Manipulating Phosphorus, Calcium, and Magnesium Utilization by Growing Lambs Using Natural Zeolite (Clinoptilolite)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Digestibility Trials and Slaughtering
2.3. Mineral Analysis:
2.3.1. Operational Parameters of the Microwave Digestion:
2.3.2. Procedure:
2.4. Statistical Analysis:
3. Results and Discussion
3.1. Performance and Nutrient Digestibility
3.2. Intestinal Metabolism of Calcium, Magnesium and Phosphorus
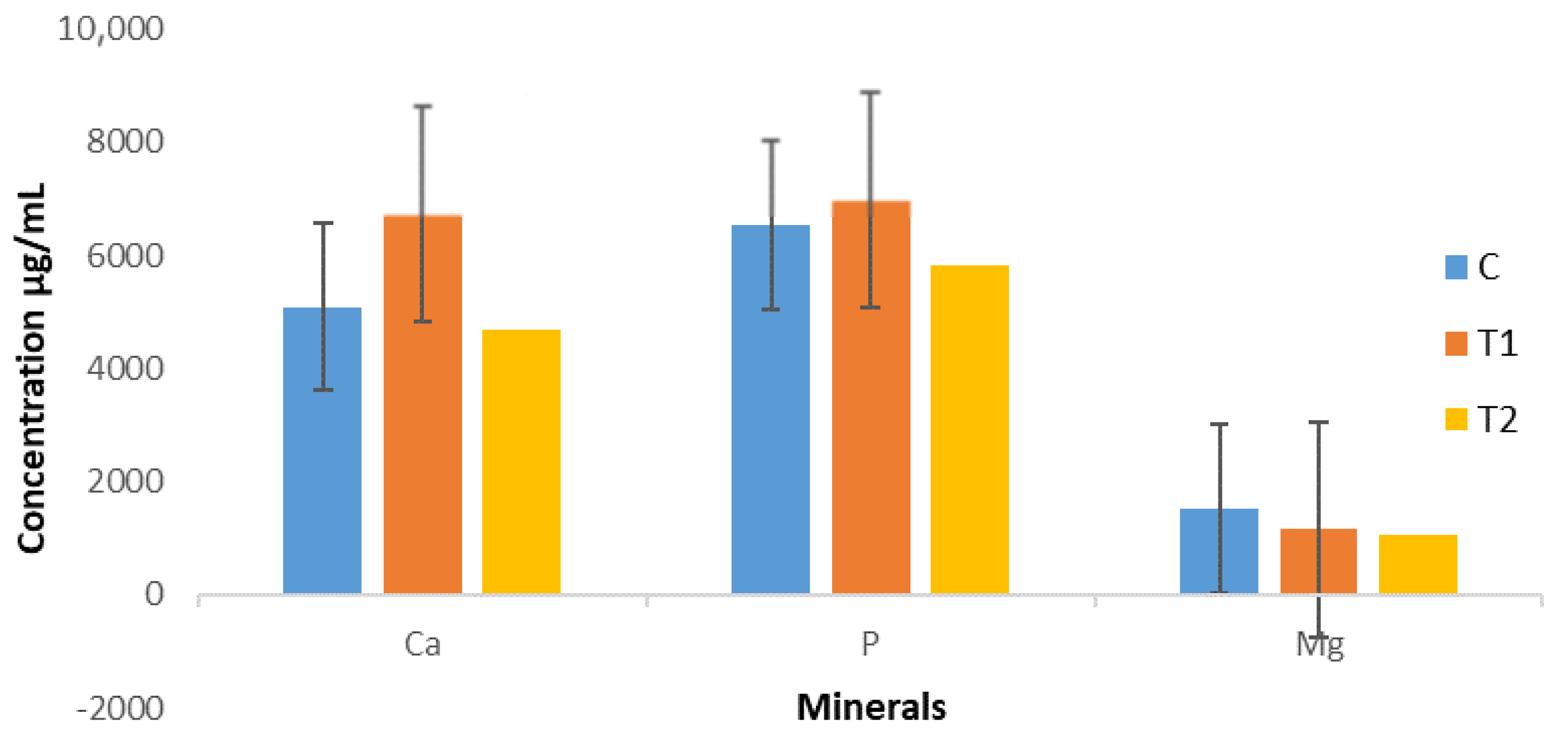
3.3. Ruminal Metabolism of Calcium, Phosphorus, and Magnesium
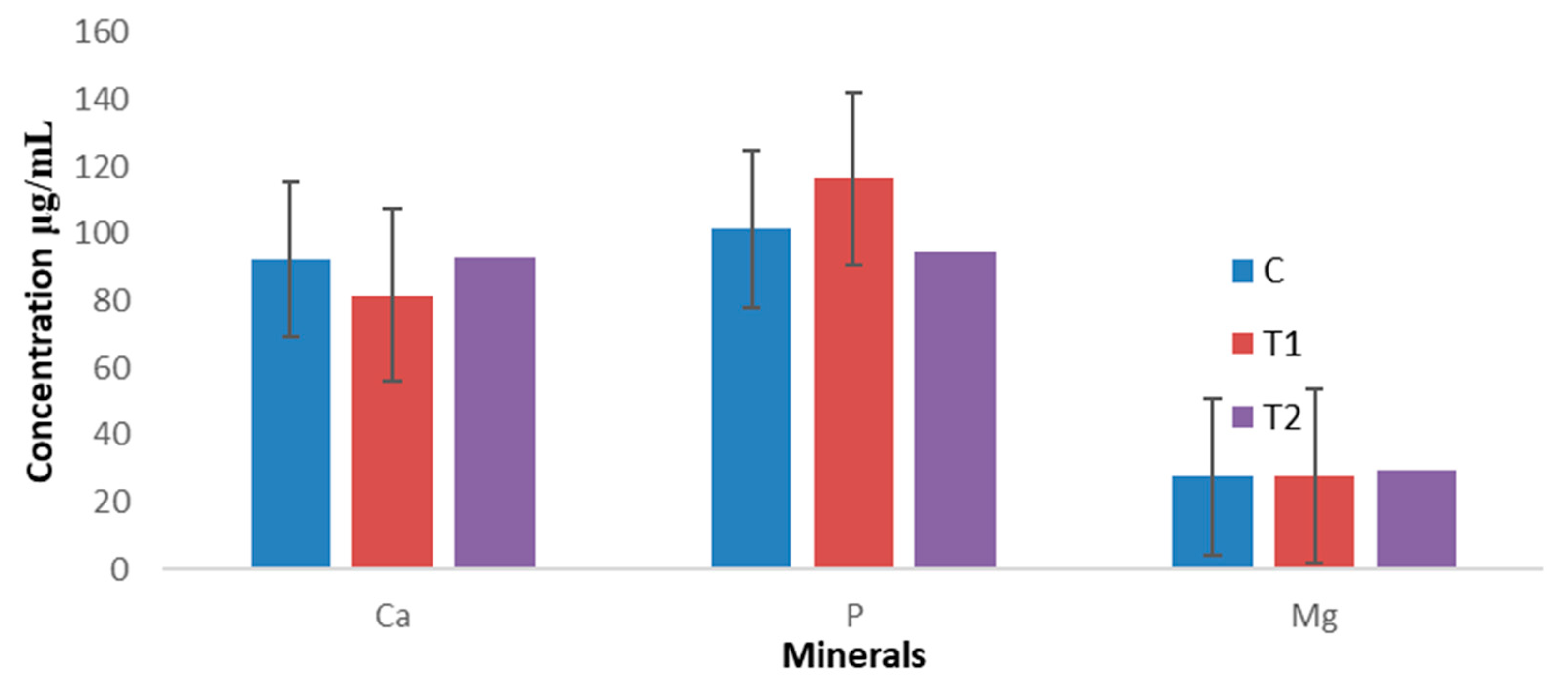
3.4. Mineral Concentration in Serum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, C.L. The use of buffers in the rations of lactating dairy cows. In Regulation of Acid-Base Balance; Hale, W.H., Meinhardt, P., Eds.; Church and Dwight Co. Inc.: Piscataway, NJ, USA, 1979. [Google Scholar]
- Muller, L.D.; Kilmer, L.H. Sodium Bicarbonate in Dairy Nutrition; National Feed Ingredients Association: West Des Moines, IA, USA, 1979; pp. 34–64. [Google Scholar]
- Snyder, T.J.; Rogers, J.A.; Muller, L.D. Effects of 1.2% sodium bicarbonate with two ratios of corn silage:grain on milk production, rumen fermentation and nutrient digestion by lactating cows. J. Dairy Sci. 1983, 66, 1290–1297. [Google Scholar] [CrossRef]
- Hu, W.; Murphy, M.R. Statistical evaluation of early- and mid-lactation dairy cow responses to dietary sodium bicarbonate addition. Anim. Feed. Sci. Technol. 2005, 119, 43–54. [Google Scholar] [CrossRef]
- Iwaniuk, M.E.; Erdman, R.A. Intake, milk production, ruminal, and feed efficiency responses to dietary cation-anion difference by lactating dairy cows. J. Dairy Sci. 2015, 98, 8973–8985. [Google Scholar] [CrossRef] [PubMed]
- Bosi, P.; Creston, D.; Casini, L. Production performance of dairy cows after the dietary addition of clinoptilolite. Ital. J. Anim. Sci. 2002, 1, 187–195. [Google Scholar] [CrossRef]
- Fillippidis, F.A.; Godelitsas, A.; Charistos, D.; Misaelides, P.; Kassoli-Fournaraki, A. The chemical behavior of natural zeolites in aqueous environments: Interactions between low-silica zeolites and 1 M NaCl solutions of different initial pH-values. Appl. Clay Sci. 1996, 11, 199–209. [Google Scholar] [CrossRef]
- European Commission Regulation. European Commission Regulation No. 1810/2005 of 4 November 2005 concerning a new authorization for 10 years of an additive in feeding stuffs, the permanent authorization of certain additives in feeding stuffs and the provisional authorization of new uses of certain additives already authorized in feeding stuffs. Off. J. Eur. Union 2005, 291, 1–7. [Google Scholar]
- Thilsing-Hansen, T.; Jørgensen, R.J.; Enemark, J.M.; Larsen, T. The effect of zeolite A supplementation in the dry period on periparturient calcium, phosphorus, and magnesium homeostasis. J. Dairy Sci. 2002, 85, 1855–1862. [Google Scholar] [CrossRef]
- Thilsing-Hansen, T.; Jørgensen, R.J.; Enemark, J.M.; Zelvyte, R.; Sederevicius, A. The effect of zeolite A supplementation in the dry period on blood mineral status around calving. Acta Vet. Scand. 2003, 97, 87–95. [Google Scholar]
- Pallesen, A.; Pallesen, F.; Jørgensen, R.J.; Thilsing, T. Effect of pre-calving zeolite, magnesium and phosphorus supplementation on periparturient serum mineral concentrations. Vet. J. 2008, 175, 234–239. [Google Scholar] [CrossRef]
- Grabherr, H.; Spolders, M.; Lebzien, P.; Hüther, L.; Flachowsky, G.; Fürll, M.; Grün, M. Effect of zeolite A on rumen fermentation and phosphorus metabolism in dairy cows. Arch. Anim. Nutr. 2009, 63, 321–336. [Google Scholar] [CrossRef]
- Grabherr, H.; Spolders, M.; Flachowsky, G.; Fürll, M. Einfluss von Zeolith A auf die Futteraufnahme von trockenstehenden Milchkühen, auf den Mengen- und Spurenelementstoffwechsel im peripartalen Zeitraum sowie auf die Milchleistung in der folgenden Laktation. Berl. Münch. Tierärztl. Wochenschr. 2008, 12, 41–52. [Google Scholar]
- Thilsing-Hansen, T.; Jørgensen, R.J. Hot topic: Prevention of parturient paresis and subclinical hypocalcemia in dairy cows by zeolite A administration in the dry period. J. Dairy Sci. 2001, 84, 691–693. [Google Scholar] [CrossRef]
- Cook, T.E.; Cilley, W.A.; Savitsky, A.C.; Wiers, B.H. Zeolite A hydrolysis and degradation. Environ. Sci. Technol. 1982, 16, 344–350. [Google Scholar] [CrossRef]
- Allen, V.G. Influence of dietary aluminum on nutrient utilization in ruminants. J. Anim. Sci. 1984, 59, 836–844. [Google Scholar] [CrossRef]
- Schwaller, D.; Wilkens, M.R.; Liesegang, A. Zeolite A effect on calcium homeostasis in growing goats. J. Anim. Sci. 2016, 94, 1576–1586. [Google Scholar] [CrossRef][Green Version]
- NRC (National Research Council). Nutrient Requirements of Small Ruminants: Sheep, Goats Cervids and New World Camelids; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- SAS Institute. SAS User’s Guide. Statistics, 8th ed.; SAS Institute Inc.: Cary, NC, USA, 2003. [Google Scholar]
- Ochodnicky, D.; Huncik, M.; Bajdal, K. Effect of Zeolite Supplement with Lamb Fattening; FAO: Rome, Italy, 1986. [Google Scholar]
- McCollum, F.T.; Galyean, M.L. Effects of clinoptilolite on rumen fermentation, digestion and feedlot performance in beef steers fed high concentrate diets. J. Anim. Sci. 1983, 56, 517–524. [Google Scholar] [CrossRef]
- Sanders, K.J.; Richardson, C.R.; Harper, S. Effects of Zeolites on Performance of Feedlot Cattle; Texas Technical University, Animal Science: Lubbock, TX, USA, 1997; Available online: http://www.zeolite-products.com/ktml2/files/uploads/Effects%20of%20zeolites%20on%20performance%20of%20feedlot%20cattle.pdf (accessed on 1 February 2021).
- Koknaroglu, H.; Toker, M.T.; Bozkurt, Y. Effect of zeolite and initial weight on feedlot performance of Brown Swiss cattle. Asian. J. Anim. Vet. Adv. 2006, 1, 49–54. [Google Scholar] [CrossRef][Green Version]
- Câmara, L.R.A.; Valadares Filho, S.C.; Leão, M.I.; Valadares, R.F.D.; Dias, M.; Gomide, A.P.C.; Barros, A.C.W.; Nascimento, V.A.; Ferreira, D.J.; Faé, J.T.; et al. Zeólita na dieta de bovinos de corte. Arq. Bras. Med. Vet. Zootec. 2012, 64, 631–639. [Google Scholar] [CrossRef][Green Version]
- Stojkovic., J.; Ilic, Z.; Ciric, S.; Ristanovic, B.; Petrovic, M.P.; Caro-Petrovic, V.; Kurcubic, V. Efficiency of zeolite basis preparation in fattening lambs diet. Biotech. Anim. Husb. 2012, 28, 545–552. [Google Scholar] [CrossRef]
- Nestorov, N. Possible applications of natural zeolites in animal husbandry. In Zeo agriculture: Use of Natural Zeolite in Agriculture and Aquaculture; International Committee on Natural Zeolites: Boulder, CO, USA, 1984; pp. 167–174. [Google Scholar]
- Nowar, M.S.; Al-Shawabkeh, K.; Khoury, H.N. Effect of feeding farm animals with Jordanian clay deposits containing montmorillonite: 1. Effect on fattening lambs performance, with special reference to blood hematology, liver and kidney functions, and parasitological and serological examinations. Zagaz. Agric. Res. 1993, 20, 651–667. [Google Scholar]
- Toprak, N.N.; Yılmaz, A.; Öztürk, E.; Yigit, O.; Cedden, F. Effect of micronized zeolite addition to lamb concentrate feeds on growth performance and some blood chemistry and metabolites. S. Afr. J. Anim. Sci. 2016, 46, 313–320. [Google Scholar]
- Enemark, J.M.; Frandsen, A.M.; Thilsing-Hansen, T.; Jørgensen, R.J. Aspects of physiological effects of sodium zeolite, A supplementation in dry, non-pregnant dairy cows fed grass silage. Acta Vet. Scand. Suppl. 2003, 97, 97–117. [Google Scholar] [PubMed]
- Kraljević Pavelić, S.; Simović Medica, J.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in vivo. Front. Pharmacol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Wheeler, D.C.; Persky, M.S.; Kestenbaum, B.; Ketteler, M.; Spiegel, D.M.; Allison, M.A.; Asplin, J.; Smits, G.; Hoofnagle, A.N.; et al. Effects of phosphate binders in moderate CKD. J. Am. Soc. Nephrol. 2012, 23, 1407–1415. [Google Scholar] [CrossRef]
- Maunder, E.M.; Pillay, A.V.; Care, A.D. Hypophosphataemia and vitamin D metabolism in sheep. Q. J. Exp. Physiol. 1986, 71, 391–399. [Google Scholar] [CrossRef]
- Scott, D.; Loveridge, N.; Nicodemo, L.; Buchan, W.; Milne, J.; Duncan, A.; Nicol, P.; Robins, S.P. Effect of diets varying in nitrogen or phosphorus content on indicators of bone growth in lambs. Exp. Physiol. 1997, 82, 193–202. [Google Scholar] [CrossRef]
- Schröder, B.; Käppner, H.; Failing, K.; Pfeffer, E.; Breves, G. Mechanisms of intestinal phosphate transport in small ruminants. Br. J. Nutr. 1985, 74, 635–648. [Google Scholar] [CrossRef]
- Kichura, T.S.; Horst, R.L.; Beitz, D.C.; Littledike, E.T. Relationships between prepartal dietary calcium and phosphorus, vitamin D metabolism, and parturient paresis in dairy cows. J. Nutr. 1982, 112, 480–487. [Google Scholar] [CrossRef]
- Barton, B.A.; Jorgensen, N.A.; DeLuca, H.F. Impact of prepartum dietary phosphorus intake on calcium homeostasis at parturition. J. Dairy Sci. 1987, 70, 1186–1191. [Google Scholar] [CrossRef]
| Parameters | C | T1 | T2 | SEM | p Value |
|---|---|---|---|---|---|
| BW, kg | 10.72 a | 11.91 a | 13.42 b | 1.2 | <0.017 |
| FCR | 4.12 b | 3.65 a | 3.45 a | 0.43 | <0.05 |
| Digestibility Coefficients | |||||
| DFI, g | 1582.5 | 1717.5 | 1722 | 47 | 0.35 |
| Fecal, g | 872.5 | 775.5 | 782.1 | 113 | 0.81 |
| Ca digestibility, % | 79.31 | 82.08 | 80.30 | 4.12 | 0.68 |
| P digestibility, % | 86.60 a | 91.23 b | 90.9 b | 3.87 | 0.04 |
| Mg digestibility, % | 88.33 | 90.61 | 90.11 | 2.54 | 0.45 |
| Tissue | Mineral | C | T1 | T2 | p Value | SEM |
|---|---|---|---|---|---|---|
| Liver | Ca | 79.53 | 93.51 | 82.13 | 0.65 | ±5.87 |
| P | 79.53 | 93.51 | 82.13 | 0.12 | ±5.87 | |
| Mg | 222.48 | 229.36 | 228.43 | 0.78 | ±3.86 | |
| Kidney | Ca | 168.97 b | 120.13 a | 123.13 ab | 0.37 | ±9.63 |
| P | 3006 | 3026.79 | 3377.39 | 0.31 | ±105.65 | |
| Mg | 216 | 216.55 | 268.17 | 0.25 | ±14.16 | |
| Muscle | Ca | 56.72 b | 45.66a | 48.49 a | 0.09 | ±2.25 |
| P | 2083 | 3123.8 | 2089 | 0.63 | ±16.83 | |
| Mg | 271.86 | 276.68 | 271.26 | 0.80 | ±3.25 |
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LivCaC | 1 | ||||||||||||||
| 2 | LivCaT1 | 0.36 | 1 | |||||||||||||
| 3 | LivCaT2 | −0.60 | 0.53 | 1 | ||||||||||||
| 4 | KiCaC | 0.75 | −0.34 | −0.98 | 1 | |||||||||||
| 5 | KiCaT1 | −0.45 | 0.67 | 0.98 | 0.93 | 1 | ||||||||||
| 6 | KiCaT2 | 0.59 | −0.54 | −0.99 * | 0.97 | −0.98 | 1 | |||||||||
| 7 | RuCaC | 0.98 | 0.56 | −0.41 | 0.58 | −0.24 | 0.40 | 1 | ||||||||
| 8 | RuCaT1 | −0.62 | −0.96 | −0.25 | 0.05 | −0.41 | 0.27 | −0.78 | 1 | |||||||
| 9 | RuCaT2 | 0.77 | 0.87 | 0.05 | 0.15 | 0.22 | −0.06 | 0.89 | −0.98 | 1 | ||||||
| 10 | MeCaC | 0.32 | 0.99 * | 0.56 | −0.38 | 0.70 | −0.58 | 0.52 | −0.94 | 0.85 | 1 | |||||
| 11 | MeCaT1 | 0.25 | 0.99 * | 0.63 | −0.45 | 0.75 | −0.64 | 0.45 | −0.91 | 0.81 | 0.99 * | 1 | ||||
| 12 | MeCaT2 | −0.79 | 0.28 | 0.96 | −0.99 | 0.90 | −0.96 | −0.64 | 0.02 | −0.22 | 0.32 | 0.40 | 1 | |||
| 13 | SeCaC | −0.98 | −0.16 | 0.76 | −0.87 | 0.62 | −0.75 | −0.91 | 0.44 | −0.62 | −0.11 | −0.03 | 0.90 | 1 | ||
| 14 | SeCaT1 | −0.79 | 0.28 | 0.96 | −0.99 | 0.90 | −0.96 | −0.64 | 0.01 | −0.22 | 0.32 | 0.40 | 0.99 * | 0.90 | 1 | |
| 15 | SeCaT2 | −0.99 | −0.47 | 0.51 | −0.67 | 0.34 | −0.49 | −0.99 * | 0.71 | −0.84 | −0.43 | −0.35 | 0.72 | 0.95 | 0.72 | 1 |
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| 1 | LivPC | 1 | ||||||||||||||
| 2 | LivPT1 | 0.72 | 1 | |||||||||||||
| 3 | LivPT2 | 0.99 * | 0.81 | 1 | ||||||||||||
| 4 | KiPC | −0.66 | −0.99 | −0.75 | 1 | |||||||||||
| 5 | KiPT1 | 0.50 | −0.24 | 0.38 | 0.32 | 1 | ||||||||||
| 6 | KiPT2 | 0.99 * | 0.77 | 0.99 * | 0.72 | 0.43 | 1 | |||||||||
| 7 | RuPC | −0.96 | −0.88 | −0.98 * | 0.84 | 0.24 | −0.98 | 1 | ||||||||
| 8 | RuPT1 | −0.40 | 0.35 | −0.27 | −0.43 | −0.99 * | −0.32 | 0.13 | 1 | |||||||
| 9 | RuPT2 | −0.99 * | −0.79 | −0.99 * | −0.72 | −0.41 | −0.99 * | 0.98 | 0.30 | 1 | ||||||
| 10 | MePC | 0.48 | 0.95 | 0.59 | −0.98 | −0.52 | 0.55 | −0.70 | 0.62 | −0.56 | 1 | |||||
| 11 | MePT1 | −0.26 | 0.48 | −0.13 | −0.56 | −0.97 | −0.18 | −0.02 | 0.98 | 0.16 | 0.73 | 1 | ||||
| 12 | MePT2 | −0.99 * | −0.77 | −0.99 * | −0.72 | −0.43 | −1 ** | 0.98 | 0.32 | 0.99 * | −0.55 | 0.18 | 1 | |||
| 13 | SePC | 0.99 | 0.60 | 0.96 | −0.53 | 0.64 | 0.97 | −0.90 | −0.54 | −0.97 | 0.33 | −0.41 | −0.97 | 1 | ||
| 14 | SePT1 | 0.74 | 0.07 | 0.65 | 0.01 | 0.95 | 0.69 | −0.53 | −0.91 | −0.67 | −0.23 | −0.84 | −0.69 | 0.41 | 1 | |
| 15 | SePT2 | 0.99 * | 0.72 | 0.99 * | −0.65 | 0.51 | 0.99 * | −0.96 | −0.40 | −0.99 * | 0.47 | −0.26 | 0.99 * | 0.87 | 0.74 | 1 |
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LivMgC | 1 | ||||||||||||||
| 2 | LivMgT1 | 0.60 | 1 | |||||||||||||
| 3 | LivMgT2 | −0.42 | 0.47 | 1 | ||||||||||||
| 4 | KiMgC | 1 ** | 0.60 | −0.42 | 1 | |||||||||||
| 5 | KiMgT1 | −0.86 | −0.11 | 0.82 | −0.85 | 1 | ||||||||||
| 6 | KiMgT2 | −0.59 | 0.29 | 0.98 | 0.59 | 0.91 | 1 | |||||||||
| 7 | RuMgC | 0.08 | −0.75 | −0.94 | −0.08 | −0.58 | −0.85 | 1 | ||||||||
| 8 | RuMgT1 | 0.90 | 0.20 | −0.77 | −0.9 | −0.99 * | −0.88 | 0.51 | 1 | |||||||
| 9 | RuMgT2 | −0.25 | −0.93 | −0.77 | −0.25 | −0.27 | −0.63 | 0.94 | 0.19 | 1 | ||||||
| 10 | MeMgC | 0.69 | 0.99 * | 0.37 | −0.68 | −0.21 | 0.18 | −0.67 | 0.31 | −0.88 | 1 | |||||
| 11 | MeMgT1 | −0.45 | −0.98 | −0.62 | −0.44 | −0.07 | −0.46 | 0.85 | −0.02 | 0.98 | −0.96 | 1 | ||||
| 12 | MeMgT2 | −0.99 * | −0.61 | 0.41 | −0.99 | 0.85 | 0.58 | −0.07 | −0.90 | 0.27 | −0.70 | 0.46 | 1 | |||
| 13 | SeMgC | 0.75 | 0.98 | 0.29 | −0.74 | −0.3 | 0.10 | −0.60 | 0.38 | −0.83 | 0.99 * | −0.93 | −0.75 | 1 | ||
| 14 | SeMgT1 | −0.88 | −0.91 | −0.07 | −0.87 | 0.5 | 0.13 | 0.40 | −0.58 | 0.69 | −0.95 | 0.82 | 0.88 | −0.97 | 1 | |
| 15 | SeMgT2 | 0.79 | 0.97 | 0.23 | −0.78 | −0.35 | 0.03 | −0.55 | 0.44 | −0.80 | 0.98 * | −0.90 | −0.79 | 0.99 * | −0.99 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, M.M.; Alhidary, I.; Adeniji, Y.A.; Alobre, M.M.; Albaadani, H.; Aljumaah, R. Manipulating Phosphorus, Calcium, and Magnesium Utilization by Growing Lambs Using Natural Zeolite (Clinoptilolite). Sustainability 2021, 13, 1539. https://doi.org/10.3390/su13031539
Abdelrahman MM, Alhidary I, Adeniji YA, Alobre MM, Albaadani H, Aljumaah R. Manipulating Phosphorus, Calcium, and Magnesium Utilization by Growing Lambs Using Natural Zeolite (Clinoptilolite). Sustainability. 2021; 13(3):1539. https://doi.org/10.3390/su13031539
Chicago/Turabian StyleAbdelrahman, Mutassim M., Ibrahim Alhidary, Yusuf A. Adeniji, Mohsen M. Alobre, Hani Albaadani, and Riyadh Aljumaah. 2021. "Manipulating Phosphorus, Calcium, and Magnesium Utilization by Growing Lambs Using Natural Zeolite (Clinoptilolite)" Sustainability 13, no. 3: 1539. https://doi.org/10.3390/su13031539
APA StyleAbdelrahman, M. M., Alhidary, I., Adeniji, Y. A., Alobre, M. M., Albaadani, H., & Aljumaah, R. (2021). Manipulating Phosphorus, Calcium, and Magnesium Utilization by Growing Lambs Using Natural Zeolite (Clinoptilolite). Sustainability, 13(3), 1539. https://doi.org/10.3390/su13031539






