Influencing Factors of the Mineral Carbonation Process of Iron Ore Mining Waste in Sequestering Atmospheric Carbon Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Mine-Waste Sampling
2.2. pH and Particle Size Distribution Analysis
2.3. Mineralogical and Chemical Analysis
2.4. Mineral Carbonation Experiment
2.5. Thermogravimetric Analysis
2.6. Statistical Analysis
3. Results and Discussion
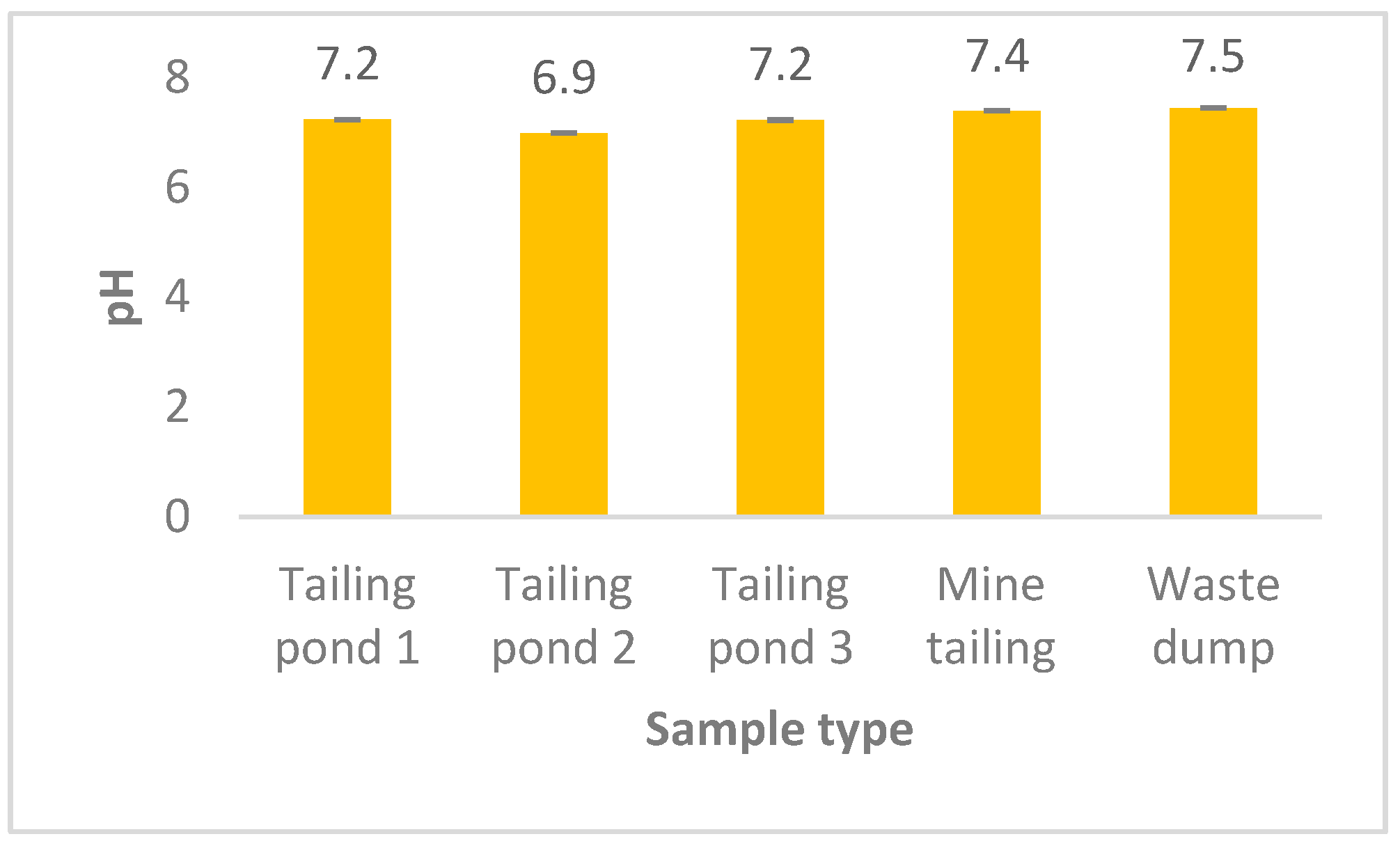
3.1. pH and Particle Size Distribution
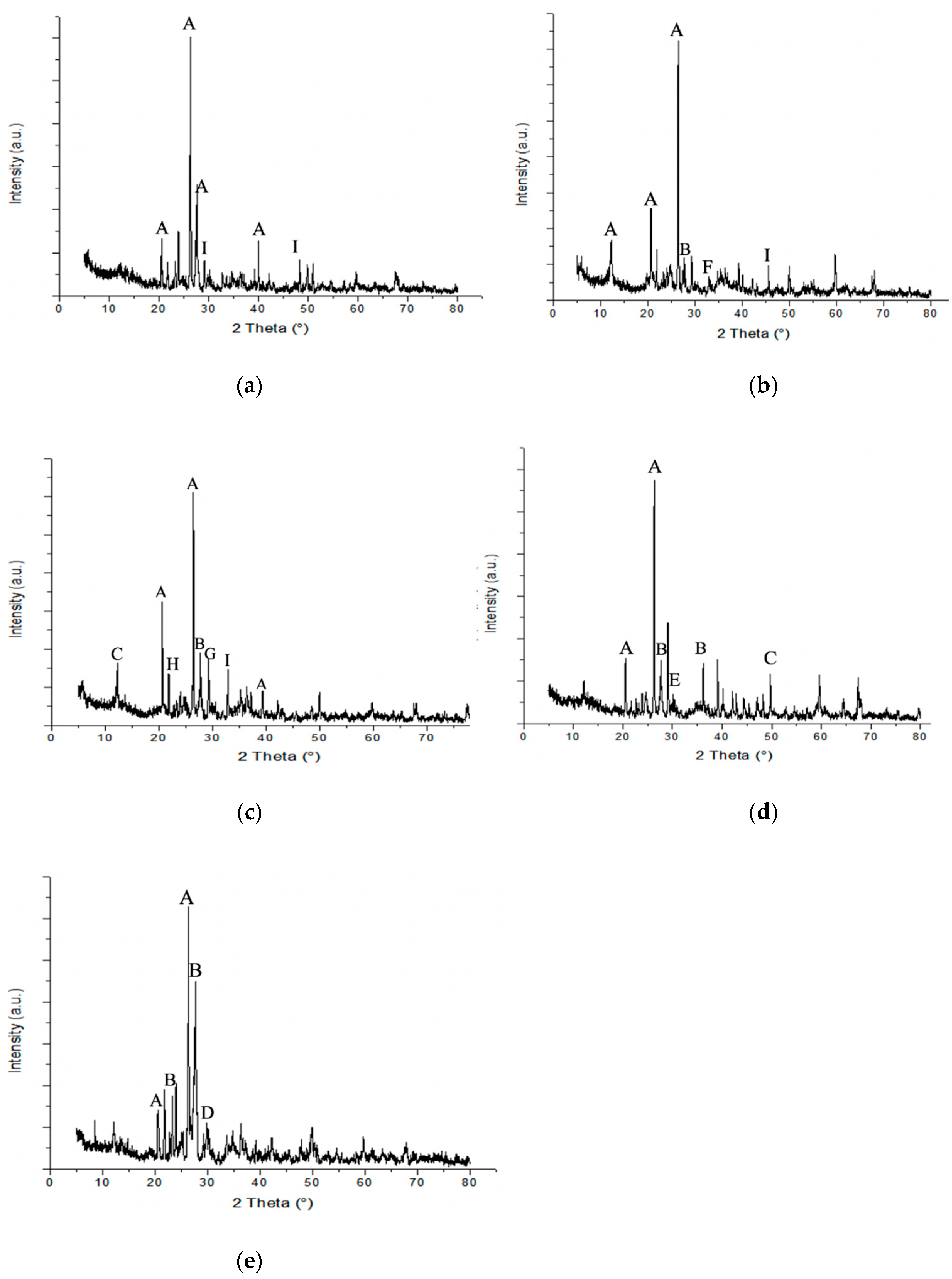
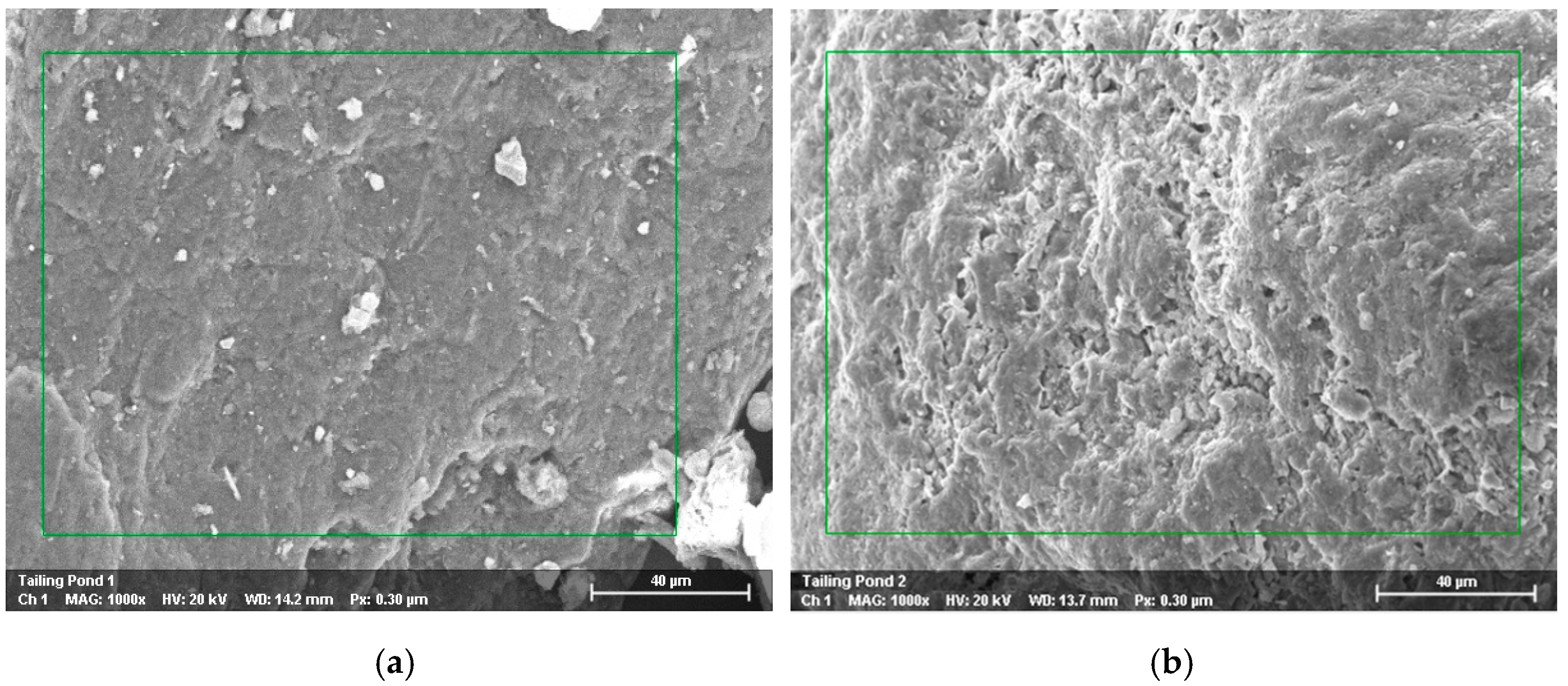
3.2. Mineralogical and Chemical Composition of Mine Waste
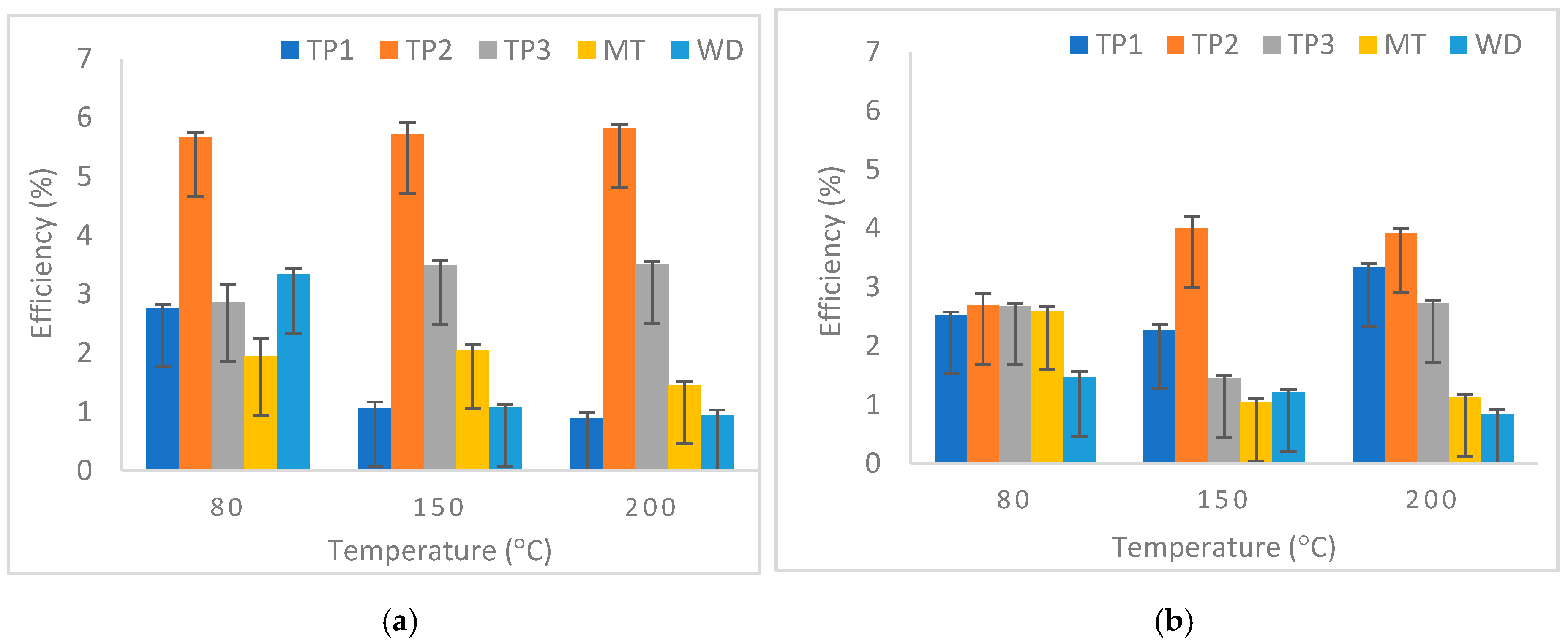
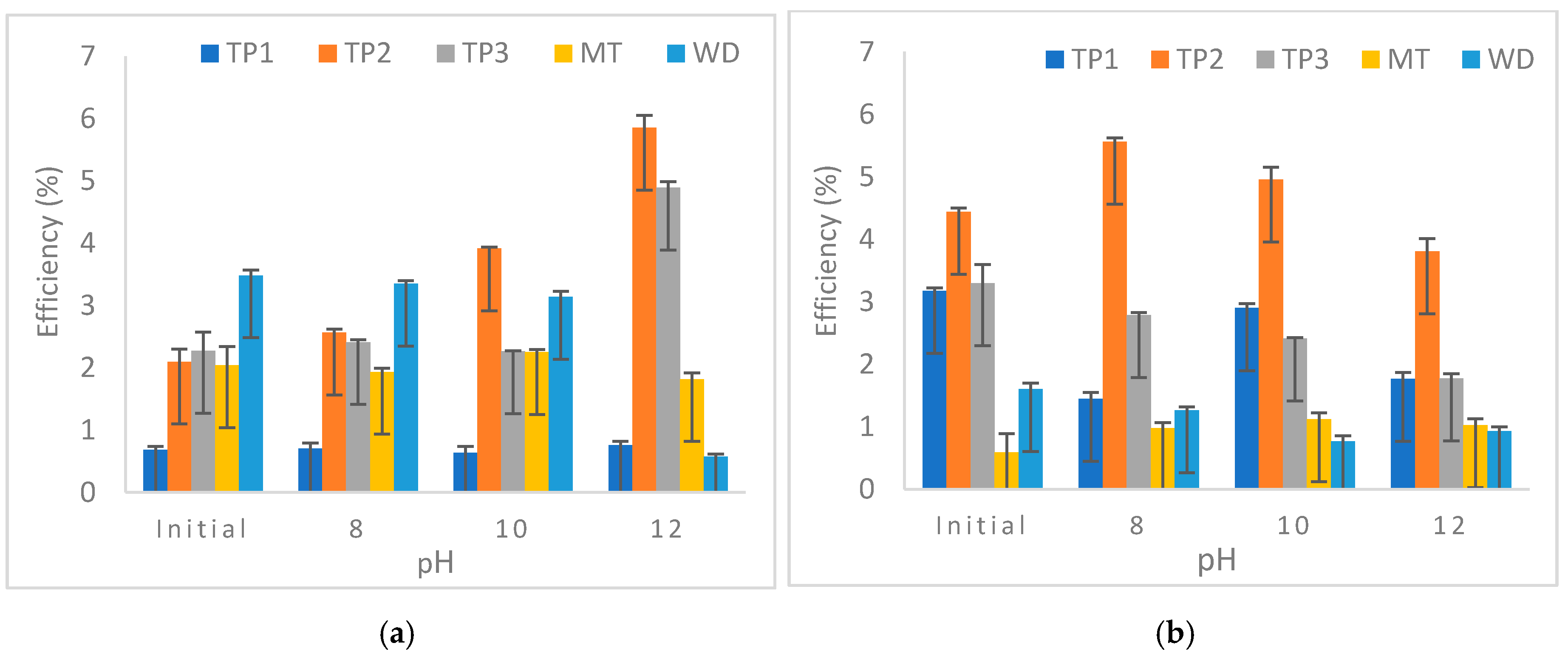
3.3. Factors Influencing the Mineral Carbonation Process of Iron Mining Waste
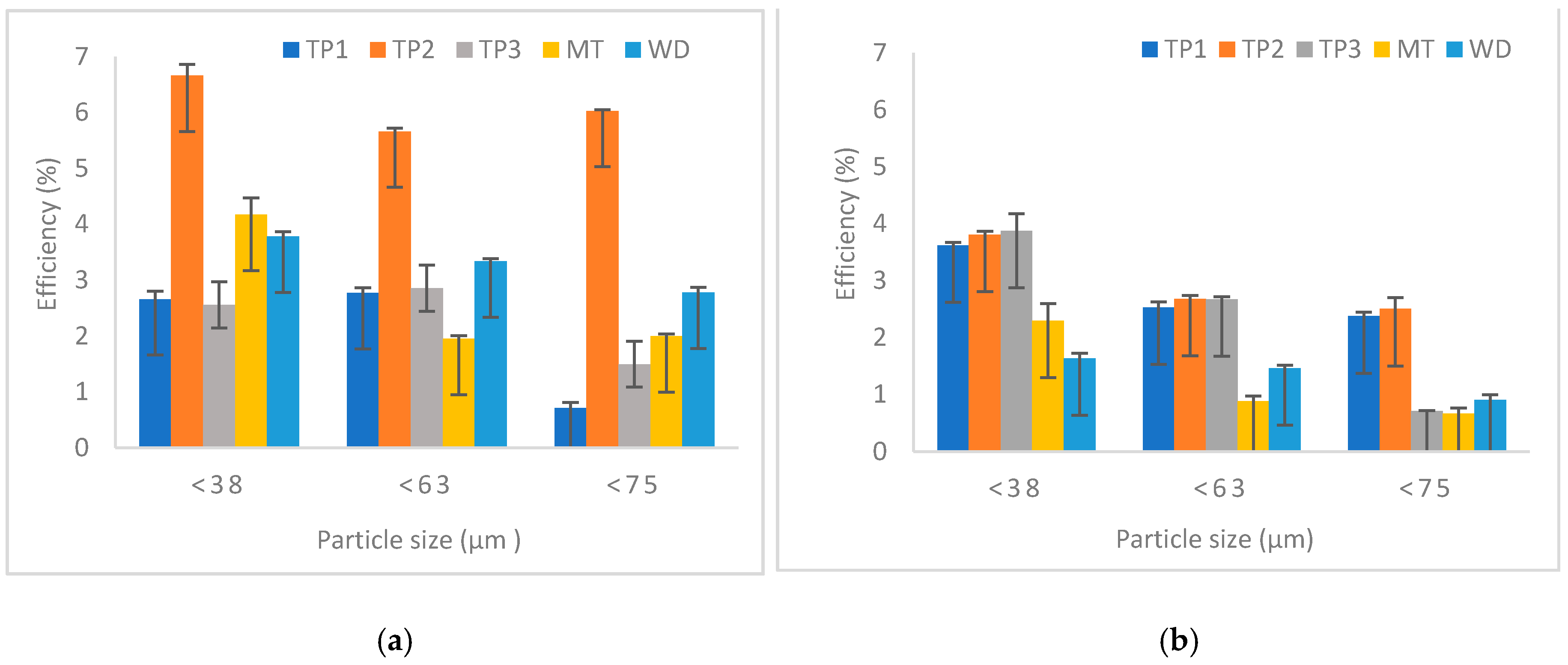
3.3.1. Effect of Particle Size
3.3.2. Effect of Temperature
3.3.3. Effect of pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majid, A.A.; Shaharudin, H.M.; Alias, S.; Adnan, E.; Hassan, A.I.A.; Ali, M.Z. Malaysian Mining Industry; Minerals and Geoscience Department Malaysia: Kuala Lumpur, Malaysia, 2013; p. 152. [Google Scholar]
- Kusin, F.M.; Awang, N.H.C.; Hasan, S.N.M.S.; Rahim, H.A.A.; Azmin, N.; Jusop, S.; Kim, K.W. Geo-ecological evaluation of mineral, major and trace elemental composition in waste rocks, soils and sediments of a gold mining area and potential associated risks. Catena 2019, 183, 104229. [Google Scholar] [CrossRef]
- Kusin, F.M.; Azani, N.N.M.; Hasan, S.N.M.S.; Sulong, N.A. Distribution of heavy metals and metalloid in surface sediments of heavily-mined area for bauxite ore in Pengerang, Malaysia and associated risk assessment. Catena 2018, 165, 454–464. [Google Scholar] [CrossRef]
- Halim, N.A.; Kusin, F.M.; Mohamed K, N. Heavy metals exposure from co-processing of hazardous wastes and associated risk assessment. Int. J. Environ. Sci. Technol. 2018, 15, 733–742. [Google Scholar] [CrossRef]
- Kiventerä, J.; Piekkari, K.; Isteri, V.; Ohenoja, K.; Tanskanen, P.; Illikainen, M. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement. J. Clean. Prod. 2019, 239, 118008. [Google Scholar] [CrossRef]
- Molahid, V.L.M.; Kusin, F.M.; Madzin, Z. Role of multiple substrates (spent mushroom compost, ochre, steel slag and limestone) in passive remediation of metal-containing acid mine drainage. Environ. Technol. 2019, 40, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Kusin, F.M.; Akhir, N.I.M.; Mohamat-Yusuff, F.; Awang, M. Greenhouse gas emissions during plantation stage of palm oil-based biofuel production addressing different land conversion scenarios in Malaysia. Environ. Sci. Pollut. Res. 2017, 24, 5293–5304. [Google Scholar] [CrossRef] [PubMed]
- Kusin, F.M.; Akhir, N.I.M.; Mohamat-Yusuff, F.; Awang, M. Nitrous oxide emission from nitrogen fertiliser application in oil palm plantation of different stages. Int. J. Glob. Warm. 2016, 9, 529–541. [Google Scholar] [CrossRef]
- Hasan, S.N.M.S.; Kusin, F.M. Potential of mining waste from metallic mineral industry for carbon sequestration. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012013. [Google Scholar] [CrossRef]
- Ibrahim, M.; El-Naas, M.; Benamor, A.; Al-Sobhi, S.; Zhang, Z. Carbon mineralization by reaction with steel-making waste: A review. Processes 2019, 7, 115. [Google Scholar] [CrossRef]
- Hasan, S.N.M.S.; Kusin, F.M.; Jusop, S.; Mohamat-Yusuff, F. The mineralogy and chemical properties of sedimentary waste rocks with carbon sequestration potential at Selinsing Gold Mine, Pahang. Pertanika J. Sci. Technol. 2019, 27, 1005–1012. [Google Scholar]
- Li, J.; Hitch, M. Mechanical activation of Magnesium Silicates for mineral carbonation, a review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- O’Conner, W.K.; Dahlin, D.C.; Nilsen, D.N.; Rush, G.E.; Walters, R.P.; Turner, P.C. Carbon Dioxide Sequestration by Direct Mineral Carbonation: Results from Recent Studies and Current Status. 2001. Available online: https://www.osti.gov/biblio/897125-carbon-dioxide-sequestration-direct-mineral-carbonation-results-from-recent-studies-current-status (accessed on 1 March 2019).
- Ohenoja, K.; Rissanen, J.; Kinnunen, P.; Illikainen, M. Direct carbonation of peat-wood fly ash for carbon capture and utilization in construction application. J. CO2 Util. 2020, 40, 101203. [Google Scholar] [CrossRef]
- Guillot, L.M.; Lloret, P.A.; Velasco, A.; Martinez, A.F.; Agudo, E.R.; Navarro, C.R. CO2 sequestration and simultaneous zeolite production by carbonation of coal fly ash: Impact on the trapping of toxic elements. J. CO2 Util. 2020, 40, 101263. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Kohlmann, J.; Mukherjee, A.B. Direct dry mineral carbonation for CO2 emissions reduction in Finland. In Proceedings of the 27th International Conference Coal Utilization Fuel Systems, Clearwater, FL, USA, 4–7 March 2002; pp. 743–754. [Google Scholar]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation: Literature review update 2003–2004; Energy Research Centre of The Netherlands: Petten, The Netherlands, 2005; ECN-C--05-022. [Google Scholar]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Influence of particle size on the carbonation of stainless steel slag for CO2 storage. Energy Procedia 2009, 1, 4859–4866. [Google Scholar] [CrossRef]
- Azdarpour, A.; Karaei, M.A.; Hamidi, H.; Mohammadian, E.; Honarvar, B. CO2 sequestration through direct aqueous mineral carbonation of red gypsum. Petroleum 2018, 4, 398–407. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Zhao, Y.; Liu, R.; Zheng, C. CO2 Sequestration by direct aqueous mineral carbonation under low-medium pressure conditions. J. Chem. Eng. Jpn. 2015, 48, 937–946. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Wang, Q.; Deng, J.; Liu, X.; Wang, J. Mineralogical and Geochemical Features of Karst Bauxites from Poci (Western Henan, China), Implications for Parental Affinity And Bauxitization. Ore Geol. Rev. 2019, 105, 295–309. [Google Scholar] [CrossRef]
- Jacobs, A.D. Quantifying the Mineral Carbonation Potential of Mine Waste Mineral: A New Parameter for Geospatial Estimation. Ph.D. Thesis, Faculty of Graduate and Postdoctoral Studies, (Mining Engineering), University of Columbia, Vancouver, CO, Canada, October 2014; p. 232. [Google Scholar]
- Kakizawa, M.; Yamasaki, A.; Yanagisawa, Y. A new CO2 disposal process via artificial weathering of calcium silicate accelerated by acetic acid. Energy 2001, 26, 341–354. [Google Scholar] [CrossRef]
- Yogo, K.; Eikou, T.; Tateaki, Y. Method for Fixing Carbon Dioxide. U.S. Patent JP2005097072, 14 April 2005. [Google Scholar]
- Sipilä, J.; Teir, S.; Zevenhoven, R. Carbon Dioxide Sequestration by Mineral Carbonation Literature Review Update 2005–2007. 2008. Available online: https://remineralize.org/wp-content/uploads/2015/10/LITR1.pdf (accessed on 16 June 2018).
- Teir, S.; Eloneva, S.; Fogelholm, C.J.; Zevenhoven, R. Dissolution of steelmaking slags in acetic acid for precipitated calcium carbonate production. Energy 2007, 32, 528–539. [Google Scholar] [CrossRef]
- Ding, W.; Chen, Q.; Sun, H.; Peng, T. Modified mineral carbonation of phosphogypsum for CO2 sequestration. J. CO2 Util. 2019, 34, 507–515. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Junin, R.; Manan, M.; Hamidi, H.; Mohammadian, E. Direct carbonation of red gypsum to produce solid carbonates. Fuel Process. Technol. 2014, 126, 429–434. [Google Scholar] [CrossRef]
- Park, A.H.A.; Fan, L.S. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Han, S.; Im, H.J.; Wee, J. Leaching and indirect mineral carbonation performance of coal fly ash-water solution system. Appl. Energy 2015, 142, 274–282. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Li, X. Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Meas. J. Int. Meas. Confed. 2017, 97, 15–22. [Google Scholar] [CrossRef]
- BS 1377-3. Method of Test for Soils for Civil Engineering Purpose-Part 3: Chemical and Electro-chemical Test; British Standards: London, UK, 1990. [Google Scholar]
- Kusin, F.M.; Hasan, S.N.M.S.; Hassim, M.A.; Molahid, V.L.M. Mineral carbonation of sedimentary mine waste for carbon sequestration and potential reutilization as cementitious material. Environ. Sci. Pollut. Res. 2020, 27, 12767–12780. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.B.S.; Talib, J. Particle-Size Analysis. In Soil Physics Analyses. Serdang; Universiti Putra Malaysia Press: Kuala Lumpur, Malaysia, 2006; pp. 1–6. [Google Scholar]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. Dynamics of carbon dioxide uptake in chrysotile mining residues—Effect of mineralogy and liquid saturation. Int. J. Greenh. Gas Control. 2013, 12, 124–135. [Google Scholar] [CrossRef]
- Harrison, A.L.; Power, I.M.; Dipple, G.M. Accelerated carbonation of brucite in mine tailings for carbon sequestration. Environ. Sci. Technol. 2013, 47, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, O.; Highfield, J.; Junin, R.; Tyrer, M.; Azdarpour, A.B. Experimental investigation and simplistic geochemical modeling of CO2 mineral carbonation using the mount tawai peridotite. Molecules 2016, 21, 353. [Google Scholar] [CrossRef]
- Hasan, S.N.M.; Kusin, F.M.; Jusop, S.; Yusuff, F.M. Potential of soil, sludge and sediment for mineral carbonation process in Selinsing gold mine, Malaysia. Minerals 2018, 8, 257. [Google Scholar] [CrossRef]
- Chang, R.; Kim, S.; Lee, S.; Choi, S.; Kim, M.; Park, Y. Calcium carbonate precipitation for CO2 storage and utilization: A review of the carbonate crystallization and polymorphism. Front. Energy Res. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Junin, R.; Hamidi, H.; Manan, M.; Mohamad-Daud, A.R. Mineral carbonation of red gypsum via pH-swing process: Effect of CO2 pressure on the efficiency and products characteristics. Chem. Eng. J. 2015, 264, 425–436. [Google Scholar] [CrossRef]
- Wang, D.; Chang, J.; Ansari, W.S. The effects of carbonation and hydration on the mineralogy and microstructure of basic oxygen furnace slag products. J. CO2 Util. 2019, 34, 87–98. [Google Scholar] [CrossRef]



| Type of Variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | Particle Size (µm) | Temperature (°C) | ||||||||
| Operating variables | Actual pH | 8 | 10 | 12 | <38 | <63 | <75 | 80 | 150 | 200 |
| Constant parameters | Particle size = 63 µm Temperature = 80 °C | pH = 8.5 Temperature = 80 °C | pH = 8.5 Particle size = 63 µm | |||||||
| Particle Size Distribution (%) | Soil Texture Class | |||
|---|---|---|---|---|
| Sample | Clay | Silt | Sand | (USDA) |
| (<2 µm) | (2–50 µm) | (>50 µm) | ||
| Tailing pond 1 | 0.23 | 2.97 | 96.80 | Sand |
| Tailing pond 2 | 30.36 | 43.98 | 25.66 | Clay loam |
| Tailing pond 3 | 11.00 | 22.27 | 66.74 | Sandy loam |
| Mine tailing | 6.80 | 11.37 | 81.84 | Loamy sand |
| No. | Minerals | Sample | ||||
|---|---|---|---|---|---|---|
| Tailing Pond 1 | Tailing Pond 2 | Tailing Pond 3 | Mine Tailing | Waste Dump | ||
| 1. | Quartz, SiO2 | √ | √ | √ | √ | √ |
| 2. | Anorthite, CaAl2Si2O8 | √ | √ | √ | √ | |
| 3. | Titanium Silicon, TiSi2 | √ | √ | |||
| 4. | Wollastonite, CaSiO3 | √ | ||||
| 5. | Diopside, (Ca(Mg,Al)(Si, Al)2O6) | √ | ||||
| 6. | Perovskite, Ca(TiO3) | √ | ||||
| 7. | Johannsenite, Ca4Mn4Si8O24 | √ | ||||
| 8. | Magnesium Aluminum Silicate, MgO·Al2O3· SiO2 | √ | ||||
| 9. | Magnetite, Fe3O4 | √ | √ | |||
| Oxide Elements | Percentage of Oxides (%) | ||||
|---|---|---|---|---|---|
| Tailing Pond 1 | Tailing Pond 2 | Tailing Pond 3 | Mine Tailing | Waste Dump | |
| SiO2 | 15.93 | 21.31 | 20.47 | 14.05 | 34.97 |
| Fe2O3 * | 61.68 | 62.95 | 61.05 | 57.79 | 39.58 |
| CaO * | 9.57 | 7.19 | 11.52 | 15.24 | 10.16 |
| MgO * | 3.50 | 2.72 | 1.32 | 6.75 | 2.10 |
| K2O | 0.97 | 1.47 | 1.49 | 1.06 | 5.31 |
| TiO2 | 0.86 | 1.45 | 0.97 | 0.77 | 1.79 |
| SO3 | 3.95 | 1.31 | 1.09 | 2.96 | 1.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, N.A.A.; Kusin, F.M.; Molahid, V.L.M. Influencing Factors of the Mineral Carbonation Process of Iron Ore Mining Waste in Sequestering Atmospheric Carbon Dioxide. Sustainability 2021, 13, 1866. https://doi.org/10.3390/su13041866
Ramli NAA, Kusin FM, Molahid VLM. Influencing Factors of the Mineral Carbonation Process of Iron Ore Mining Waste in Sequestering Atmospheric Carbon Dioxide. Sustainability. 2021; 13(4):1866. https://doi.org/10.3390/su13041866
Chicago/Turabian StyleRamli, Noor Allesya Alis, Faradiella Mohd Kusin, and Verma Loretta M. Molahid. 2021. "Influencing Factors of the Mineral Carbonation Process of Iron Ore Mining Waste in Sequestering Atmospheric Carbon Dioxide" Sustainability 13, no. 4: 1866. https://doi.org/10.3390/su13041866
APA StyleRamli, N. A. A., Kusin, F. M., & Molahid, V. L. M. (2021). Influencing Factors of the Mineral Carbonation Process of Iron Ore Mining Waste in Sequestering Atmospheric Carbon Dioxide. Sustainability, 13(4), 1866. https://doi.org/10.3390/su13041866





