Nanobiotechnology for Agriculture: Smart Technology for Combating Nutrient Deficiencies with Nanotoxicity Challenges
Abstract
:1. Introduction
2. Nutrient Deficiency—Causes and Implication
3. Traditional Chemical vs. Nanofertilizers
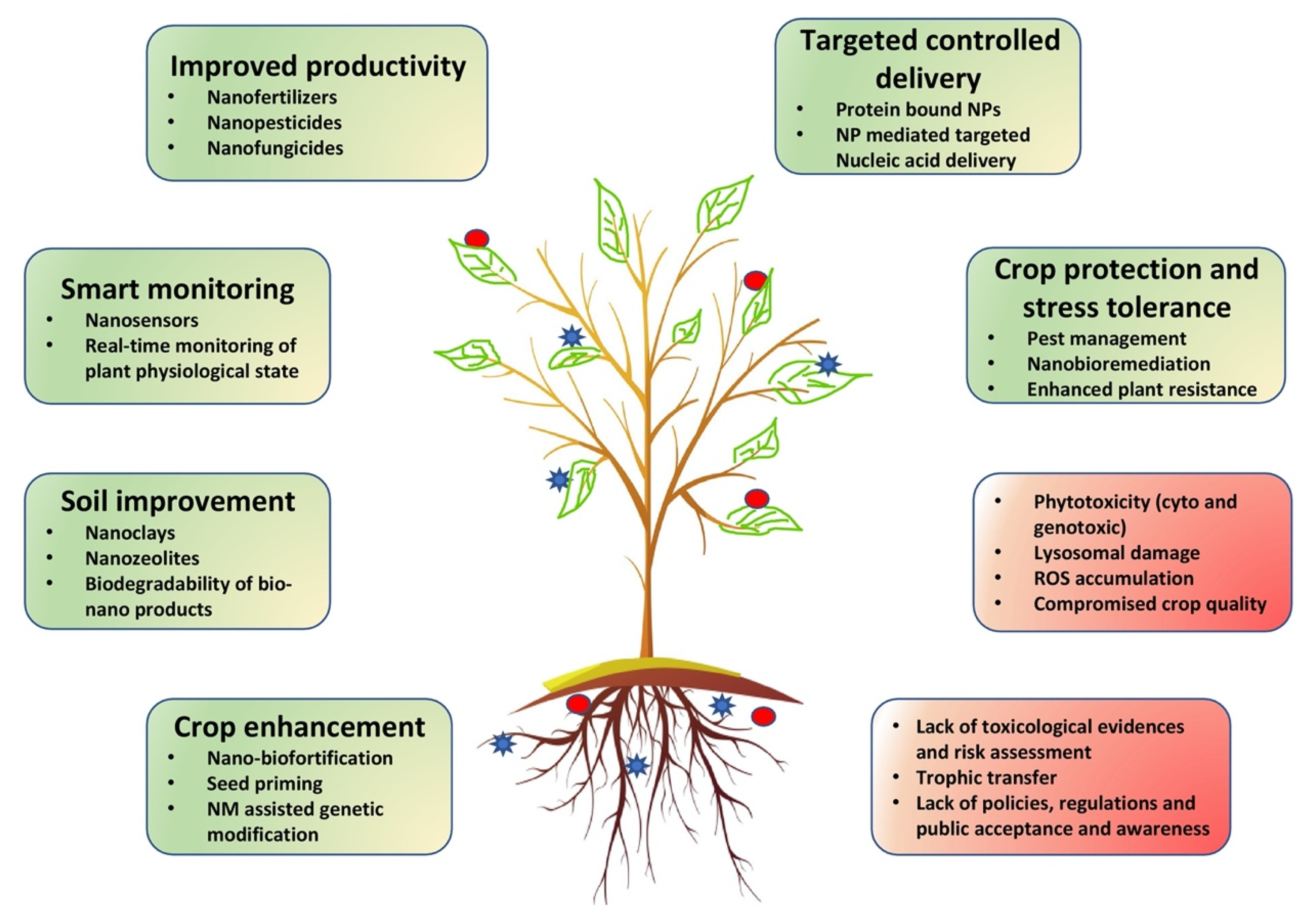
4. Advanced Use of Nanotechnology to Develop Nanofertilizers
5. Nano-Nutrient Uptake and Regulation in Plants
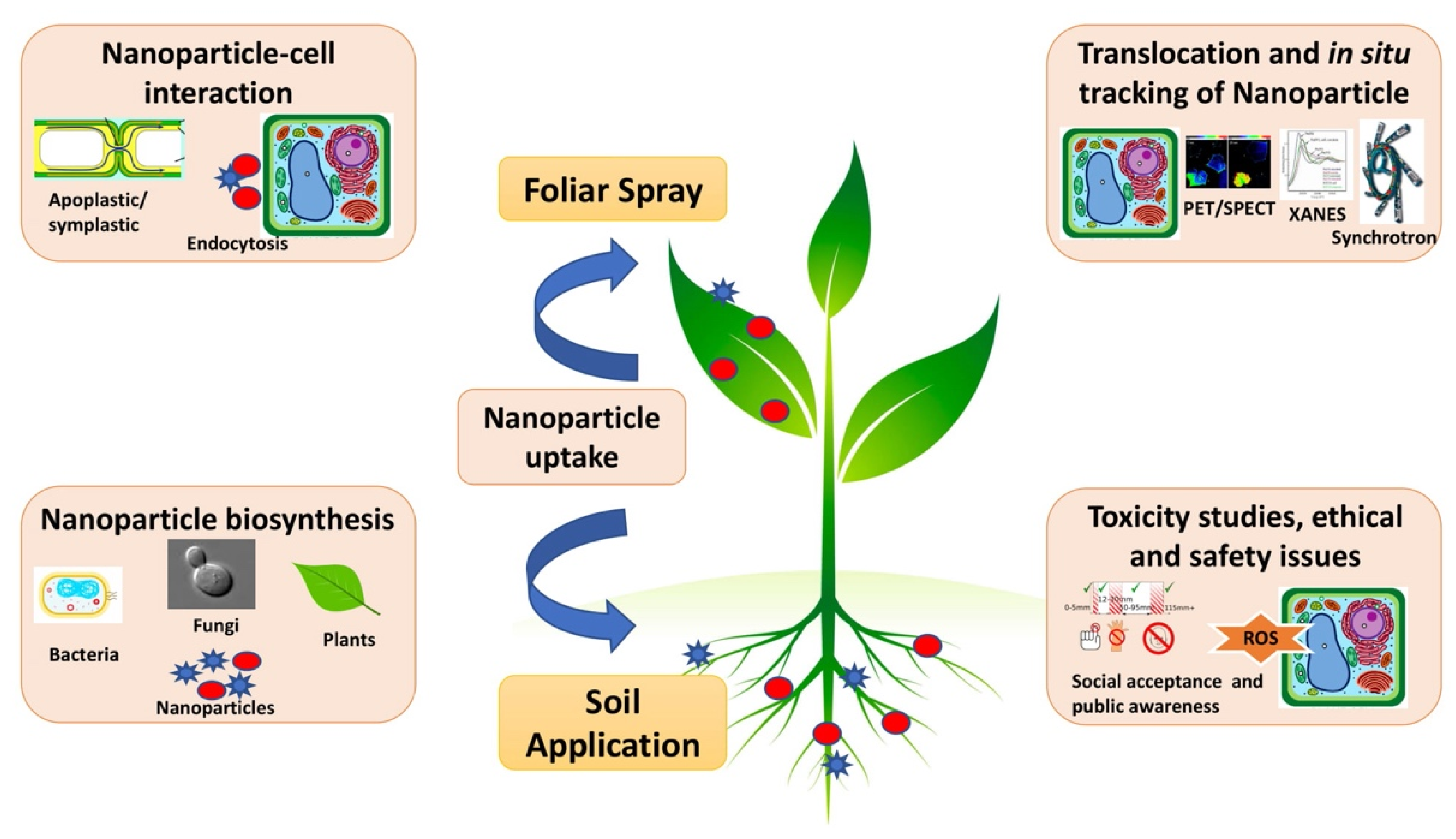
6. In Situ Visualization and Tracking of NPs
7. Smart Target Delivery System (Based on Environmental Triggers and Biological Demands)
8. Toxicity Studies Related to NPs Uptake by Plants
9. Ethical and Safety Issues Surrounding the Use of Nanoparticles for Enhanced Plant Productivity
10. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J. Nanotechnology for sustainable food production: Promising opportunities and scientific challenges. Environ. Sci. Nano 2017, 4, 767–781. [Google Scholar] [CrossRef]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and their smart delivery system. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 81–101. [Google Scholar]
- Hasler, K.; Bröring, S.; Omta, S.W.F.; Olfs, H.-W. Life cycle assessment (LCA) of different fertilizer product types. Eur. J. Agron. 2015, 69, 41–51. [Google Scholar] [CrossRef]
- Connor, D.J.; Loomis, R.S.; Cassman, K.G. Crop Ecology: Productivity and Management in Agricultural Systems; Cambridge University Press: Cambridge, UK, 2011; ISBN 1139500325. [Google Scholar]
- Kumar, D.; Patel, K.P.; Ramani, V.P.; Shukla, A.K.; Meena, R.S. Management of Micronutrients in Soil for the Nutritional Security. In Nutrient Dynamics for Sustainable Crop Production; Meena, R.S., Ed.; Springer Nature: Singapore, 2020; pp. 103–134. [Google Scholar]
- Chhowalla, M. Slow Release Nanofertilizers for Bumper Crops. ACS Cent. Sci. 2017, 3, 156–157. [Google Scholar] [CrossRef]
- Jha, Z.; Behar, N.; Sharma, S.N.; Chandel, G.; Sharma, D.K.; Pandey, M.P. Nanotechnology: Prospects of agricultural advancement. Nano Vis. 2011, 1, 88–100. [Google Scholar]
- Pradhan, S.; Mailapalli, D.R. Interaction of Engineered Nanoparticles with the Agri-environment. J. Agric. Food Chem. 2017, 65, 8279–8294. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef]
- Ombódi, A.; Saigusa, M. Broadcast application versus band application of polyolefin-coated fertilizer on green peppers grown on andisol. J. Plant Nutr. 2000, 23, 1485–1493. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Monreal, C.; McGill, W.B.; Nyborg, M. Spatial heterogeneity of substrates: Effects on hydrolysis, immobilization and nitrification of urea-N. Can. J. Soil Sci. 1986, 66, 499–511. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef]
- Cui, H.X.; Sun, C.J.; Liu, Q.; Jiang, J.; Gu, W. Applications of nanotechnology in agrochemical formulation, perspectives, challenges and strategies. In Proceedings of the International Conference on Nanoagri, Sao pedro, Brazil, 20–25 June 2010; pp. 28–33. [Google Scholar]
- Sasson, Y.; Levy-Ruso, G.; Toledano, O.; Ishaaya, I. Nanosuspensions: Emerging novel agrochemical formulations. In Insecticides Design Using Advanced Technologies; Ishaaya, I., Nauen, R., Horowitz, A.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–39. [Google Scholar]
- Reineke, J. Nanotoxicity: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2012; ISBN 1627030026. [Google Scholar]
- Zhao, L.; Sun, Y.; Hernandez-Viezcas, J.A.; Hong, J.; Majumdar, S.; Niu, G.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Monitoring the environmental effects of CeO2 and ZnO nanoparticles through the life cycle of corn (Zea mays) plants and in situ μ-XRF mapping of nutrients in kernels. Environ. Sci. Technol. 2015, 49, 2921–2928. [Google Scholar] [CrossRef]
- Zahra, Z.; Waseem, N.; Zahra, R.; Lee, H.; Badshah, M.A.; Mehmood, A.; Choi, H.-K.; Arshad, M. Growth and metabolic responses of rice (Oryza sativa L.) cultivated in phosphorus-deficient soil amended with TiO2 nanoparticles. J. Agric. Food Chem. 2017, 65, 5598–5606. [Google Scholar] [CrossRef]
- Khan, M.N. Nano-titanium Dioxide (Nano-TiO2) mitigates NaCl stress by enhancing antioxidative enzymes and accumulation of compatible solutes in tomato (Lycopersicon esculentum Mill.). J. Plant Sci. 2016, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pošćić, F.; Mattiello, A.; Fellet, G.; Miceli, F.; Marchiol, L. Effects of cerium and titanium oxide nanoparticles in soil on the nutrient composition of barley (Hordeum vulgare L.) kernels. Int. J. Environ. Res. Public Health 2016, 13, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, B.; Shabbir, A.; Jaleel, H.; Khan, M.M.A.; Sadiq, Y. Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr. Plant Biol. 2018, 13, 6–15. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Rico, C.M.; Morales, M.I.; Barrios, A.C.; McCreary, R.; Hong, J.; Lee, W.-Y.; Nunez, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effect of cerium oxide nanoparticles on the quality of rice (Oryza sativa L.) grains. J. Agric. Food Chem. 2013, 61, 11278–11285. [Google Scholar] [CrossRef]
- Rico, C.M.; Barrios, A.C.; Tan, W.; Rubenecia, R.; Lee, S.C.; Varela-Ramirez, A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Physiological and biochemical response of soil-grown barley (Hordeum vulgare L.) to cerium oxide nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 10551–10558. [Google Scholar] [CrossRef]
- Rico, C.M.; Lee, S.C.; Rubenecia, R.; Mukherjee, A.; Hong, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). J. Agric. Food Chem. 2014, 62, 9669–9675. [Google Scholar] [CrossRef]
- Du, W.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Zhu, J.; Peralta-Videa, J.R.; Guo, H. Physiological and biochemical changes imposed by CeO2 nanoparticles on wheat: A life cycle field study. Environ. Sci. Technol. 2015, 49, 11884–11893. [Google Scholar] [CrossRef]
- Servin, A.D.; Morales, M.I.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Munoz, B.; Zhao, L.; Nunez, J.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron verification of TiO2 accumulation in cucumber fruit: A possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ. Sci. Technol. 2013, 47, 11592–11598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peralta-Videa, J.R.; Rico, C.M.; Hernandez-Viezcas, J.A.; Sun, Y.; Niu, G.; Servin, A.; Nunez, J.E.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus). J. Agric. Food Chem. 2014, 62, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.I.; Rico, C.M.; Hernandez-Viezcas, J.A.; Nunez, J.E.; Barrios, A.C.; Tafoya, A.; Flores-Marges, J.P.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J. Agric. Food Chem. 2013, 61, 6224–6230. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sun, Y.; Morelius, E.; Tamez, C.; Bandyopadhyay, S.; Niu, G.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): A life cycle study. Front. Plant Sci. 2016, 6, 1242. [Google Scholar] [CrossRef] [Green Version]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Prasad, T.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Tavallali, V.; Rowshan, V.; Bahmanzadegan, A. Variations in sweet basil in response to Green synthesized Zinc-Amino nano complexes. J. Clean. Prod. 2018, 196, 452–459. [Google Scholar] [CrossRef]
- López-Vargas, E.R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef] [Green Version]
- Hernández, H.H.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; Juárez-Maldonado, A. Cu Nanoparticles in chitosan-PVA hydrogels as promoters of growth, productivity and fruit quality in tomato. Emir. J. Food Agric. 2017, 29, 573–580. [Google Scholar]
- Zhao, L.; Huang, Y.; Hu, J.; Zhou, H.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress. Environ. Sci. Technol. 2016, 50, 2000–2010. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Hu, J.; Huang, Y.; Wang, H.; Adeleye, A.; Ortiz, C.; Keller, A.A. 1H NMR and GC–MS based metabolomics reveal nano-Cu altered cucumber (Cucumis sativus) fruit nutritional supply. Plant Physiol. Biochem. 2017, 110, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef] [Green Version]
- Sheykhbaglou, R.; Sedghi, M.; Shishevan, M.T.; Sharifi, R.S. Effects of nano-iron oxide particles on agronomic traits of soybean. Not. Sci. Biol. 2010, 2, 112–113. [Google Scholar] [CrossRef] [Green Version]
- Deepa, M.; Sudhakar, P.; Nagamadhuri, K.V.; Reddy, K.B.; Krishna, T.G.; Prasad, T.N.V.K.V. First evidence on phloem transport of nanoscale calcium oxide in groundnut using solution culture technique. Appl. Nanosci. 2015, 5, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Yugandhar, P.; Savithramma, N. Green synthesis of calcium carbonate nanoparticles and their effects on seed germination and seedling growth of Vigna mungo (L.) Hepper. Int. J. Adv. Res. 2013, 1, 89–103. [Google Scholar]
- Delfani, M.; Baradarn Firouzabadi, M.; Farrokhi, N.; Makarian, H. Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun. Soil Sci. Plant Anal. 2014, 45, 530–540. [Google Scholar] [CrossRef]
- Shams, G.; Ranjbar, M.; Amiri, A. Effect of silver nanoparticles on concentration of silver heavy element and growth indexes in cucumber (Cucumis sativus L. negeen). J. Nanoparticle Res. 2013, 15, 1630. [Google Scholar] [CrossRef]
- Song, U.; Jun, H.; Waldman, B.; Roh, J.; Kim, Y.; Yi, J.; Lee, E.J. Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 2013, 93, 60–67. [Google Scholar] [CrossRef]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Cécillon, L.; Bureau, S.; Barthès, V.; Ouerdane, L.; Carrière, M.; Sarret, G. Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation. J. Hazard. Mater. 2014, 264, 98–106. [Google Scholar] [CrossRef]
- Kumar, V.; Guleria, P.; Kumar, V.; Yadav, S.K. Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci. Total Environ. 2013, 461, 462–468. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Pantidos, N. Biological Synthesis of Metallic Nanoparticles by Bacteria, Fungi and Plants. J. Nanomed. Nanotechnol. 2014, 05. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Herricks, T.; Sun, Y.; Xia, Y. Polyol synthesis of silver nanoparticles: Use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons. Nano Lett. 2004, 4, 1733–1739. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef]
- Kah, M. Nanopesticides and nanofertilizers: Emerging contaminants or opportunities for risk mitigation? Front. Chem. 2015, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—a review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Li, J.; Tian, B.; Li, T.; Dai, S.; Weng, Y.; Lu, J.; Xu, X.; Jin, Y.; Pang, R.; Hua, Y. Biosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using protein extracts of Deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018, 13, 1411. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J. Urea-Hydroxyapatite Nanohybrids for Slow Release of Nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Sharonova, N.L.; Yapparov, A.K.; Khisamutdinov, N.S.; Ezhkova, A.M.; Yapparov, I.A.; Ezhkov, V.O.; Degtyareva, I.A.; Babynin, E. V Nanostructured water-phosphorite suspension is a new promising fertilizer. Nanotechnol. Russ. 2015, 10, 651–661. [Google Scholar] [CrossRef]
- Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Understanding the role of nanomaterials in agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 271–288. [Google Scholar]
- Tarafdar, J.C.; Raliya, R.; Rathore, I. Microbial synthesis of phosphorous nanoparticle from tri-calcium phosphate using Aspergillus tubingensis TFR-5. J. Bionanosci. 2012, 6, 84–89. [Google Scholar] [CrossRef]
- Kubavat, D.; Trivedi, K.; Vaghela, P.; Prasad, K.; Vijay Anand, G.K.; Trivedi, H.; Patidar, R.; Chaudhari, J.; Andhariya, B.; Ghosh, A. Characterization of a chitosan-based sustained release nanofertilizer formulation used as a soil conditioner while simultaneously improving biomass production of Zea mays L. Land Degrad. Dev. 2020, 31, 2734–2746. [Google Scholar] [CrossRef]
- Gerdini, F.S. Effect of nano potassium fertilizer on some parchment pumpkin (Cucurbita pepo) morphological and physiological characteristics under drought conditions. Intl. J. Farm. Alli Sci. 2016, 5, 367–371. [Google Scholar]
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A. Metallic nanoparticle (TiO2 and Fe3O4) application modifies rhizosphere phosphorus availability and uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. Biosynthesis and characterization of zinc, magnesium and titanium nanoparticles: An eco-friendly approach. Int. Nano Lett. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in Plant Science: To Make a Long Story Short. Front. Bioeng. Biotechnol. 2019, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jahangirian, H.; Rafiee-Moghaddam, R.; Jahangirian, N.; Nikpey, B.; Jahangirian, S.; Bassous, N.; Saleh, B.; Kalantari, K.; Webster, T.J. Green Synthesis of Zeolite/Fe2O3 Nanocomposites: Toxicity & Cell Proliferation Assays and Application as a Smart Iron Nanofertilizer. Int. J. Nanomed. 2020, 15, 1005. [Google Scholar]
- Mandal, N.; Datta, S.C.; Manjaiah, K.M.; Dwivedi, B.S.; Kumar, R.; Aggarwal, P. Evaluation of zincated nanoclay polymer composite in releasing Zn and P and effect on soil enzyme activities in a wheat rhizosphere. Eur. J. Soil Sci. 2019, 70, 1164–1182. [Google Scholar] [CrossRef]
- Reguera, G. Harnessing the power of microbial nanowires. Microb. Biotechnol. 2018, 11, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Noruzi, M. Electrospun nanofibres in agriculture and the food industry: A review. J. Sci. Food Agric. 2016, 96, 4663–4678. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Krishnan, P.; Kotnala, R.K.; Sumana, G. Recent developments in biosensors to combat agricultural challenges and their future prospects. Trends Food Sci. Technol. 2019, 88, 157–178. [Google Scholar] [CrossRef]
- Alidoust, D.; Isoda, A. Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol. Plant. 2013, 35, 3365–3375. [Google Scholar] [CrossRef]
- Salehi, H.; Chehregani, A.; Lucini, L.; Majd, A.; Gholami, M. Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci. Total Environ. 2018, 616, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302. [Google Scholar] [CrossRef] [Green Version]
- Al-Salim, N.; Barraclough, E.; Burgess, E.; Clothier, B.; Deurer, M.; Green, S.; Malone, L.; Weir, G. Quantum dot transport in soil, plants, and insects. Sci. Total Environ. 2011, 409, 3237–3248. [Google Scholar] [CrossRef] [PubMed]
- Chichiriccò, G.; Poma, A. Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 2015, 5, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Feng, Y.; Lin, X.; Wang, J. Arbuscular mycorrhizal fungi alleviate the negative effects of iron oxide nanoparticles on bacterial community in rhizospheric soils. Front. Environ. Sci. 2016, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.; McLean, J.; McManus, P.; Britt, D. Soil chemistry influences the phytotoxicity of metal oxide nanoparticles. Int. J. Nanotechnol. 2017, 14, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Comerford, N.B. Soil factors affecting nutrient bioavailability. In Nutrient Acquisition by Plants; BassiriRad, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–14. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology 5th Edition Sinauer Associates; Sinauer Associates, Inc.: Sunderland, UK, 2010. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011; ISBN 0123849063. [Google Scholar]
- Lawlor, D.W.; Mengel, K.; Kirkby, E.A. Principles of plant nutrition. Ann. Bot. 2004, 93, 479–480. [Google Scholar] [CrossRef] [Green Version]
- Larue, C.; Laurette, J.; Herlin-Boime, N.; Khodja, H.; Fayard, B.; Flank, A.-M.; Brisset, F.; Carriere, M. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): Influence of diameter and crystal phase. Sci. Total Environ. 2012, 431, 197–208. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Q.; Huang, Y.; Keller, A.A. Response at Genetic, Metabolic, and Physiological Levels of Maize (Zea mays) Exposed to a Cu(OH)2 Nanopesticide. ACS Sustain. Chem. Eng. 2017, 5, 8294–8301. [Google Scholar] [CrossRef] [Green Version]
- Palocci, C.; Valletta, A.; Chronopoulou, L.; Donati, L.; Bramosanti, M.; Brasili, E.; Baldan, B.; Pasqua, G. Endocytic pathways involved in PLGA nanoparticle uptake by grapevine cells and role of cell wall and membrane in size selection. Plant Cell Rep. 2017, 36, 1917–1928. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Lv, Z.; Cui, L.; Mao, H.; Kopittke, P.M. Using Synchrotron-Based Approaches to Examine the Foliar Application of ZnSO4 and ZnO Nanoparticles for Field-Grown Winter Wheat. J. Agric. Food Chem. 2018, 66, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Åberg, C.; Dawson, K.A.; Salvati, A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Ferrari, M. The role of specific and non-specific interactions in receptor-mediated endocytosis of nanoparticles. Biomaterials 2007, 28, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.-S.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci, F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008, 7, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.M.; McNerny, D.Q.; Baker, J.R.; Orr, B.G.; Holl, M.M.B. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef]
- Lin, S.; Reppert, J.; Hu, Q.; Hudson, J.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 2009, 5, 1128–1132. [Google Scholar] [CrossRef]
- Zhai, G.; Walters, K.S.; Peate, D.W.; Alvarez, P.J.J.; Schnoor, J.L. Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar. Environ. Sci. Technol. Lett. 2014, 1, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racuciu, M. Iron oxide nanoparticles coated with β-cyclodextrin polluted of Zea mays plantlets. Nanotechnol. Dev. 2012, 2, e6-e6. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Lombi, E.; Sun, S.; Scheckel, K.G.; Malysheva, A.; McKenna, B.A.; Menzies, N.W.; Zhao, F.-J.; Kopittke, P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano 2017, 4, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Carmona, A.; Llorens, I.; Solari, P.L. X-ray absorption spectroscopy of biological samples. A tutorial. J. Anal. At. Spectrom. 2012, 27, 2054–2065. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Punshon, T.; Paterson, D.J.; Tappero, R.V.; Wang, P.; Blamey, F.P.C.; van der Ent, A.; Lombi, E. Synchrotron-based X-ray fluorescence microscopy as a technique for imaging of elements in plants. Plant Physiol. 2018, 178, 507–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hettiarachchi, G.M.; Donner, E.; Doelsch, E. Application of Synchrotron Radiation-based Methods for Environmental Biogeochemistry: Introduction to the Special Section. J. Environ. Qual. 2017, 46, 1139–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; He, X.; Zhang, H.; Ma, Y.; Zhang, P.; Ding, Y.; Zhao, Y. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 2011, 3, 816–822. [Google Scholar] [CrossRef]
- Bystrzejewska-Piotrowska, G.; Asztemborska, M.; Stęborowski, R.; Polkowska-Motrenko, H.; Danko, B.; Ryniewicz, J. Application of neutron activation for investigation of Fe3O4 nanoparticles accumulation by plants. Nukleonika 2012, 57, 427–430. [Google Scholar]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Tan, Z.; Hu, L.; Yu, S.; Liu, J.; Jiang, G. Isotope tracers to study the environmental fate and bioaccumulation of metal-containing engineered nanoparticles: Techniques and applications. Chem. Rev. 2017, 117, 4462–4487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreyling, W.G.; Abdelmonem, A.M.; Ali, Z.; Alves, F.; Geiser, M.; Haberl, N.; Hartmann, R.; Hirn, S.; De Aberasturi, D.J.; Kantner, K. In vivo integrity of polymer-coated gold nanoparticles. Nat. Nanotechnol. 2015, 10, 619. [Google Scholar] [CrossRef] [Green Version]
- Lamb, J.; Holland, J.P. Advanced methods for radiolabeling multimodality nanomedicines for SPECT/MRI and PET/MRI. J. Nucl. Med. 2018, 59, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Abou, D.S.; Pickett, J.E.; Thorek, D.L.J. Nuclear molecular imaging with nanoparticles: Radiochemistry, applications and translation. Br. J. Radiol. 2015, 88, 20150185. [Google Scholar] [CrossRef]
- Naderi, M.R.; Danesh-Shahraki, A. Nanofertilizers and their roles in sustainable agriculture. Int. J. Agric. Crop Sci. (IJACS) 2013, 5, 2229–2232. [Google Scholar]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Aziz, H.M.M.A.; Hasaneen, M.N.A.; Omer, A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span. J. Agric. Res. 2016, 14, 17. [Google Scholar]
- Fleischer, C.C.; Payne, C.K. Nanoparticle surface charge mediates the cellular receptors used by protein-nanoparticle complexes. J. Phys. Chem. B 2012, 116, 8901–8907. [Google Scholar] [CrossRef] [Green Version]
- Doorley, G.W.; Payne, C.K. Nanoparticles act as protein carriers during cellular internalization. Chem. Commun. 2012, 48, 2961–2963. [Google Scholar] [CrossRef] [PubMed]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Toy, R.; Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef]
- Dehaini, D.; Fang, R.H.; Zhang, L. Biomimetic strategies for targeted nanoparticle delivery. Bioeng. Transl. Med. 2016, 1, 30–46. [Google Scholar] [CrossRef]
- Ge, C.; Du, J.; Zhao, L.; Wang, L.; Liu, Y.; Li, D.; Yang, Y.; Zhou, R.; Zhao, Y.; Chai, Z.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 16968–16973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melby, E.S.; Lohse, S.E.; Park, J.E.; Vartanian, A.M.; Putans, R.A.; Abbott, H.B.; Hamers, R.J.; Murphy, C.J.; Pedersen, J.A. Cascading Effects of Nanoparticle Coatings: Surface Functionalization Dictates the Assemblage of Complexed Proteins and Subsequent Interaction with Model Cell Membranes. ACS Nano 2017, 11, 5489–5499. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sheibani, S.; Milani, A.S.; Rezaee, F.; Gauberti, M.; Dinarvand, R.; Vali, H. Crucial role of the protein corona for the specific targeting of nanoparticles. Nanomedicine 2015, 10, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Bertrand, N.; Zope, H.; Farokhzad, O.C. Emerging understanding of the protein corona at the nano-bio interfaces. Nano Today 2016, 11, 817–832. [Google Scholar] [CrossRef] [Green Version]
- Spielman-Sun, E.; Avellan, A.; Bland, G.D.; Clement, E.T.; Tappero, R.V.; Acerbo, A.S.; Lowry, G.V. Protein coating composition targets nanoparticles to leaf stomata and trichomes. Nanoscale 2020, 12, 3630–3636. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Abdelmonem, A.M.; Behzadi, S.; Clement, J.H.; Dutz, S.; Ejtehadi, M.R.; Hartmann, R.; Kantner, K.; Linne, U.; Maffre, P. Temperature: The “ignored” factor at the nanobio interface. ACS Nano 2013, 7, 6555–6562. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanoparticle Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Pradhan, S.; Patra, P.; Das, S.; Chandra, S.; Mitra, S.; Dey, K.K.; Akbar, S.; Palit, P.; Goswami, A. Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: A detailed molecular, biochemical, and biophysical study. Environ. Sci. Technol. 2013, 47, 13122–13131. [Google Scholar] [CrossRef] [PubMed]
- Vroman, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical-Chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Bewersdorff, T.; Vonnemann, J.; Kanik, A.; Haag, R.; Haase, A. The influence of surface charge on serum protein interaction and cellular uptake: Studies with dendritic polyglycerols and dendritic polyglycerol-coated gold nanoparticles. Int. J. Nanomed. 2017, 12, 2001. [Google Scholar] [CrossRef] [Green Version]
- Nandhakumar, S.; Dhanaraju, M.D.; Sundar, V.D.; Heera, B. Influence of surface charge on the in vitro protein adsorption and cell cytotoxicity of paclitaxel loaded poly (ε-caprolactone) nanoparticles. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 249–258. [Google Scholar] [CrossRef]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of protein modification on the protein corona on nanoparticles and nanoparticle-cell interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein− nanoparticle interactions: Opportunities and challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef]
- Macazo, F.C.; Minteer, S.D. Mechanistic Studies of Protein-Based, Metal Nanoparticle Biosynthesis. Meet. Abstr. 2018, MA2018-01, 2052. [Google Scholar]
- Nagayama, S.; Ogawara, K.; Fukuoka, Y.; Higaki, K.; Kimura, T. Time-dependent changes in opsonin amount associated on nanoparticles alter their hepatic uptake characteristics. Int. J. Pharm. 2007, 342, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem.Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef]
- Yang, J.A.; Lohse, S.E.; Murphy, C.J. Tuning cellular response to nanoparticles via surface chemistry and aggregation. Small 2014, 10, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. Checking the Biocompatibility of Plant-Derived Metallic Nanoparticles: Molecular Perspectives. Trends Biotechnol. 2016, 34, 440–449. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772. [Google Scholar] [CrossRef]
- Senapati, V.A.; Kansara, K.; Shanker, R.; Dhawan, A.; Kumar, A. Monitoring characteristics and genotoxic effects of engineered nanoparticle–protein corona. Mutagenesis 2017, 32, 479–490. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Song, F.; Liu, R.; Guo, H.; Olsen, D.W.H.; Cohen, Y.; Emili, A.; Chan, W.C.W. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 2014, 8, 2439–2455. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; Unrine, J.M.; Rao, W.; Wirick, S.; Bertsch, P.M. Bioavailability of gold nanomaterials to plants: Importance of particle size and surface coating. Environ. Sci. Technol. 2012, 46, 8467–8474. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Raliya, R.; Mahawar, H.; Rathore, I. Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum). Agric. Res. 2014, 3, 257–262. [Google Scholar] [CrossRef]
- Mukherjee, A.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Rico, C.M.; Zhao, L.; Gardea-Torresdey, J.L. Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 2014, 6, 132–138. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Dwivedi, S.P.; Siddiqui, M.H.; Prasad, T. In vitro studies on oxidative stress-independent, Ag nanoparticles-induced cell toxicity of Candida albicans, an opportunistic pathogen. Int. J. Nanomed. 2018, 13, 91. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-Based Nanotoxicity and Detoxification Pathways in Higher Plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar] [CrossRef]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef]
- Rico, C.M.; Hong, J.; Morales, M.I.; Zhao, L.; Barrios, A.C.; Zhang, J.Y.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effect of cerium oxide nanoparticles on rice: A study involving the antioxidant defense system and in vivo fluorescence imaging. Environ. Sci. Technol. 2013, 47, 5635–5642. [Google Scholar] [CrossRef] [PubMed]
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.-J. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, E2451–E2456. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, F.; Schulin, R.; Limbach, L.K.; Stark, W.; Bürge, D.; Nowack, B. Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture. Chemosphere 2013, 91, 512–520. [Google Scholar] [CrossRef]
- Taylor, A.F.; Rylott, E.L.; Anderson, C.W.N.; Bruce, N.C. Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS ONE 2014, 9, e93793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruotolo, R.; Maestri, E.; Pagano, L.; Marmiroli, M.; White, J.C.; Marmiroli, N. Plant response to metal-containing engineered nanomaterials: An omics-based perspective. Environ. Sci. Technol. 2018, 52, 2451–2467. [Google Scholar] [CrossRef]
- Majumdar, S.; Almeida, I.C.; Arigi, E.A.; Choi, H.; VerBerkmoes, N.C.; Trujillo-Reyes, J.; Flores-Margez, J.P.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Environmental effects of nanoceria on seed production of common bean (Phaseolus vulgaris): A proteomic analysis. Environ. Sci. Technol. 2015, 49, 13283–13293. [Google Scholar] [CrossRef]
- Mubarak Ali, D.; Arunkumar, J.; Pooja, P.; Subramanian, G.; Thajuddin, N.; Alharbi, N.S. Synthesis and characterization of biocompatibility of tenorite nanoparticles and potential property against biofilm formation. Saudi Pharm. J. 2015, 23, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, K.; Kim, S.T.; Yan, B.; Miranda, O.R.; Alfonso, F.S.; Shlosman, D.; Rotello, V.M. Surface functionality of nanoparticles determines cellular uptake mechanisms in mammalian cells. Small 2013, 9, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Marmiroli, N.; White, J.C. Nanotoxicology and environmental risk assessment of engineered nanomaterials (ENMs) in plants. Front. Plant Sci. 2016, 7, 1370. [Google Scholar] [CrossRef] [Green Version]
- Lombi, E.; Donner, E.; Dusinska, M.; Wickson, F. A One Health approach to managing the applications and implications of nanotechnologies in agriculture. Nat. Nanotechnol. 2019, 14, 523–531. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. One Health: Food and Agriculture Organization of the United Nations Strategic Action Plan. Available online: http://www.fao.org/documents/card/en/c/e193889c-28d5-55a0-9c03-beeac89e5960/ (accessed on 28 January 2021).
- Mitter, N.; Hussey, K. Moving policy and regulation forward for nanotechnology applications in agriculture. Nat. Nanotechnol. 2019, 14, 508–510. [Google Scholar] [CrossRef]

| Nutrient | Nanoparticles | Plant Species | Application Method | Concentration | Effect on Plant | References |
|---|---|---|---|---|---|---|
| Titanium | TiO2 | Oryza sativa (rice) | Root | 750 mg kg−1 | Increased P in root, shoot and grain, amino acids, fatty acids | [18] |
| Solanum lycopersicum (tomato) | Root | 20 mg L−1 | Increased amino acids, total phenolics, antioxidant capacity | [19] | ||
| Hordeum vulgare (barley) | Root | 500 mg kg−1 | Increased P, Ca, Mg, Zn, Mn, amino acids | [20] | ||
| Mentha piperita (peppermint) | Foliar | 150 mg L−1 | Increased N, chlorophyll, menthol, menthone | [21] | ||
| Solanum lycopersicum (tomato) | Root and foliar | 100 mg kg−1 | Increased lycopene | [22] | ||
| Cerium | CeO2 | Oryza sativa (rice) | Root | 500 mg kg−1 | Increased K, Ca, Na, protein albumin, total sugars | [23] |
| Hordeum vulgare (barley) | Root | 500 mg kg−1 | Increased P, K, Ca, Mg, S, Cu, Fe, Zn, Mn, amino acids, fatty acids | [24] | ||
| Triticum aestivum (wheat) | Root | 400 mg kg−1 | Increased P, K, Fe, amino acids, fatty acids, total sugars | [25,26] | ||
| Cucumis sativus (cucumber) | Root | 750 mg kg−1 400 mg kg−1 | Increased K, Ca, Mg, S, P, Fe, Mn, Zn, total sugars, starches, proteins | [27,28] | ||
| Coriandrum sativum (cilantro) | Root | 125 mg kg−1 | Increased Ce, catalase, and ascorbate peroxidase activities | [29] | ||
| Zinc | ZnO | Pisum sativum (pea) | Root | 250 mg kg−1 | Increased P, Fe, Zn, Mn, total sugars | [30] |
| Cucumis sativus (cucumber) | Root | 400 mg kg−1 | Increased K, Mg, Fe, Mn, Zn, S, prolamin, globulin, glutelin | [28] | ||
| Zea mays (maize) | Foliar | 100 mg L−1 | Increased Zn, germination, growth, yield | [31] | ||
| Solanum lycopersicum (tomato) | Root and Foliar | 10 mg L−1 | Increased lycopene | [32] | ||
| Arachis hypogaea (peanut) | Foliar | 1000 mg L−1 | Increased Zn, chlorophyll, root biomass, yield | [33] | ||
| Zn-amino acid nano complex | Ocimum basilicum (sweet basil) | Foliar | 1500 mg L−1 | Increased catechin, hesperetin | [34] | |
| Copper | Cu | Solanum lycopersicum (tomato) | Root and Foliar | 250 mg L−1 | Increased K, total proteins, vitamin C, total phenols, flavonoids, lycopene, antioxidant capacity | [35,36] |
| Cucumis sativus (cucumber) | Root | 400 mg kg−1 | Increased Cu, Fe, sugars, organic acids, amino acids, fatty acids | [37,38] | ||
| Iron | Fe2O3 | Arachis hypogaea (peanut) | Root | 1000 mg kg−1 | Increased Zn, growth, biomass | [39] |
| Glycine max (soybean) | Foliar | 750 mg L−1 | [40] | |||
| Calcium | CaO | Arachis hypogaea (peanut) | Foliar | 500 mg L−1 | Increased Ca, root development | [41] |
| CaCO3 | Vigna mungo (Black gram) | Seed | 750 mg L−1 | Increased root and shoot growth, biomass | [42] | |
| Magnesium | Mg | Vigna unguiculata (cowpea) | Foliar | 500 mg L−1 | Increased photosynthesis, growth, yield | [43] |
| Silver | Ag | Cucumis sativus (cucumber) | Foliar | 3000 mg L−1 | Increased growth, fruit yield, biomass, total soluble solids in fruit | [44] |
| Solanum lycopersicum (tomato) | Root | 1000 mg L−1 | Increased superoxide dismutase activity | [45] | ||
| Lactuca sativa (lettuce) | Foliar | 100 mg kg−1 | Increased Ag content | [46] | ||
| Gold | Au | Arabidopsis thaliana (thale cress) | Root | 10 mg L−1 | Increased seed germination, growth, yield | [47] |
| Brassica juncea (brown mustard) | Foliar | 10 mg L−1 | Increased germination, yield, protection from oxidative damage | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chugh, G.; Siddique, K.H.M.; Solaiman, Z.M. Nanobiotechnology for Agriculture: Smart Technology for Combating Nutrient Deficiencies with Nanotoxicity Challenges. Sustainability 2021, 13, 1781. https://doi.org/10.3390/su13041781
Chugh G, Siddique KHM, Solaiman ZM. Nanobiotechnology for Agriculture: Smart Technology for Combating Nutrient Deficiencies with Nanotoxicity Challenges. Sustainability. 2021; 13(4):1781. https://doi.org/10.3390/su13041781
Chicago/Turabian StyleChugh, Gaurav, Kadambot H. M. Siddique, and Zakaria M. Solaiman. 2021. "Nanobiotechnology for Agriculture: Smart Technology for Combating Nutrient Deficiencies with Nanotoxicity Challenges" Sustainability 13, no. 4: 1781. https://doi.org/10.3390/su13041781






