1. Introduction
Concern about global warming has resulted in an effort to reduce the emissions of methane and other greenhouse gases (GHGs) associated with human activities. Landfills are the largest point source of human-related methane emissions in California, emitting an estimated 41% of total methane to the atmosphere [
1]. The Governor of California signed SB 1383 in 2016 to reduce the placement of food and other organic waste in landfills by 75% by the year 2025, based on the 2014 baseline [
2]. With the passage SB 1383, waste management providers are evaluating alternatives to the landfilling of source separated organics (SSOs).
Technologies that have been used to process SSO feedstocks include anaerobic digestion (AD), pyrolysis, and thermal conversion to biodiesel, depending on the desired end product. Of these technologies, anaerobic digestion is among the oldest and is used most commonly for the conversion of organic waste to a biofuel. To accommodate the SSO load, stand-alone digestion facilities are now being developed at landfills, transfer stations, and other strategic waste management locations. In addition, many medium and large-scale wastewater treatment plants with excess digestion capacity are accepting, or planning to accept, imported SSO feedstocks to generate revenue and increase biogas production.
Two important design and operational considerations in the application of AD for the processing of high nitrogen feedstocks are: (1) The discharge of high ammonium effluent and (2) the buildup of ammonia to inhibitory levels within the digester. Digester effluent with high ammonium concentrations can impact effluent discharge permits and increase costs for wastewater treatment, while inhibitory ammonia concentrations reduce methane production. The ionized ammonia (i.e., ammonium) that accumulates in anaerobic digesters has an equilibrium shift towards unionized ammonia as the pH and temperature increase [
3]. Ammonium and ammonia are both inhibitors of the AD process, but unionized ammonia is considered much more toxic than ammonium to the methanogenesis phase [
4,
5]. Ammonia levels should remain below 80 mg/L, while ammonium levels can range between 1500–3000 mg/L without inhibition [
6]. Typically, a high pH value is a self-limiting condition due to the fact that the inhibition of methanogens allows for an increase in volatile acids and the subsequent reduction in pH within the digester. Changes in the feedstock, retention time, and organic loading rate can also impact the digester pH.
The cost associated with the management of digester effluent with a high nitrogen content can be a barrier to the implementation of AD facilities. One potential alternative for the management of high ammonium effluent is to provide a degree of pretreatment such that the digester effluent can be used beneficially or returned to wastewater processing facilities without a negative impact. The performance of two pilot studies designed to remove ammonium from different types of digester effluents are presented and discussed in this paper.
2. Nitrogen in Concentrated Waste Streams
Within urban and surrounding areas there are a number of low water content organic waste streams, referred to as urban concentrates, which contain nutrients, energy, etc. Recovering and reusing products derived from these urban concentrates is a fundamental concept in developing a circular economy for sustainable waste management [
7]. One common example is the use of urban concentrates as feedstocks in AD processes for biogas production. Nitrogen released in the form of ammonium during AD has been identified as a challenge when returned to wastewater treatment processes, but also feasible for recovery. Sources, characteristics, and management of the nitrogen contained in concentrated urban feedstocks for AD are discussed in this section.
2.1. Sources of Concentrated AD Feedstocks
Within urban settings and the surrounding areas, large amounts of organic feedstocks are produced that could be used for biogas production. While there are many sources of potential feedstock for AD facilities, the need for odor and vector control, material handling and hauling, removal of contaminants, and pre-processing are common constraints. A summary of concentrated waste streams associated with urban areas that can be used as AD feedstock is presented in
Table 1.
2.2. Characteristics of Selected AD Feedstocks
Most concentrated feedstocks suitable for processing by anaerobic digestion have a high methane yield potential, but also have a high nitrogen content. Potential feedstock, as shown in
Table 2, can have a nitrogen content ranging from nearly 0 to more than 10%. During the digestion process, a portion of the nitrogen content from proteins, urea, and nucleic acids is released as ammonium into the solution, resulting in digester effluent flows with high ammonium concentrations. Ammonium nitrogen, the primary form of nitrogen present in anaerobic digesters, is a concern when these digester effluent flows are discharged to wastewater treatment facilities without pre-treatment [
3]. The nitrogen concentration in an AD system can be controlled partially by removing nitrogen containing feedstocks or by altering feedstock carbon to nitrogen (C:N) ratios [
8]. A high nitrogen feedstock can be blended and co-digested with low nitrogen materials to reduce the concentration of ammonium produced during the AD process [
9]. Other benefits of co-digestion include the dilution of the toxic compounds, pH adjustment, and control of solids loading [
10]. Feedstock blending can also have the benefit of supplementing limited trace elements, which may be deficient and could therefore inhibit the digestion process. However, feedstock blending will not be favorable to many facilities as it increases the operation cost for feed management, storage, pretreatment, and operations requirements [
3].
2.3. Management of Ammonium in the Waste Stream from AD
A variety of approaches have been applied for the management of digester effluent. Strategies for nitrogen removal potential in AD systems can be implemented for pre-treatment, in situ, or post-treatment of digester effluent [
12]. The most common approach for managing ammonium has been to simply blend the digester effluent with the influent to the local wastewater treatment process. Two problems associated with blending a concentrated effluent with influent wastewater is that the constituents (e.g., ammonia, phosphorus, etc.) in the digester effluent are diluted and potentially not recoverable, and the shock loading of ammonium can disrupt biological wastewater treatment processes. Pre-treatment processes that have been used for managing ammonium in digester effluent flows include physiochemical treatment with air or steam stripping in packed columns, and biological treatment consisting of nitrification-denitrification, nitritation-denitritation, or partial nitritation-anaerobic ammonium oxidation [
3]. While these technologies have been proven successful at various scales, the high capital and operation costs, overall energetics, chemical requirements, and maintenance needs make the implementation of these technologies infeasible in many facilities. It is further noted that the biological processes may be challenging to implement at full scale, are subject to upset conditions due to changes in loading or toxicity, are relatively expensive to operate, and cannot be used for nitrogen recovery [
13].
Examples of air and steam stripping in packed columns have been reported in the literature. However, chemical addition for pH adjustment has been required for high levels of ammonium removal [
14,
15,
16]. Air and steam stripping can be implemented either as a pre-treatment [
17] or as a post-treatment [
18,
19,
20]. To maintain the process pH for nitrogen recovery in conventional stripping, the addition of a base is needed with the most common example being sodium hydroxide (NaOH) as well as calcium oxide (CaO) [
3,
21]. Sodium hydroxide reacts with ammonium bicarbonate in the feed solution to create sodium carbonate, but at a higher relative operational cost. Calcium oxide is significantly cheaper, but may increase the fouling rate within the stripping column, resulting in added maintenance costs [
3]. Chemical addition for pH control increases the cost of ammonium recovery and salt concentration in effluent water. There is limited information in the literature on successful operation of ammonia stripping processes without pH adjustment.
The application of thermal stripping of ammonium from digester effluents, i.e., digestate and centrate, is considered in this paper. Previous lab-scale vacuum thermal stripping systems with acid absorption have been demonstrated for the recovery of ammonium from dairy manure digestate [
22,
23]. However, scaled up and continuous thermal stripping in a tray column under a vacuum for ammonium extraction from digester effluents has not been reported in the literature. Based on preliminary tests, it was determined that at elevated temperatures ammonium could be extracted from digestate and centrate directly, without the addition of caustic chemicals. It was also found that temperature control and level of vacuum were key parameters in controlling the process efficiency. The methodology and the results obtained are discussed in the following sections.
3. Methodology
Two pilot studies were conducted using a thermal stripping process designed for the extraction of ammonium from concentrated liquid and slurried waste streams. The process equipment used in this study, the waste characteristics, and the test parameters are discussed below. Results are presented in the following section.
3.1. Process Equipment and Experimental Setup
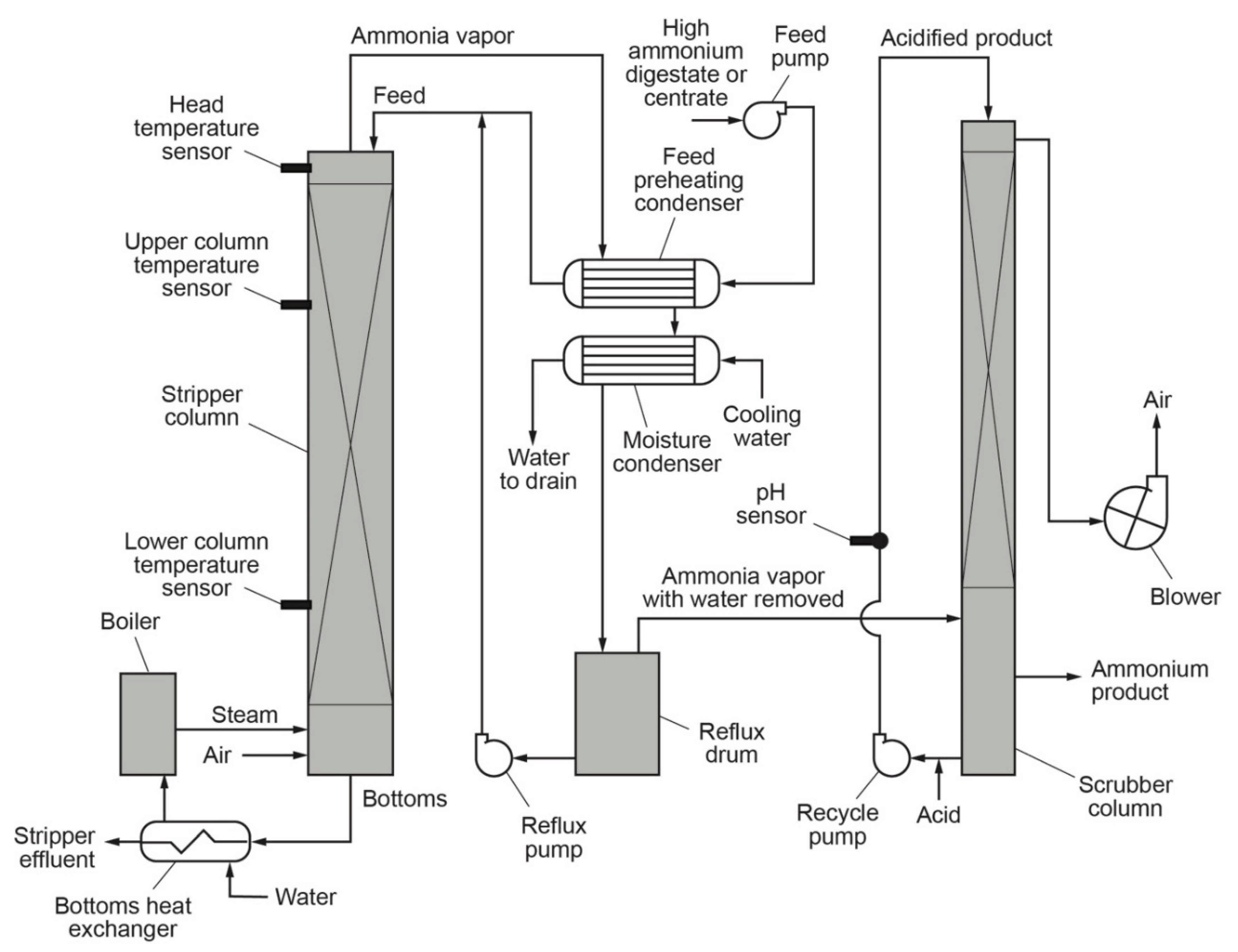
The process equipment, provided by Advanced Environmental Methods LLC, used to investigate the potential for nutrient recovery from digester return flows is shown schematically in
Figure 1. The raw digestate or centrate is delivered to the top of the stripping column and flows vertically down the column through a series of perforated trays or stages. Air and steam are introduced in the bottom of the column and flow upwards, counter to the feed flow. As the feed moves through the column, carbon dioxide vapor is released, causing the pH of the liquid to rise. With the increased pH, ammonia is released from the solution and swept out of the column. The vapor is absorbed in an acid scrubber using sulfuric acid or another acid, resulting in a strong ammonium solution. The treated effluent drains to the WWTP. During the stripping process, a portion of the alkalinity is removed as carbon dioxide. The remaining alkalinity discharged via the bottoms is minimal and can be used as an alkalinity source to buffer nitrification reactions in biological nutrient removal [
3].
3.2. Waste Characteristics
Feed samples consisted of screened final digestate from a thermophilic food waste digester (digestate) and centrate from a co-digestion process (centrate). The characteristics of the digestate samples and test parameters used to evaluate the thermal stripping process described in this study are summarized below.
Digestate Sample. The liquid digestate waste stream of interest in this study was obtained from treated digestate holding tanks. The digestate results from separate acid and methane phase fermentation, followed by solids separation in a screw press with a 1 mm screen. The digestate samples were collected and transported in 1000 L IBC tote containers to the pilot site location at the University of California Davis wastewater treatment plant (UCD WWTP). The characteristics of digestate used in this study are summarized in
Table 3.
Centrate Sample. The centrate sample was obtained from a facility that blends food residues and food processing waste with primary and secondary wastewater process solids in anaerobic digesters to increase the biogas production. A total of eleven centrate subsamples were collected sequentially from a centrate drain, analyzed for ammonium and conductivity, and then blended together for a composite feed sample (see
Table 3). One consequence of augmenting AD with food waste is increased nutrients, primarily ammonium, in digestate and centrate. As shown in
Figure 2, the centrate ammonium content ranged from around 400–1200 mg N/L and a correlation is apparent between EC and ammonium. Based on this correlation, it is expected that EC could be used for process monitoring.
3.3. Test Parameters
All the samples were evaluated at a process feed flowrate of 0.7 L/min (stripping column loading of 55 L/m
2-min). To evaluate the potential benefits of caustic addition (50% NaOH) for ammonia stripping, the test variables for the digestate study included caustic addition. Other parameters which were varied include the operational temperature at the lower temperature sensor (i.e., 50, 60, 70, 80, and 90 °C) and the vacuum applied (0.6 and 0.9 m of water column, measured at the blower inlet using a manometer). Loading parameters applied for the test samples are summarized in
Table 4. Preliminary studies with the pilot system were used for the determination of optimal design and loading parameters to maximize ammonia recovery. Results from these studies are outside the scope of this paper and not described further.
Process Temperature and Vacuum Setpoints. For the centrate samples, a range of process temperature and vacuum setpoints were used to characterize the process performance. The stripping process was evaluated at temperatures of 70, 78, 83, and 92 °C, while the vacuum was set at either 0.6 or 0.9 m of water column. The process was operated at a feed flowrate setting of 0.7 L/min for all testing of the centrate samples. The composite centrate was screened (6 mesh) prior to treatment in the stripping process.
Steam Usage. The steam usage is characterized using the feed to steam ratio (F/S), defined as the feed flowrate divided by the flow of water converted to steam. A high F/S value is favorable as it represents a lower relative energy input for steam production.
Sample Testing. The centrate and process bottom samples were tested individually for ammonium nitrogen, pH, and EC. Ammonium was measured by the Salicylate Method [
24], while EC and pH were determined in the field using a handheld meter (Ultrameter II, #6PFC).
4. Experimental Results
The findings from this study include observations related to performance with and without caustic addition to the digestate, without caustic addition to the centrate, at a range of temperatures, and at two different vacuum set points.
4.1. Ammonium Removal from the Digestate
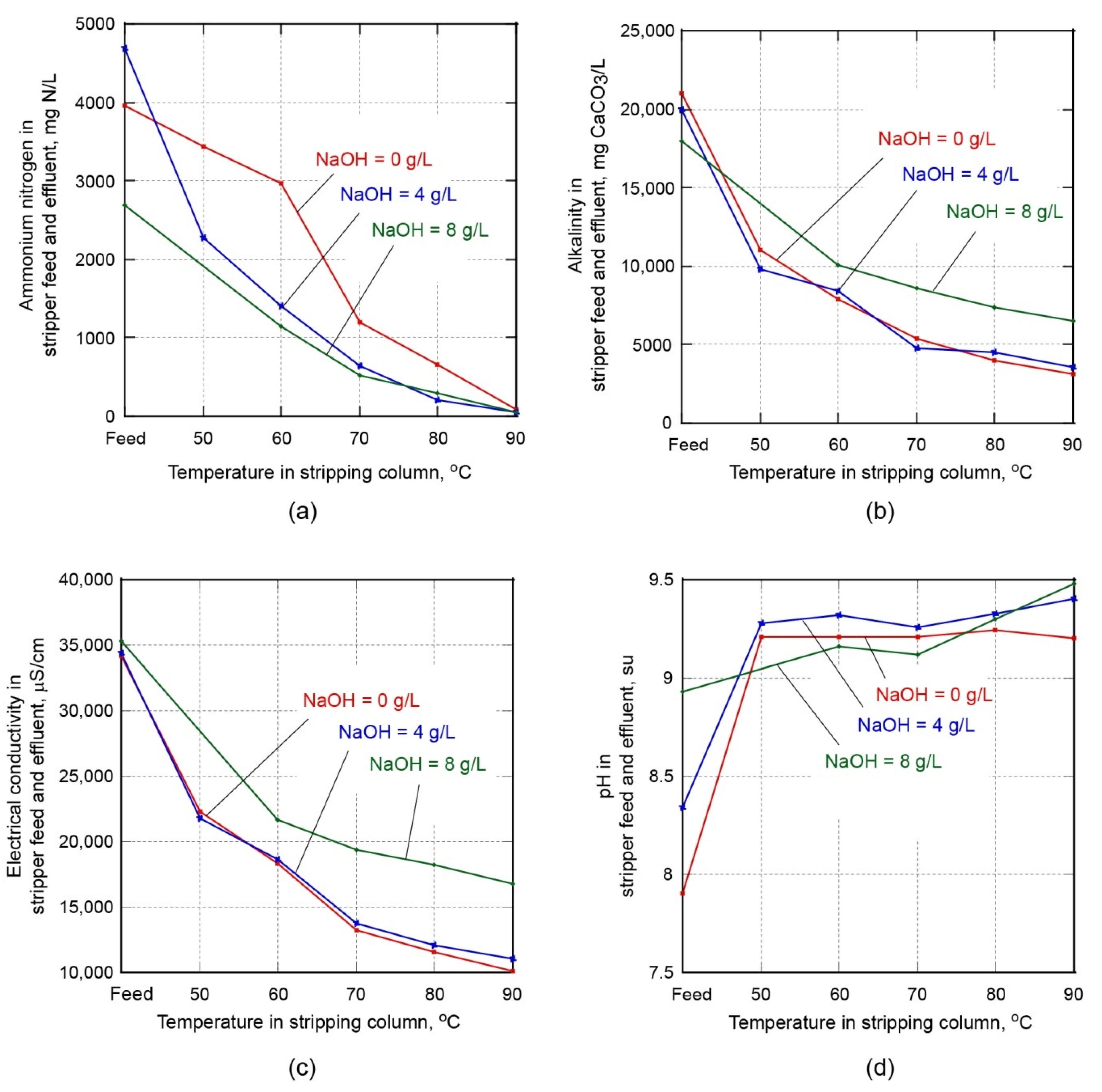
The testing of the digestate ammonia stripping process was focused on evaluating the operation at selected process temperatures at the lower column temperature sensor (50, 60, 70, 80, and 90 °C), a vacuum of 0.6 m water column, and three levels of feed caustic addition (0, 4, and 8 g NaOH/L). The results from the test conditions are summarized in
Figure 3. As shown in
Figure 3, the performance improved with increasing temperature and caustic addition. However, it was noted that around 90 °C the ammonium removal performance was not improved with the addition of caustic.
As shown in
Figure 3, several trends are apparent in the data obtained from the caustic addition pilot testing. The ammonium, alkalinity, and conductivity present in the feed solution were increasingly reduced at higher operating temperatures, as constituents volatilize and are stripped from the feed flow. The volatile constituents are entrained with the vapor phase and removed at the top of the column and condensed or captured in a downstream scrubber (see
Figure 1).
Based on the results of the caustic addition study, an operating temperature at the lower column temperature sensor of 85–90 °C without caustic addition was identified as a feasible operating point due to the potentially low operating costs associated with no chemical addition.
4.2. Ammonium Removal from the Centrate
The raw centrate subsamples had ammonium concentration values ranging from about 400–1300 mg N/L. The ammonium concentration of the composite feed was 950 mg N/L (historically, centrate N is 1400–1800 mg/L at this location). It is expected that the ammonium concentration varies in the centrate over time due to variations in digester operations and process water use during dewatering.
The range of operations applied during the pilot test are summarized in
Table 2. Steam is the source of process heat and is essential for proper operation. For the pilot test, the feed to steam ratio (F/S) varied from 3–6, representing a steam flowrate range of 0.23–0.11 L/min, respectively.
The thermal stripping process was found to be capable of removing a significant fraction of the centrate ammonium. For a process vacuum of 0.9 m water column, 90–98% of the centrate ammonium is removed at process temperatures ranging from 70–78 °C, respectively. Similarly, operations at a vacuum of 0.6 m water column ranged from 90–98% of ammonium N removal at temperatures ranging from 78–92 °C, respectively. The ammonium removal efficiency declined with increased F/S value and the corresponding reduction in process temperature.
4.3. Performance Summary
A summary of performance data from digestate and centrate pilot test studies is shown in
Figure 4. As shown in
Figure 4, the results from ammonium removal from both a concentrated food waste digestate and co-digestion centrate, without pH adjustment, can be described using the same curve. The application of increased vacuum in the column was found to improve the efficiency of ammonium recovery. It was also observed that elevated temperatures (>90 °C) and elevated vacuum (>0.9 m) are more likely to cause foaming in the column and should be avoided.
5. Discussion
The results obtained with the thermal stripping process are considered with respect to process performance, applications, and economics.
5.1. Process Performance
- -
The thermal stripping process is adaptable to rapid startup and shutdown and the process appears to be easy to control and not subject to upsets. The process can be tuned to remove nearly all or a portion of the digestate or centrate ammonium.
- -
The thermal stripping process was found to remove more than 90% of centrate and digestate ammonium when operated at a feed to steam ratio (F/S) less than 5 and a vacuum equal to or greater than 0.6 m water column.
- -
Process performance for the treatment of centrate is similar to the performance observed for the treatment of screened food waste digestate under similar conditions and without any caustic addition.
5.2. Process Applications and Economics
- -
The stripping process is applicable for the removal of ammonium from liquids and thin slurries without column fouling or chemical addition.
- -
The thermal process is operated at temperatures that will result in a high level of thermal disinfection and is suitable for meeting requirements for pasteurization of digestate.
- -
The ammonia vapor generated by the thermal stripping process is readily captured in various acids depending on the market opportunities. In the pilot study, sulfuric acid was used to collect ammonia vapor to form ammonium sulfate. For the final product, for each kilogram of nitrogen recovered, about 4.8 kg of ammonium sulfate (21% nitrogen and 24% sulfur) can be produced.
- -
With an acid scrubber, the thermal stripping process can produce a commercial ammonium fertilizer with a market value that can generate revenue. While nutrient recovery may be considered as an environmentally sustainable option for fertilizer production, the production process should also be operationally feasible and cost competitive with traditional chemical fertilizers. The economic viability of this process will be dependent on operating costs and market demand.
- -
Steam can be generated using waste heat or a portion of the biogas output from the digestion process.
- -
Ammonium recovery from digestate or centrate will enhance the performance of wastewater treatment processes where anaerobic digester flows are returned to the headworks. Removing a significant ammonium load from the wastewater treatment system will improve the overall economics of digestate management and improve the quality of effluent water.
6. Conclusions
Based on the results from two pilot studies, it has been demonstrated that thermal stripping processes can extract ammonium efficiently from concentrated liquid and slurried waste streams. It was determined that at elevated temperatures ammonium could be extracted from digestate and centrate directly, with limited pre-treatment and without the addition of caustic chemicals. It was also found that the process temperature and level of applied vacuum were key parameters in controlling the process efficiency. The key findings for the pilot studies are:
- -
Increasing the process temperature and addition of caustic both improved the ammonium extraction efficiency. However, the ammonium removal performance did not improve at process temperatures around 90 °C, even with the addition of caustic. At temperatures in excess of 90 °C, foaming was observed in the column.
- -
Ammonia recovery efficiency was improved by increasing the vacuum applied to the system. At a vacuum of 0.9 m water column, the thermal stripping process was able to recover 90–98% of the ammonium at process temperatures ranging from 70–78 °C, respectively. However, at a vacuum of 0.6 m water column the ammonium removal ranged from 90–98% at temperatures ranging from 78–92 °C, respectively. At elevated temperatures and a vacuum greater than 0.9 m water column, foaming was observed in the column.
- -
For the thermal stripping process under consideration in this paper, to achieve high levels of ammonium recovery from digester effluent without chemical addition to raise the pH value, it is necessary to operate at an increased vacuum and temperatures ranging between 85–90 °C.
By removing nitrogen and other constituents from the effluent of anaerobic digesters, it is technically and economically feasible to discharge the treated waste stream to a local wastewater collection system. Digestate nutrient recovery systems will make it possible to discharge the treated waste stream to wastewater collection systems such that small-scale anaerobic digestion facilities can be distributed throughout communities for the generation of electricity and resource recovery in a distributed system, reducing transportation costs. However, and most importantly, it will make available a process that can be used to reduce the discharge of SSO feedstock material to landfills, produce energy, and recover useful products.
Author Contributions
Conceptualization, H.L., R.A. and G.T.; methodology, R.A. and H.L.; software, H.L.; validation, J.H.; formal analysis, H.L. and G.T.; investigation, H.L. and J.H.; resources, R.A. and G.T.; data curation, H.L. and J.H.; writing—original draft preparation, H.L.; writing—review and editing, J.H., R.A. and G.T.; visualization, H.L. and G.T.; supervision, G.T. and R.A.; project administration, H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Michael Fan and Joseph Yonkoski at the University of California Davis Department of Utilities, and Yun Shang at East Bay Municipal Utility District, for their support of this study.
Conflicts of Interest
H.L. and R.A. declare that they are managing members of Advanced Environmental Methods LLC (AEM), a company founded to support the development of sustainable fertilizers, and provider of the equipment used for this study.
References
- Duren, R.M.; Thorpe, A.K.; Foster, K.T.; Rafiq, T.; Hopkins, F.M.; Yadav, V.; Brian, D.B.; David, R.T.; Stephen, C.; Nadia, K.C.; et al. California’s Methane Super-Emitters. Nature 2019, 575, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CalRecycle. Short-Lived Climate Pollutants (SLCP): Organic Waste Methane Emissions Reductions. Available online: www.calrecycle.ca.gov/Climate/SLCP/ (accessed on 16 April 2019).
- Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Gallert, C.; Bauer, S.; Winter, J. Effect of ammonia on the anaerobic degradation of protein by a mesophilic and thermophilic biowaste population. Appl. Microbiol. Biotechnol. 1998, 50, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Çalli, B.; Mertoglu, B.; Inanc, B.; Yenigun, O. Effects of high free ammonia concentrations on the performances of anaerobic bioreactors. Process Biochem. 2005, 40, 1285–1292. [Google Scholar] [CrossRef]
- Marchaim, U. Biogas Processes for Sustainable Development; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; Available online: http://www.fao.org/3/t0541e/T0541E00.htm (accessed on 12 December 2020).
- Mohan, S.V.; Amulya, K.; Modestra, J.A. Urban biocycles—Closing metabolic loops for resilient and regenerative ecosystem: A perspective. Bioresour. Technol. 2020, 306, 123098. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.; Bolton, K.; Wong, J.; Pandey, A. Sustainable Resource Recovery and Zero Waste Approaches; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.; Rajagopalan, G. Understanding Impacts of Co-Digestion: Digester Chemistry, Gas Production, De-Waterability, Solids Production, Cake Quality, and Economics; Water Environment & Reuse Foundation: Alexandria, VA, USA, 2017. [Google Scholar]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Charatrization of Food Waste as Feedstock for Anaerobic Digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Mutegoa, E.; Hilonga, A.; Njau, K. Approaches to the Mitigation of Ammonia Inhibition During Anaerobic Di-gestion—A Review. Water Pract. Technol. 2020, 15, 551–570. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Reducing Nutrients in the San Francisco Bay Through Additional Wastewater Treatment Plant Sidestream Treatment; EPA-R9-WTR3-13-001; United States Environmental Protection Agency: Washington, DC, USA, 2017; Region 9.
- Menkveld, H.; Broeders, E. Recovery of ammonia from digestate as fertilizer. Water Pract. Technol. 2018, 13, 382–387. [Google Scholar] [CrossRef]
- Serna-Maza, A.; Heaven, S.; Banks, C. Biogas stripping of ammonia from fresh digestate from a food waste digester. Bioresour. Technol. 2015, 190, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency. Bench-Scale Evaluation of Ammonia Removal from Wastewater by Steam Stripping; EPA-600-s2-91-046; United States Environmental Protection Agency: Washington, DC, USA, 1992.
- Zhang, L.; Yong-Woo, L.; Deokjin, J. Ammonia Stripping for Enhanced Biomethanization of Piggery Wastewater. J. Hazard. Mater. 2012, 200, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Sugiura, N.; Feng, C.; Maekawa, T. Pretreatment of anaerobic digestion effluent with ammonia stripping and biogas purification. J. Hazard. Mater. 2007, 145, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Bonmatí, A.; Flotats, X. Air Stripping of Ammonia from Pig Slurry: Characterization and Feasibility as a Pre- or Post-Treatment to Mesophilic Anaerobic Digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Zeng, L.; Mangan, C.; Li, X. Ammonia recovery from anaerobically digested cattle manure by steam stripping. Water Sci. Technol. 2006, 54, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zheng, X.; Yang, X.; Dai, T.; Zhou, J.; He, J.; Luo, X. Recovery of NH3-N from mature leachate via negative pressure steam-stripping pretreatment and its benefits on MBR systems: A pilot scale study. J. Clean. Prod. 2018, 203, 918–925. [Google Scholar] [CrossRef]
- Ukwuani, A.T.; Tao, W. Developing a vacuum thermal stripping—Acid absorption process for ammonia recovery from anaerobic digester effluent. Water Res. 2016, 106, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ukwuani, A.T. Coupling thermal stripping and acid absorption for ammonia recovery from dairy manure: Ammonia volatilization kinetics and effects of temperature, pH and dissolved solids content. Chem. Eng. J. 2015, 280, 188–196. [Google Scholar] [CrossRef]
- Willis, R.B.; Montgomery, M.E.; Allen, P.R. Improved Method for Manual, Colorimetric Determination of Total Kjeldahl Nitrogen Using Salicylate. J. Agric. Food Chem. 1996, 44, 1804–1807. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).