the operational life or mitigating the degradation rates to an acceptable level [108]. The realistic operating conditions include load cycling, thermal cycling and impurities in the fuel and air, which will influence the chemical and mechanical stability of the fuel cell systems and components. Maintaining steady-state operation is important to obtain prolonged durability, and can only be achieved through appropriate system design and an optimized control strategy encompassing all elements of the power system. In addition, marine fuel cell power systems operate in a sea water environment. Accordingly, some precautions need to be taken to prevent ingression of salt mist in the cathode air since the performance of the fuel cells is proved to be degraded by the sea mist [19].
6.5. Operability
Operability could be reflected by start-up time and transient dynamic response. Considering the fuel cell stack, the start-up time ranges from a few seconds for a PEMFC to tens of minutes for a SOFC since high temperature fuel cells need more time for stack and reformer preheating [106]. However, for maritime applications, a long start-up time is not a significant flaw and could be accepted to some extent. After all, several hours are normally required for engine standby of large maritime ships powered by diesel engines at present. Dynamic response characteristics reflect the response of fuel cell power systems to external load changes. The transient response time ranges from less than 10 s for PEMFC to 15 min for SOFC [106]. Meanwhile, the transient response time of reforming systems is typically a few minutes. Therefore, for standalone fuel cell systems, batteries or supercapacitors are required to offset the sudden changes of external loads since the transient response time of batteries or supercapacitors is normally less than 10 s [112]. When the working conditions of the fuel cell stack adapt to the external loads, the batteries or supercapacitors no longer contribute power to the grid.
6.6. Economic Costs
The application of any new technology onboard a ship has a cost associated with it, which can of course act to inhibit the transition to novel power and propulsion systems. However, estimating the premium of innovation of a ship by applying the net present value formula is an effective tool which could assist in the evaluation of the financial performance on acquisition and operational decisions [113]. The development of fuel cells for maritime applications commenced half a century ago, but high costs are commonly regarded as the primary factor restricting their widespread use. High costs emanate from the following aspects:
(i) High stack costs. The unit prices of fuel cell stacks are more than 50 $/kW for PEMFC and about 4000–9000 $/kW for MCFC and SOFC at present [34], which are significantly more expensive than conventional diesel engine power plants. Achieving reduced stack costs depends on the use of cheaper electrode materials rather than precious metal catalysts. In addition, with increasing annual production volume, the unit prices would decrease gradually due to economies of scale. For example, the SOFC stack cost is expected to reduce to a competitive price, 170 $/kW, through mass production and cell scale-up [114,115]. In addition, increased system efficiency with cell scale-up will also decrease operational cost.
(ii) Shorter plant lifetime. The maximum stack lifetime of MCFC and SOFC is commonly about 40,000 h [17,106]. In any case, stack lifetimes are significantly shorter than the lifetime of 20–30 years of conventional diesel engine power plants, which further increases the plant investment cost. Fuel pretreatment, electrode material technologies and stable operating conditions are helpful to mitigate stack degradation.
(iii) Higher cost of auxiliary systems and components. Fuel storage units, reforming units, WHR units, monitoring systems and power electronics are more expensive than that for conventional diesel engine power plants. However, mass production would lower the component costs and the increased energy efficiencies witnessed with fuel cell hybrid systems would save some operational cost.
(iv) Investment cost for onshore infrastructure. Currently, the infrastructure for production, transport, storage and bunkering of renewable fuels is insufficient, and without regulatory intervention, the huge investment required would manifest itself in increased fuel prices. The prices of renewable fuels discussed in this paper are commonly higher than conventional marine fuels. However, with increasingly stringent regulations on local emissions in Emission Control Areas and near port areas, infrastructure for eco-friendly fuels is gradually becoming indispensable.
(v) Operational cost. During the operating phase, fuel consumption is the main cost. Other operational costs include repair and maintenance, educational and training cost for special professional skills and higher wages for crews with higher skill levels. Fuel cost depends on fuel consumption and fuel price. The former is related to energy efficiency and the latter is linked to infrastructure investment as well as market supply and demand. Fuel cell stacks have higher electrical efficiencies than conventional power plants. If a GT or power turbine are installed to recover waste heat, the overall efficiency of the system will be increased [17]. However, auxiliary systems and components such as reforming, cooling, recirculating and controlling are associated with pumps, fans/blowers, sensors and controllers (usually referred to as the balance of plant, BoP), which will consume parasitic power [34]. Thus, the net system efficiency will decrease significantly. Moreover, the net system efficiency is further reduced when the plant operates at partial load conditions. Overall, energy efficiency is associated with system design [75], predetermined control strategies [75], stack degradation [116] and operating conditions [72,75]. Research and development on tailored system designs for specific maritime applications, identifying degradation mechanisms and optimized operating and control strategies are required for a higher system efficiency.
7. Conclusions and Implications
Fuel cell power systems for maritime applications were reviewed in terms of their types, characteristics, potential zero carbon or carbon-neutral fuels and notable demonstration projects. The challenges with regard to power capacity, safety, reliability, durability, operability and costs were analyzed. The following conclusions and implications can be summarized and are expected to provide useful reference for research communities and industrial organizations in the maritime sector.
(1) Existing demonstration projects were originally aimed at reducing NOx and SOx emissions, meaning CO2 emissions were not the main consideration. This led to diesel, LNG and fossil-based methanol being the chosen fuel options for several projects. However, with the goal of achieving low or zero carbon maritime transportation, the prospects for carbon-containing fossil fuels onboard ships are not particularly optimistic due to the extra space and energy demands for carbon capture and conditioning and for the temporary storage of captured CO2. Therefore, the selection of marine fuel cell power systems and marine fuels should be considered simultaneously. The demonstration projects to date show that the refueling interval for power systems with hydrogen storage could not extend beyond 2–3 days due to the low volumetric energy density of hydrogen, otherwise the necessary increase in space utilized for hydrogen storage would significantly decrease the power density of onboard power systems and the payloads of ships. Consequently, direct hydrogen storage is probably only viable for domestic or short-sea shipping. Deep-sea shipping, by comparison, requires marine fuels with higher volumetric energy density, such as ammonia, renewable methane and methanol.
(2) Considering energy efficiency, power capacity and sensitivity to fuel/oxidant impurities, PEMFC/HT-PEMFC, MCFC and SOFC are the most promising options for maritime applications. Due to the existence of reforming units and WHR units, MCFC and SOFC power systems have lower power densities but higher energy efficiencies compared to a PEMFC power system. Therefore, for the maritime sector, quantifying the power density of different fuel cell power systems (including their storage volume for different marine fuels), determining specific fuel consumption and comparing with conventional marine power systems are important research issues at present. Moreover, the advantages of each power system are dependent upon their application scenarios, and the guidelines for design and applications need to be addressed.
(3) Due to the possible integration of reforming units and WHR units, MCFC and SOFC power systems can be characterized by a number of layout options. These include the choice of a standalone or hybrid system, indirect hybrid system or direct hybrid system, atmospheric system or pressurized system, external reforming or internal reforming, etc. A large number of system design and optimization parameters must be considered to maximize performance depending on the specific applications. In addition, optimized operating and control strategies should be employed to improve energy efficiency, reliability and durability. Moreover, hybrid systems coupling fuel cells with batteries, solar PV or diesel generators could effectively improve reliability and durability of fuel cell stacks, and consequently decrease the costs; due to localized cooling, heat and power demands onboard ships, a co- and tri-generation system coupling fuel cells with GT and HVAC is a key consideration to maximize fuel efficiency. An overall system optimization and energy management scheme study, which is clearly ship specific and application dependent, is an important future research requirement.
(4) The technical feasibility of fuel cell power systems for maritime applications, in terms of power capacity, safety, reliability, durability and operability, has been verified by significant amounts of research and existing demonstration projects. The significant advantages are mainly characterized by reduced emissions, increased efficiency and quiet operation, which are all attractive for the sustainability of future shipping. However, the disadvantages in terms of power capacity, durability and economic costs are noteworthy as well. The maximum power output of the demonstration projects conducted within the maritime industry is only a few hundred kW, which is far from meeting the requirements of ocean shipping. In addition, the durability tests of practical application scenarios onboard ships are scarce. More convincing results are dependent on the accumulation of more real-world data. Currently, short plant lifetime, high initial investment and operational costs are the main obstacles preventing widespread use of fuel cells in the maritime sector. However, large-scale applications in transport sectors in future are expected to significantly reduce the costs to an acceptable level. In addition, strict regulatory requirements, investment in infrastructure for fuel bunkering and development of design rules and operational guidelines should simultaneously accompany the development of this technology.
Author Contributions
Conceptualization, H.C.; methodology, H.X.; formal analysis, H.X. and C.S.; investigation, H.X. and C.S.; writing-original draft, H.X. and C.S.; resources, S.S.; Writing-review & editing, S.S.; supervision, S.S.; project administration, H.C.; funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the China Scholarship Council, grant number 2019-44, and the Fundamental Research Funds for the Central Universities, grant number 3132019330.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AFC | Alkaline Fuel Cell |
| C | Carbon |
| CCB | Catalytic Combustion Burner |
| CCS | Carbon Capture and Storage |
| CH3OH | Methanol |
| CH4 | Methane |
| CnHm | Hydrocarbons |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| DMFC | Direct Methanol Fuel Cell |
| GHG | Greenhouse Gases |
| GT | Gas Turbine |
| H2 | Hydrogen |
| H2O | Water |
| Hex | Heat Exchanger |
| HT-PEMFC | High Temperature Proton Exchange Membrane Fuel Cell |
| HVAC | Heating, Ventilation and Air Conditioning |
| IMO | International Maritime Organization |
| LH2 | Liquefied Hydrogen |
| APU | Auxiliary Power Unit |
| LNG | Liquefied Natural Gas |
| MCFC | Molten Carbonate Fuel Cell |
| N2 | Nitrogen |
| NG | Natural Gas |
| NH3 | Ammonia |
| NOx | Nitrogen Oxides |
| O2 | Oxygen |
| ORC | Organic Rankine Cycle |
| PAFC | Phosphoric Acid Fuel Cell |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| PM | Particulate Matters |
| PV | Photovoltaic |
| S | Sulphur |
| SNG | Synthetic Natural Gas |
| SOFC | Solid Oxide Fuel Cell |
| SOx | Sulphur Oxides |
| ST | Steam Turbine |
| V | Volts |
| WHR | Waste Heat Recovery |
References
- IMO. Third IMO GHG Study 2014; Technical Report; International Maritime Organization: London, UK, 2015. [Google Scholar]
- IMO. Amendments to the Annex of the Protocol of 1997 to Amend the International Convention for the Prevention of Pollution from Ships, 1973, as Modified by the Protocol of 1978 Relating Thereto; Resolution MEPC.203(62); IMO: London, UK, 15 July 2011. [Google Scholar]
- IMO. 2012 Guidelines on the Method of Calculation of the Attained Energy Efficiency Design Index (EEDI) for New Ships; Resolution MEPC.212(63); IMO: London, UK, 2 March 2012. [Google Scholar]
- IMO. 2012 Guidelines for the Development of a Ship Energy Efficiency Management Plan (SEEMP); Resolution MEPC.213(63); IMO: London, UK, 2 March 2012. [Google Scholar]
- IMO. Initial IMO Strategy on Reduction of GHG Emissions from Ships; Resolution MEPC.304(72); IMO: London, UK, 13 April 2018. [Google Scholar]
- Bouman, E.A.; Lindstad, E.; Rialland, A.I.; Strømman, A.H. State-of-the-art technologies, measures, and potential for reducing GHG emissions from shipping—A review. Transport. Res. Part D Transport. Environ. 2017, 52, 408–421. [Google Scholar] [CrossRef]
- Xing, H.; Spence, S.; Chen, H. A comprehensive review on countermeasures for CO2 emissions from ships. Renew. Sustain. Energy Rev. 2020, 134, 110222. [Google Scholar] [CrossRef]
- CE Delft; UMAS. Study on Methods and Considerations for the Determination of Greenhouse Gas Emission Reduction for International Shipping; Final Report Prepared for the European Commission; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- McConnell, V.P. Now, voyager? The increasing marine use of fuel cells. Fuel Cells Bull. 2010, 2010, 12–17. [Google Scholar] [CrossRef]
- Sattler, G. Fuel cells going on-board. J. Power Sources 2000, 86, 61–67. [Google Scholar] [CrossRef]
- Tronstad, T.; Åstrand, H.H.; Haugom, G.P.; Langfeldt, L. Study on the Use of Fuel cells in Shipping; Report to European Maritime Safety Agency by DNV GL; DNV GL: Oslo, Norway, 2017. [Google Scholar]
- Leo, T.J.; Durango, J.A.; Navarro, E. Exergy analysis of PEM fuel cells for marine applications. Energy 2010, 35, 1164–1171. [Google Scholar] [CrossRef]
- Ghenai, C.; Bettayeb, M.; Brdjanin, B.; Hamid, A.K. Hybrid solar PV/PEM fuel cell/diesel generator power system for cruise ship: A case study in Stockholm, Sweden. Case Stud. Therm. Eng. 2019, 14, 100497. [Google Scholar] [CrossRef]
- Alkaner, S.; Zhou, P. A comparative study on life cycle analysis of molten carbon fuel cells and diesel engines for marine application. J. Power Sources 2006, 158, 188–199. [Google Scholar] [CrossRef]
- Roh, G.; Kim, H.; Jeon, H.; Yoon, K. Fuel Consumption and CO2 emission reductions of ships powered by a fuel-cell-based hybrid power source. J. Mar. Sci. Eng. 2019, 7, 230. [Google Scholar] [CrossRef]
- Evrin, R.A.; Dincer, I. Thermodynamic analysis and assessment of an integrated hydrogen fuel cell system for ships. Int. J. Hydrogen Energy 2019, 44, 6919–6928. [Google Scholar] [CrossRef]
- Baldi, F.; Moret, S.; Tammi, K.; Maréchal, F. The role of solid oxide fuel cells in future ship energy systems. Energy 2020, 194, 116811. [Google Scholar] [CrossRef]
- Bassam, A.M.; Phillips, A.B.; Turnock, S.R.; Wilson, P.A. Development of a multi-scheme energy management strategy for a hybrid fuel cell driven passenger ship. Int. J. Hydrogen Energy 2017, 42, 623–635. [Google Scholar] [CrossRef]
- Sasank, B.V.; Rajalakshmi, N.; Dhathathreyan, K.S. Performance analysis of polymer electrolyte membrane (PEM) fuel cell stack operated under marine environmental conditions. J. Mar. Sci. Technol. 2016, 21, 471–478. [Google Scholar] [CrossRef]
- Klebanoff, L.E.; Pratt, J.W.; LaFleur, C.B. Comparison of the safety-related physical and combustion properties of liquid hydrogen and liquid natural gas in the context of the SF-BREEZE high-speed fuel-cell ferry. Int. J. Hydrogen Energy 2017, 42, 757–774. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Yan, X.; Malekian, R.; Li, Z. A study on a numerical simulation of the leakage and diffusion of hydrogen in a fuel cell ship. Renew. Sustain. Energy Rev. 2018, 97, 177–185. [Google Scholar] [CrossRef]
- Aarskog, F.G.; Hansen, O.R.; Strømgren, T.; Ulleberg, Ø. Concept risk assessment of a hydrogen driven high speed passenger ferry. Int. J. Hydrogen Energy 2020, 45, 1359–1372. [Google Scholar] [CrossRef]
- Ahn, J.; Noh, Y.; Joung, T.; Lim, Y.; Kim, J.; Seo, Y.; Chang, D. Safety integrity level (SIL) determination for a maritime fuel cell system as electric propulsion in accordance with IEC 61511. Int. J. Hydrogen Energy 2019, 44, 3185–3194. [Google Scholar] [CrossRef]
- Ahn, J.; Noh, Y.; Park, S.H.; Choi, B.I.; Chang, D. Fuzzy-based failure mode and effect analysis (FMEA) of a hybrid molten carbonate fuel cell (MCFC) and gas turbine system for marine propulsion. J. Power Sources 2017, 364, 226–233. [Google Scholar] [CrossRef]
- Jeon, H.; Park, K.; Kim, J. Comparison and verification of reliability assessment techniques for fuel cell-based hybrid power system for ships. J. Mar. Sci. Eng. 2020, 8, 74. [Google Scholar] [CrossRef]
- Choi, C.H.; Yu, S.; Han, I.-S.; Kho, B.-K.; Kang, D.-G.; Lee, H.Y.; Seo, M.-S.; Kong, J.-W.; Kim, G.; Ahn, J.-W.; et al. Development and demonstration of PEM fuel-cell-battery hybrid system for propulsion of tourist boat. Int. J. Hydrogen Energy 2016, 41, 3591–3599. [Google Scholar] [CrossRef]
- Jeong, J.; Seo, S.; You, H.; Chang, D. Comparative analysis of a hybrid propulsion using LNG-LH2 complying with regulations on emissions. Int. J. Hydrogen Energy 2018, 43, 3809–3821. [Google Scholar] [CrossRef]
- Bensaid, S.; Specchia, S.; Federici, F.; Saracco, G.; Specchia, V. MCFC-based marine APU: Comparison between conventional ATR and cracking coupled with SR investigated inside the stack pressurized vessel. Int. J. Hydrogen Energy 2009, 34, 2026–2042. [Google Scholar] [CrossRef]
- Ahn, J.; Park, S.H.; Lee, S.; Noh, Y.; Chang, D. Molten carbonate fuel cell (MCFC)-based hybrid propulsion systems for a liquefied hydrogen tanker. Int. J. Hydrogen Energy 2018, 43, 7525–7537. [Google Scholar] [CrossRef]
- Tse, L.K.C.; Wilkins, S.; McGlashan, N.; Urban, B.; Martinez-Botas, R. Solid oxide fuel cell/gas turbine trigeneration system for marine applications. J. Power Sources 2011, 196, 3149–3162. [Google Scholar] [CrossRef]
- Díaz-de-Baldasano, M.C.; Mateos, F.J.; Núñez-Rivas, L.R.; Leo, T.J. Conceptual design of offshore platform supply vessel based on hybrid diesel generator-fuel cell power plant. Appl. Energy 2014, 116, 91–100. [Google Scholar] [CrossRef]
- Ahn, J.; Park, S.H.; Noh, Y.; Choi, B.I.; Ryu, J.; Chang, D.; Brendstrup, K.L.M. Performance and availability of a marine generator-solid oxide fuel cell-gas turbine hybrid system in a very large ethane carrier. J. Power Sources 2018, 399, 199–206. [Google Scholar] [CrossRef]
- De-Troya, J.J.; Alvarez, C.; Fernandez-Garrido, C.; Carral, L. Analysing the possibilities of using fuel cells in ships. Int. J. Hydrogen Energy 2016, 41, 2853–2866. [Google Scholar] [CrossRef]
- Van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef]
- Dai, W.; Wang, H.; Yuan, X.Z.; Martin, J.J.; Yang, D.; Qiao, J.; Ma, J. A review on water balance in the membrane electrode assembly of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 9461–9478. [Google Scholar] [CrossRef]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Ahmed, M.; Dincer, I. A review on methanol crossover in direct methanol fuel cells: Challenges and achievements. Int. J. Energy Res. 2011, 35, 1213–1228. [Google Scholar] [CrossRef]
- McPhail, S.J.; Aarva, A.; Devianto, H.; Bove, R.; Moreno, A. SOFC and MCFC: Commonalities and opportunities for integrated research. Int. J. Hydrogen Energy 2011, 36, 10337–10345. [Google Scholar] [CrossRef]
- Kulkarni, A.; Giddey, S. Materials issues and recent developments in molten carbonate fuel cells. J. Solid State Electrochem. 2012, 16, 3123–3146. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Ammonia-fed solid oxide fuel cells for power generation—A review. Int. J. Energy Res. 2009, 33, 943–959. [Google Scholar] [CrossRef]
- Schinas, O.; Stefanakos, C.N. Selecting technologies towards compliance with MARPOL Annex VI: The perspective of operators. Transport. Res. Part D Transport. Environ. 2014, 28, 28–40. [Google Scholar] [CrossRef]
- Inal, O.B.; Deniz, C. Assessment of fuel cell types for ships: Based on multi-criteria decision analysis. J. Clean. Prod. 2020, 265, 121734. [Google Scholar] [CrossRef]
- Office of Energy Efficiency & Renewable Energy. Fuel Cells. Available online: https://www.energy.gov/eere/fuelcells/fuel-cells (accessed on 24 June 2020).
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Chaubey, R.; Sahu, S.; James, O.O.; Maity, S. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 2013, 23, 443–462. [Google Scholar] [CrossRef]
- Office of Energy Efficiency & Renewable Energy. Hydrogen Production: Electrolysis. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis (accessed on 24 June 2020).
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)-Assessment based on chemical and economic properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Lan, R.; Christophe, T.G.P.; Tao, S. Solid-state electrochemical synthesis of ammonia: A review. J. Solid State Electrochem. 2011, 15, 1845–1860. [Google Scholar]
- Cinti, G.; Discepoli, G.; Sisani, E.; Desideri, U. SOFC operating with ammonia: Stack test and system analysis. Int. J. Hydrogen Energy 2016, 41, 13583–13590. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; Koch, A.M.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Svanberg, M.; Ellis, J.; Lundgren, J.; Landälv, I. Renewable methanol as a fuel for the shipping industry. Renew. Sustain. Energy Rev. 2018, 94, 1217–1228. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Fuel Cell Technologies Office of U.S. Department of Energy. Hydrogen Fuel Quality Specifications for Polymer Electrolyte Fuel Cells in Road Vehicles; Report to the Safety, Codes and Standards Program; U.S. Department of Energy: Washington, DC, USA, 2016.
- Peters, R.; Dahl, R.; Klüttgen, U.; Palm, C.; Stolten, D. Internal reforming of methane in solid oxide fuel cell systems. J. Power Sources 2002, 106, 238–244. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Han, Y.; Li, M.; Chen, W. A state machine strategy based on droop control for an energy management system of PEMFC-battery-supercapacitor hybrid tramway. Int. J. Hydrogen Energy 2016, 41, 16148–16159. [Google Scholar] [CrossRef]
- Frangopoulos, C.A.; Nakos, L.G. Development of a model for thermoeconomic design and operation optimization of a PEM fuel cell system. Energy 2006, 31, 1501–1519. [Google Scholar] [CrossRef]
- Arsalis, A. Modeling and simulation of a 100 kWe HT-PEMFC subsystem integrated with an absorption chiller subsystem. Int. J. Hydrogen Energy 2012, 37, 13484–13490. [Google Scholar] [CrossRef]
- Bischoff, M. Molten carbonate fuel cells: A high temperature fuel cell on the edge to commercialization. J. Power Sources 2006, 160, 842–845. [Google Scholar] [CrossRef]
- Wee, J.-H. Molten carbonate fuel cell and gas turbine hybrid systems as distributed energy resources. Appl. Energy 2011, 88, 4252–4263. [Google Scholar] [CrossRef]
- Ovrum, E.; Dimopoulos, G. A validated dynamic model of the first marine molten carbonate fuel cell. Appl. Therm. Eng. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Dimopoulos, G.G.; Stefanatos, I.C.; Kakalis, N.M.P. Exergy analysis and optimisation of a marine molten carbonate fuel cell system in simple and combined cycle configuration. Energy Convers. Manag. 2016, 107, 10–21. [Google Scholar] [CrossRef]
- Lunghi, P.; Bove, R.; Desideri, U. Analysis and optimization of hybrid MCFC gas turbines plants. J. Power Sources 2003, 118, 108–117. [Google Scholar] [CrossRef]
- Oh, K.S.; Kim, T.S. Performance analysis on various system layouts for the combination of an ambient pressure molten carbonate fuel cell and a gas turbine. J. Power Sources 2006, 158, 455–463. [Google Scholar] [CrossRef]
- Grillo, O.; Magistri, L.; Massardo, A.F. Hybrid systems for distributed power generation based on pressurisation and heat recovering of an existing 100 kW molten carbonate fuel cell. J. Power Sources 2003, 115, 252–267. [Google Scholar] [CrossRef]
- Liu, A.; Weng, Y. Performance analysis of a pressurized molten carbonate fuel cell/micro-gas turbine hybrid system. J. Power Sources 2010, 195, 204–213. [Google Scholar] [CrossRef]
- Yoshiba, F. Kawagoe 300 kW class MCFC/TCG compact system: Thermal efficiency and endurance test results. J. Fuel Cell Sci. Technol. 2008, 5, 021010. [Google Scholar] [CrossRef]
- Sciacovelli, A.; Verda, V. Sensitivity analysis applied to the multi-objective optimization of a MCFC hybrid plant. Energy Convers. Manag. 2012, 60, 180–187. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Buonomano, A.; Calise, F.; d’Accadia, M.D.; Palombo, A.; Vicidomini, M. Hybrid solid oxide fuel cells-gas turbine systems for combined heat and power: A review. Appl. Energy 2015, 156, 32–85. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, S.H.; Li, G.; Ho, H.K.; Li, J.; Feng, Z. A review of integration strategies for solid oxide fuel cells. J. Power Sources 2010, 195, 685–702. [Google Scholar] [CrossRef]
- Arsalis, A. Thermoeconomic modeling and parametric study of hybrid SOFC-gas turbine-steam turbine power plants ranging from 1.5 to 10 MWe. J. Power Sources 2008, 181, 313–326. [Google Scholar] [CrossRef]
- Lee, Y.D.; Ahn, K.Y.; Morosuk, T.; Tsatsaronis, G. Exergetic and exergoeconomic evaluation of a solid-oxide fuel-cell-based combined heat and power generation system. Energy Convers. Manag. 2014, 85, 154–164. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.D.; Ahn, K.Y. Performance analysis of an SOFC/HCCI engine hybrid system: System simulation and thermo-economic comparison. Int. J. Hydrogen Energy 2014, 39, 1799–1810. [Google Scholar] [CrossRef]
- Masoud, R. Thermodynamic analysis of an integrated solid oxide fuel cell cycle with a Rankine cycle. Energy Convers. Manag. 2010, 51, 2724–2732. [Google Scholar]
- Pierobon, L.; Rokni, M.; Larsen, U.; Haglind, F. Thermodynamic analysis of an integrated gasification solid oxide fuel cell plant combined with an organic Rankine cycle. Renew. Energy 2013, 60, 226–234. [Google Scholar] [CrossRef]
- Ozcan, H.; Dincer, I. Thermodynamic analysis of an integrated SOFC, solar ORC and absorption chiller for tri-generation applications. Fuel Cells 2013, 13, 781–793. [Google Scholar] [CrossRef]
- Tuo, H. Energy and exergy-based working fluid selection for organic Rankine cycle recovering waste heat from high temperature solid oxide fuel cell and gas turbine hybrid systems. Int. J. Energy Res. 2013, 37, 1831–1841. [Google Scholar] [CrossRef]
- Al-Sulaiman, F.A.; Dincer, I.; Hamdullahpur, F. Thermoeconomic optimization of three trigeneration systems using organic Rankine cycles: Part I—Formulations. Energy Convers. Manag. 2013, 69, 199–208. [Google Scholar] [CrossRef]
- Roberts, R.; Brouwer, J.; Jabbari, F.; Junker, T.; Ghezel-Ayagh, H. Control design of an atmospheric solid oxide fuel cell/gas turbine hybrid system: Variable versus fixed speed gas turbine operation. J. Power Sources 2006, 161, 484–491. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, T.S. Comparison between pressurized design and ambient pressure design of hybrid solid oxide fuel cell gas turbine systems. J. Power Sources 2006, 163, 490–499. [Google Scholar] [CrossRef]
- Obara, S. Dynamic-characteristics analysis of an independent microgrid consisting of a SOFC triple combined cycle power generation system and large-scale photovoltaics. Appl. Energy 2015, 141, 19–31. [Google Scholar] [CrossRef]
- Meratizaman, M.; Monadizadeh, S.; Amidpour, M. Techno-economic assessment of high efficient energy production (SOFC-GT) for residential application from natural gas. J. Nat. Gas Sci. Eng. 2014, 21, 118–133. [Google Scholar] [CrossRef]
- Haseli, Y.; Dincer, I.; Naterer, G.F. Thermodynamic analysis of a combined gas turbine power system with a solid oxide fuel cell through exergy. Thermochim. Acta 2008, 480, 1–9. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Borgogna, G.; Speranza, E.; Lamberti, T.; Traverso, A.N.; Magistri, L.; Gadducci, E.; Massardo, A.F.; Olivieri, P. Design and development of a laboratory for the study of PEMFC system for marine applications. E3S Web Conf. 2019, 113, 02020. [Google Scholar] [CrossRef]
- Markowski, J.; Pielecha, I. The potential of fuel cells as a drive source of maritime transport. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 012019. [Google Scholar] [CrossRef]
- MTU Friedrichshafen GmbH. First yacht with certified fuel cell propulsion. Fuel Cells Bull. 2003, 2003, 4–5. [Google Scholar] [CrossRef]
- Green Car Congress. Hydrogen Hybrid Canal Boat. 24 September 2007. Available online: https://www.greencarcongress.com/2007/09/hydrogen-hybrid.html (accessed on 24 June 2020).
- Hydrogenics Corporation. Turkish fuel cell boat powered, refuelled by Hydrogenics tech. Fuel Cells Bull. 2012, 2012, 2–3. [Google Scholar]
- Yazici, M.S.; Hatipoglu, M. Hydrogen and fuel cell demonstration in Turkey. Energy Procedia 2012, 29, 683–689. [Google Scholar] [CrossRef]
- Zemships. One Hundred Passengers and Zero Emissions: The First Ever Passenger Vessel to Sail Propelled by Fuel Cells. Available online: https://ec.europa.eu/environment/life/project/Projects/index.cfm?fuseaction=home.showFile&rep=file&fil=Zemships_Brochure_EN.pdf (accessed on 24 June 2020).
- Chakraborty, S.; Dzielendziak, A.S.; Köroğlu, T.; Yang, K. Evaluation of Smart Eco-Friendly Public Transport Options in Coastal Cities: Towards a Green Future for the City of Southampton; LRF Collegium 2013 Series; University of Southampton: Southampton, UK, 2013; Volume 2. [Google Scholar]
- Pratt, J.W.; Klebanoff, L.E. Feasibility of the SF-BREEZE: A Zero-Emission, Hydrogen Fuel Cell, High-Speed Passenger Ferry; SANDIA Report; SAND2016-9719; Sandia National Laboratories: Livermore, CA, USA, 2016.
- German e4ships project reports on fuel cell maritime demos. Fuel Cells Bull. 2016, 2016, 5, 11. [CrossRef]
- E4ships. Fuel Cells in Marine Applications (2009–2016). December 2016. Available online: https://www.e4ships.de/english/news/ (accessed on 24 June 2020).
- Ahn, J.; You, H.; Ryu, J.; Chang, D. Strategy for selecting an optimal propulsion system of a liquefied hydrogen tanker. Int. J. Hydrogen Energy 2017, 42, 5366–5380. [Google Scholar] [CrossRef]
- METHAPU prototypes methanol SOFC for ships. Fuel Cells Bull. 2008, 2008, 4–5.
- Green Car Congress. Project to Start Trials on Ship-Board Methanol SOFC APU. 18 March 2008. Available online: https://www.greencarcongress.com/2008/03/project-to-star.html (accessed on 24 June 2020).
- Strazza, C.; Borghi, A.D.; Costamagna, P.; Traverso, A.; Santin, M. Comparative LCA of methanol-fuelled SOFCs as auxiliary power systems on-board ships. Appl. Energy 2010, 87, 1670–1678. [Google Scholar] [CrossRef]
- Ellamla, H.R.; Staffell, I.; Bujlo, P.; Pollet, B.G.; Pasupathi, S. Current status of fuel cell based combined heat and power systems for residential sector. J. Power Sources 2015, 293, 312–328. [Google Scholar] [CrossRef]
- De Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and degradation issues of PEM fuel cell components. Fuel Cells 2008, 1, 3–22. [Google Scholar] [CrossRef]
- Hawkes, A.D.; Brett, D.J.L.; Brandon, N.P. Fuel cell micro-CHP techno-economics: Part 2-Model application to consider the economic and environmental impact of stack degradation. Int. J. Hydrogen Energy 2009, 34, 9558–9569. [Google Scholar] [CrossRef]
- Shabani, B.; Hafttananian, M.; Khamani, S.; Ramiar, A.; Ranjbar, A.A. Poisoning of proton exchange membrane fuel cells by contaminants and impurities: Review of mechanisms, effects, and mitigation strategies. J. Power Sources 2019, 427, 21–48. [Google Scholar] [CrossRef]
- Khatib, F.N.; Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; El-Hassan, Z.; Durrant, A.; Thompson, J.; Olabi, A.G. Material degradation of components in polymer electrolyte membrane (PEM) electrolytic cell and mitigation mechanisms: A review. Renew. Sustain. Energy Rev. 2019, 111, 1–14. [Google Scholar] [CrossRef]
- Yokokawa, H.; Tu, H.; Iwanschitz, B.; Mai, A. Fundamental mechanisms limiting solid oxide fuel cell durability. J. Power Sources 2008, 182, 400–412. [Google Scholar] [CrossRef]
- Maru, H.; Singhal, S.C.; Stone, C.; Wheeler, D. 1–10 kW Stationary Combined Heat and Power Systems Status and Technical Potential; Independent Review Report for U.S. Department of Energy Hydrogen and Fuel Cells Program; The National Renewable Energy Laboratory: Washington, DC, USA, 2010.
- Schinas, O. Financing ships of innovative technology. In Finance and Risk Management for International Logistics and the Supply Chain; Gong, S., Cullinane, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 8; pp. 167–192. [Google Scholar] [CrossRef]
- Scataglini, R.; Mayyas, A.; Wei, M.; Chan, S.H.; Lipman, T.; Gosselin, D.; D’Alessio, A.; Breunig, H.; Colella, W.G.; James, B.D. A Total Cost of Ownership Model for Solid Oxide Fuel Cells in Combined Heat and Power and Power-Only Applications; A Report by Lawrence Berkeley National Laboratory under Contract No. DE-AC02-05CH11231; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2015.
- Thijssen, J. The impact of scale-up and production volume on SOFC stack cost. Presented at the 7th Annual SECA Workshop and Peer Review, Philadelphia, PA, USA, 12–14 September 2006. [Google Scholar]
- Staffell, I. Fuel Cells for Domestic Heat and Power: Are They Worth It? Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2009. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).