Variations of Structural and Functional Traits of Azolla pinnata R. Br. in Response to Crude Oil Pollution in Arid Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Acclimation and Growth Conditions of Azolla Pinnata
2.3. Petroleum Oil Pollution Treatment
2.4. Plant Cover
2.5. Photosynthetic Pigments
2.6. Genotoxic Effects of Crude Petroleum Oil
2.7. Plant Anatomy and Chloroplast Structure
2.8. Statistical Analysis
3. Results
3.1. Plant Cover
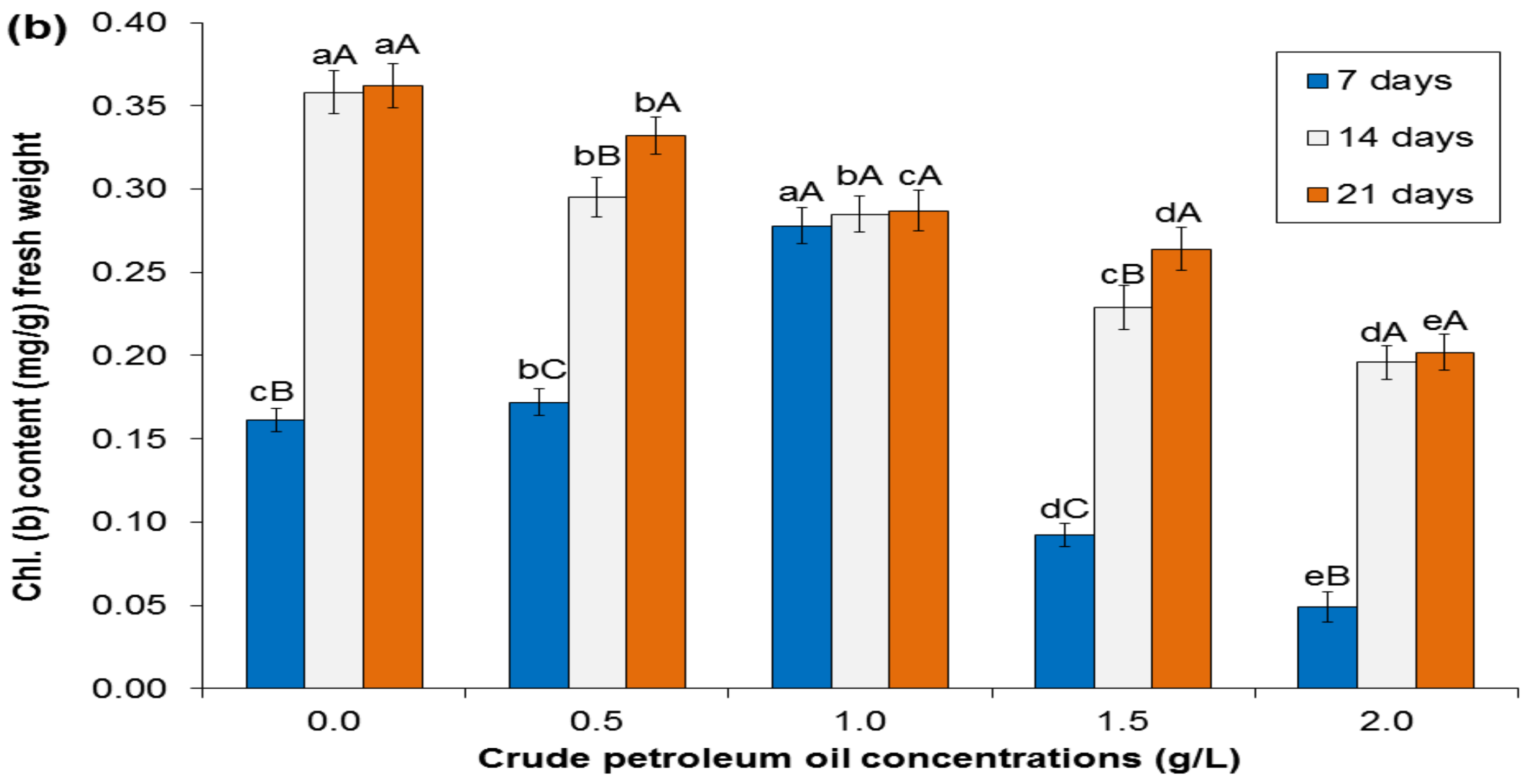
3.2. Chlorophyll Content
3.3. Carotenoid Content and Comet Image
3.4. Anatomical Structure of the Frond
3.5. The Ultrastructure of Frond Chloroplasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eshagberi, G.O. Toxic Effects of Water Soluble Fractions of Crude Oil, Diesel and Gasoline on Ceratophyllum Demersum. Int. J. Health Med. 2017, 2, 2518-0630. [Google Scholar] [CrossRef][Green Version]
- Margesin, R.; Walder, G.; Schinner, F. Bioremediation Assessment of a BTEX-Contaminated Soil. Acta Biotechnol. 2003, 23, 29–36. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Diekmann, M.; Ayad, G. Impact of plant invasions on ecosystems and native gene pools. In Environment 2000 and Beyond; Hegazy, A.K., Ed.; Horus for Computer and Printing: Cairo, Egypt, 1999. [Google Scholar]
- Mendelssohn, I.A.; Andersen, G.L.; Baltz, D.M.; Caffey, R.H.; Carman, K.R.; Fleeger, J.W.; Joye, S.B.; Lin, Q.; Maltby, E.; Overton, E.B.; et al. Oil Impacts on Coastal Wetlands: Implications for the Mississippi River Delta Ecosystem after the Deepwater Horizon Oil Spill. Bioscience 2012, 62, 562–574. [Google Scholar] [CrossRef]
- Yavari, S.; Malakahmad, A.; Sapari, N.B. A Review on Phytoremediation of Crude Oil Spills. Water Air Soil Pollut. 2015, 226, 1–18. [Google Scholar] [CrossRef]
- Williams, S.D.; Ladd, D.E.; Farmer, J. Fate and Transport of Petroleum Hydrocarbons in Soil and Ground Water at Big South Fork National River and Recreation Area, Tennessee and Kentucky, 2002–2003; US Department of the Interior, US Geological Survey: Washington, DC, USA, 2006; pp. 2005–5104. [Google Scholar]
- Venosa, A.D.; Zhu, X. Biodegradation of Crude Oil Contaminating Marine Shorelines and Freshwater Wetlands. Spill Sci. Technol. Bull. 2003, 8, 163–178. [Google Scholar] [CrossRef]
- Lee, R.F.; Page, D.S. Petroleum hydrocarbons and their effects in subtidal regions after major oil spills. Mar. Pollut. Bull. 1997, 34, 928–940. [Google Scholar] [CrossRef]
- Kingston, P.F. Long-term Environmental Impact of Oil Spills. Spill Sci. Technol. Bull. 2002, 7, 53–61. [Google Scholar] [CrossRef]
- Moubasher, H.; Hegazy, A.K.; Mohamed, N.H.; Moustafa, Y.; Kabiel, H.; Hamad, A. Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Das, K.; Mukherjee, A.K. Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from North-East India. Bioresour. Technol. 2007, 98, 1339–1345. [Google Scholar] [CrossRef]
- Lin, Q.; Mendelssohn, I.A. Evaluation of tolerance limits for restoration and phytoremediation with spartina alterniflorain crude oil-contaminated coastal salt marshes. Int. Oil Spill Conf. Proc. 2008, 2008, 869–873. [Google Scholar] [CrossRef]
- Ibañez, S.G.; Paisio, C.E.; Wevar Oller, A.L.; Talano, M.A.; González, P.S.; Medina, M.I.; Agostini, E. Overview and new insights of genetically engineered plants for improving phytoremediation. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 1. [Google Scholar]
- Jan, S.; Parray, J.A. Biodiversity Prospecting for Phytoremediation of Metals in the Environment. In Approaches to Heavy Metal Tolerance in Plants; Springer International Publishing: Cham, Switzerland, 2016; pp. 103–110. [Google Scholar]
- Kösesakal, T.; Ünlü, V.S.; Külen, O.; Memon, A.; Yuksel, B. Evaluation of the phytoremediation capacity of Lemna minor L. incrude oil spiked cultures. Turk. J. Boil. 2015, 39, 479–484. [Google Scholar] [CrossRef]
- Azab, E.; Hegazy, A.K.; Gobouri, A.A.; Elkelish, A. Impact of Transgenic Arabidopsis thaliana Plants on Herbicide Isoproturon Phytoremediation through Expressing Human Cytochrome P450-1A2. Biology 2020, 9, 362. [Google Scholar] [CrossRef]
- Kebeish, R.; Azab, E.; Peterhaensel, C.; El-Basheer, R. Engineering the metabolism of the phenylurea herbicide chlortoluron in genetically modified Arabidopsis thaliana plants expressing the mammalian cytochrome P450 enzyme CYP1A2. Environ. Sci. Pollut. Res. 2014, 21, 8224–8232. [Google Scholar] [CrossRef] [PubMed]
- Azab, E.; Hegazy, A.K.; El-Sharnouby, M.E.; ElSalam, H.E.A. Phytoremediation of the organic Xenobiotic simazine by p450-1a2 transgenicArabidopsis thalianaplants. Int. J. Phytoremediation 2016, 18, 738–746. [Google Scholar] [CrossRef]
- Azab, E.; Kebeish, R.; Hegazy, A. Expression of the human gene CYP1A2 enhances tolerance and detoxification of the phenylurea herbicide linuron in Arabidopsis thaliana plants and Escherichia coli. Environ. Pollut. 2018, 238, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Azab, E.; Soror, A.-F.S. Physiological Behavior of the Aquatic Plant Azolla sp. in Response to Organic and Inorganic Fertilizers. Plants 2020, 9, 924. [Google Scholar] [CrossRef]
- Sood, A.; Uniyal, P.L.; Prasanna, R.; Ahluwalia, A.S. Phytoremediation Potential of Aquatic Macrophyte, Azolla. Ambio 2011, 41, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L. Flora of Egypt Checklist, Revised Annotated Edition; Al Hadara Publishing: Cairo, Egypt, 2009. [Google Scholar]
- Yoshida, S.; Institute, I.R.R. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Los Baños, Philippines, 1976. [Google Scholar]
- Zhu, J.; Peng, Z.; Liu, X.; Deng, J.; Zhang, Y.; Hu, W. Response of Aquatic Plants and Water Quality to Large-Scale Nymphoides peltata Harvest in a Shallow Lake. Water 2019, 11, 77. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagenes. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy Principles and Techniques for Biologists. Available online: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=25575 (accessed on 1 March 1991).
- Baruah, P.; Saikia, R.R.; Baruah, P.P.; Deka, S. Effect of crude oil contamination on the chlorophyll content and morpho-anatomy of Cyperus brevifolius (Rottb.) Hassk. Environ. Sci. Pollut. Res. 2014, 21, 12530–12538. [Google Scholar] [CrossRef] [PubMed]
- Gilde, K.; Pinckney, J.L. Sublethal Effects of Crude Oil on the Community Structure of Estuarine Phytoplankton. Chesap. Sci. 2012, 35, 853–861. [Google Scholar] [CrossRef]
- Atta, A.M.; Mohamed, N.H.; Hegazy, A.K.; Moustafa, Y.M.; Mohamed, R.R.; Safwat, G.; Diab, A.A. Green Technology for Remediation of Water Polluted with Petroleum Crude Oil: Using of Eichhornia crassipes (Mart.) Solms Combined with Magnetic Nanoparticles Capped with Myrrh Resources of Saudi Arabia. Nanomaterials 2020, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Al-Baldawi, I.A.; Abdullah, S.; Suja, F.; Anuar, N.; Idris, M. Preliminary Test of Hydrocarbon Exposure on Azolla pinnata in Phytoremediation Process. Available online: http://www.ipcbee.com/vol33/047-ICEEB2012-FB015.pdf (accessed on 10 October 2020).
- Pokora, W.; Tukaj, Z. The combined effect of anthracene and cadmium on photosynthetic activity of three Desmodesmus (Chlorophyta) species. Ecotoxicol. Environ. Saf. 2010, 73, 1207–1213. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 6. [Google Scholar] [CrossRef]
- Fahmy, G.M.; Hegazy, A.K.; Hassan, H.T. Phenology, Pigment Content and Diurnal Change of Proline in Green and Senescing Leaves of Three Zygophyllum Species. Flora 1990, 184, 423–436. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Alzurfi, S.K.L.; Alasedi, K.K.; Abdulraheem, N.I. Effect different concentrations of crude oil on the pigment content and protein content of Hydrilla verticillata plant. Iraqi J. Sci. 2019, 60, 2141–2148. [Google Scholar]
- Odukoya, J.; Lambert, R.; Sakrabani, R. Impact of Crude Oil on Yield and Phytochemical Composition of Selected Green Leafy Vegetables. Int. J. Veg. Sci. 2019, 25, 554–570. [Google Scholar] [CrossRef]
- Noori, A.; Maivan, H.Z.; Alaie, E.; Newman, L.A. Leucanthemum vulgare lam. crude oil phytoremediation. Int. J. Phytoremediation 2018, 20, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Zakari, S.A.; Asad, M.-A.-U.; Han, Z.; Zhao, Q.; Cheng, F. Relationship of Nitrogen Deficiency-Induced Leaf Senescence with ROS Generation and ABA Concentration in Rice Flag Leaves. J. Plant Growth Regul. 2020, 39, 1503–1517. [Google Scholar] [CrossRef]
- Shi, X.; Wu, H.; Shi, J.; Xue, S.J.; Wang, D.; Wang, W.; Cheng, A.; Gong, Z.; Chen, X.; Wang, C. Effect of modifier on the composition and antioxidant activity of carotenoid extracts from pumpkin (Cucurbita maxima) by supercritical CO2. LWT 2013, 51, 433–440. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Wang, H.; Zhan, X.; Xing, B. Carotenoid and superoxide dismutase are the most effective antioxidants participating in ROS scavenging in phenanthrene accumulated wheat leaf. Chemosphere 2018, 197, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Pilon, C.; Soratto, R.P.; Broetto, F.; Fernandes, A.M. Foliar or Soil Applications of Silicon Alleviate Water-Deficit Stress of Potato Plants. Agron. J. 2014, 106, 2325–2334. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Serpeloni, J.M.; Barcelos, G.R.M.; Angeli, J.P.F.; Mercadante, A.Z.; Bianchi, M.L.P.; Antunes, L.M.G. Dietary carotenoid lutein protects against DNA damage and alterations of the redox status induced by cisplatin in human derived HepG2 cells. Toxicol. Vitr. 2012, 26, 288–294. [Google Scholar] [CrossRef]
- Ben Ghnaya, A.; Charles, G.; Hourmant, A.; Ben Hamida, J.; Branchard, M. Physiological behaviour of four rapeseed cultivar (Brassica napus L.) submitted to metal stress. Comptes Rendus Biol. 2009, 332, 363–370. [Google Scholar] [CrossRef]
- Balliana, A.G.; Moura, B.B.; Inckot, R.C.; Bona, C. Development of Canavalia ensiformis in soil contaminated with diesel oil. Environ. Sci. Pollut. Res. 2016, 24, 979–986. [Google Scholar] [CrossRef]
- Kumar, D.; Kaatánek, P.; Adhikary, S.P. Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr. Sci. 2018, 115, 234. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Mondal, A.; Bhunia, B.; Bandyopadhyay, T.K.; Jaladi, P.; Oinam, G.; Indrama, T. Purification, characterization and biotechnological potential of new exopolysaccharide polymers produced by cyanobacterium Anabaena sp. CCC 745. Polymer 2019, 178, 121695. [Google Scholar] [CrossRef]
- Sikorski, Z.; Kolakowska, A. Chemical, Biological, and Functional Aspects of Food Lipids; CRC Press: Boca Raton, FL, USA, 2011; p. 512. [Google Scholar] [CrossRef]
- Skała, E.; Sitarek, P.; Różalski, M.; Krajewska, U.; Szemraj, J.; Wysokińska, H.; Śliwiński, T. Antioxidant and DNA Repair Stimulating Effect of Extracts from Transformed and Normal Roots ofRhaponticum carthamoidesagainst Induced Oxidative Stress and DNA Damage in CHO Cells. Oxid. Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Samary Atyah, B.; Al-Mayaly, I.K.A. Biodegradation of Crude Oil by Anabaena variabilis Isolated from Al-Dora Refinery. J. Pharm. Biol. Sci. 1018, 13, 51–56. [Google Scholar]
- Burritt, D.J. The polycyclic aromatic hydrocarbon phenanthrene causes oxidative stress and alters polyamine metabolism in the aquatic liverwortRiccia fluitansL. Plant Cell Environ. 2008, 31, 1416–1431. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Weisman, D.; Ye, Y.-B.; Cui, B.; Huang, Y.-H.; Colón-Carmona, A.; Wang, Z.-H. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 2009, 176, 375–382. [Google Scholar] [CrossRef]
- Uheda, E.; Maejima, K.; Shiomi, N. Localization of Glutamine Synthetase Isoforms in Hair Cells of Azolla Leaves. Plant Cell Physiol. 2004, 45, 1087–1092. [Google Scholar] [CrossRef]
- Oka, M.; Shimoda, Y.; Sato, N.; Inoue, J.; Yamazaki, T.; Shimomura, N.; Fujiyama, H. Abscisic acid substantially inhibits senescence of cucumber plants (Cucumis sativus) grown under low nitrogen conditions. J. Plant Physiol. 2012, 169, 789–796. [Google Scholar] [CrossRef]
- Meng, S.; Peng, J.-S.; He, Y.-N.; Zhang, G.-B.; Yi, H.-Y.; Fu, Y.-L.; Gong, J.-M. Arabidopsis NRT1.5 Mediates the Suppression of Nitrate Starvation-Induced Leaf Senescence by Modulating Foliar Potassium Level. Mol. Plant 2016, 9, 461–470. [Google Scholar] [CrossRef]
- Niittylä, T.; Messerli, G.; Trevisan, M.; Chen, J.; Smith, A.M.; Zeeman, S.C. A Previously Unknown Maltose Transporter Essential for Starch Degradation in Leaves. Science 2004, 303, 87–89. [Google Scholar] [CrossRef]
- Yu, T.-S.; Kofler, H.; Häusler, R.E.; Hille, D.; Flügge, U.-I.; Zeeman, S.C.; Smith, A.M.; Kossmann, J.; Lloyd, J.; Ritte, G.; et al. The Arabidopsis sex1 Mutant Is Defective in the R1 Protein, a General Regulator of Starch Degradation in Plants, and Not in the Chloroplast Hexose Transporter. Plant Cell 2001, 13, 1907–1918. [Google Scholar] [CrossRef]
- Edner, C.; Li, J.; Albrecht, T.; Mahlow, S.; Hejazi, M.; Hussain, H.; Kaplan, F.; Guy, C.; Smith, S.M.; Steup, M.; et al. Glucan, Water Dikinase Activity Stimulates Breakdown of Starch Granules by Plastidial β-Amylases. Plant Physiol. 2007, 145, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, M.; Fettke, J.; Haebel, S.; Edner, C.; Paris, O.; Frohberg, C.; Steup, M.; Ritte, G. Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J. 2008, 55, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.C.; Delatte, T.; Messerli, G.; Umhang, M.; Stettler, M.; Mettler, T.; Streb, S.; Reinhold, H.; Kötting, O. Starch breakdown: Recent discoveries suggest distinct pathways and novel mechanisms. Funct. Plant Biol. 2007, 34, 465–473. [Google Scholar] [CrossRef]
- Stettler, M.; Eicke, S.; Mettler, T.; Messerli, G.; Hörtensteiner, S.; Zeeman, S.C. Blocking the Metabolism of Starch Breakdown Products in Arabidopsis Leaves Triggers Chloroplast Degradation. Mol. Plant 2009, 2, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.R.; Frost, E.; Vidi, P.-A.; Kessler, F.; Staehelin, L.A. Plastoglobules Are Lipoprotein Subcompartments of the Chloroplast That Are Permanently Coupled to Thylakoid Membranes and Contain Biosynthetic Enzymes. Plant Cell 2006, 18, 1693–1703. [Google Scholar] [CrossRef]
- Besagni, C.; Kessler, F. A mechanism implicating plastoglobules in thylakoid disassembly during senescence and nitrogen starvation. Planta 2013, 237, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Gaude, N.; Bréhélin, C.; Tischendorf, G.; Kessler, F.; Dörmann, P. Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. Plant J. 2007, 49, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Biosynthesis, accumulation and emission of carotenoids, α-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance. Photosynth. Res. 2007, 92, 163–179. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, A.A.; Hafez, R.M.; Hegazy, A.K.; Fattah, A.M.A.-E.; Mohamed, N.H.; Mustafa, Y.M.; Gobouri, A.A.; Azab, E. Variations of Structural and Functional Traits of Azolla pinnata R. Br. in Response to Crude Oil Pollution in Arid Regions. Sustainability 2021, 13, 2142. https://doi.org/10.3390/su13042142
Mostafa AA, Hafez RM, Hegazy AK, Fattah AMA-E, Mohamed NH, Mustafa YM, Gobouri AA, Azab E. Variations of Structural and Functional Traits of Azolla pinnata R. Br. in Response to Crude Oil Pollution in Arid Regions. Sustainability. 2021; 13(4):2142. https://doi.org/10.3390/su13042142
Chicago/Turabian StyleMostafa, Aya A., Rehab M. Hafez, Ahmad K. Hegazy, Azza M. Abd-El Fattah, Nermen H. Mohamed, Yasser M. Mustafa, Adil A. Gobouri, and Ehab Azab. 2021. "Variations of Structural and Functional Traits of Azolla pinnata R. Br. in Response to Crude Oil Pollution in Arid Regions" Sustainability 13, no. 4: 2142. https://doi.org/10.3390/su13042142
APA StyleMostafa, A. A., Hafez, R. M., Hegazy, A. K., Fattah, A. M. A.-E., Mohamed, N. H., Mustafa, Y. M., Gobouri, A. A., & Azab, E. (2021). Variations of Structural and Functional Traits of Azolla pinnata R. Br. in Response to Crude Oil Pollution in Arid Regions. Sustainability, 13(4), 2142. https://doi.org/10.3390/su13042142






