Review of Sewage Sludge as a Soil Amendment in Relation to Current International Guidelines: A Heavy Metal Perspective
Abstract
:1. Introduction
2. Review Methodology
3. Legislation
4. Sewage Sludge and Biosolids Analysis
5. Soil Analyses
6. Food Crops
7. Phytoremediation and Bioenergetics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iglesias, M.; Marguí, E.; Camps, F.; Hidalgo, M. Extractability and crop transfer of potentially toxic elements from mediterranean agricultural soils following long-term sewage sludge applications as a fertilizer replacement to barley and maize crops. Waste Manag. 2018, 75, 312–318. [Google Scholar] [CrossRef]
- Wang, Q.; Shaheen, S.M.; Jiang, Y.; Li, R.; Slaný, M.; Abdelrahman, H.; Kwon, E.; Bolan, N.; Rinklebe, J.; Zhang, Z. Fe/Mn- and P-modified drinking water treatment residuals reduced Cu and Pb phytoavailability and uptake in a mining soil. J. Hazard Mater. 2021, 403. [Google Scholar] [CrossRef]
- European Commission. Protection of the Environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Communities 1986, 4, 6–12. [Google Scholar]
- Burducea, M.; Zheljazkov, V.D.; Lobiuc, A.; Pintilie, C.A.; Virgolici, M.; Silion, M.; Asandulesa, M.; Burducea, I.; Zamfirache, M.M. Biosolids application improves mineral composition and phenolic profile of basil cultivated on eroded soil. Sci. Hortic. 2019, 249, 407–418. [Google Scholar] [CrossRef]
- Demirbas, A.; Edris, G.; Alalayah, W.M. Sludge production from municipal wastewater treatment in sewage treatment plant. Energy Sources Part. A Recover. Util. Environ. Eff. 2017, 39, 999–1006. [Google Scholar] [CrossRef]
- Mohamed, B.; Mounia, K.; Aziz, A.; Ahmed, H.; Rachid, B.; Lotfi, A. Sewage sludge used as organic manure in Moroccan sunflower culture: Effects on certain soil properties, growth and yield components. Sci. Total Environ. 2018, 627, 681–688. [Google Scholar] [CrossRef]
- Yuan, Z.; Pratt, S.; Batstone, D.J. Phosphorus recovery from wastewater through microbial processes. Curr. Opin. Biotechnol. 2012, 23, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowski, F.; de Souza Lauretto, M.; Nardocci, A.C.; Sato, M.I.Z.; Razzolini, M.T.P. Assessing the probability of infection by Salmonella due to sewage sludge use in agriculture under several exposure scenarios for crops and soil ingestion. Sci. Total Environ. 2016, 568, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lloret, E.; Pastor, L.; Pradas, P.; Pascual, J.A. Semi full-scale thermophilic anaerobic digestion (TAnD) for advanced treatment of sewage sludge: Stabilization process and pathogen reduction. Chem. Eng. J. 2013, 232, 42–50. [Google Scholar] [CrossRef]
- Dabrowska, L.; Rosińska, A. Change of PCBs and forms of heavy metals in sewage sludge during thermophilic anaerobic digestion. Chemosphere 2012, 88, 168–173. [Google Scholar] [CrossRef]
- Yang, L.; Liu, W.; Zhu, D.; Hou, J.; Ma, T.; Wu, L.; Zhu, Y.; Christie, P. Application of biosolids drives the diversity of antibiotic resistance genes in soil and lettuce at harvest. Soil Biol. Biochem. 2018, 122, 131–140. [Google Scholar] [CrossRef]
- García-Santiago, X.; Franco-Uría, A.; Omil, F.; Lema, J.M. Risk assessment of persistent pharmaceuticals in biosolids: Dealing with uncertainty. J. Hazard. Mater. 2016, 302, 72–81. [Google Scholar] [CrossRef]
- Shamuyarira, K.K.; Gumbo, J.R. Assessment of heavy metals in municipal sewage sludge: A case study of Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2014, 11, 2569–2579. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.L.; Northcott, G.L.; Stern, G.A.; Tomy, G.T.; Jones, K.C. PAHs, PCBs, PCNs, organochlorine pesticides, synthetic musks, and polychlorinated n-alkanes in U.K. sewage sludge: Survey results and implications. Environ. Sci. Technol. 2003, 37, 462–467. [Google Scholar] [CrossRef]
- Navarro, I.; de la Torre, A.; Sanz, P.; Fernández, C.; Carbonell, G.; Martínez, M. de los Á. Environmental risk assessment of perfluoroalkyl substances and halogenated flame retardants released from biosolids-amended soils. Chemosphere 2018, 210, 147–155. [Google Scholar] [CrossRef]
- FAO. Agricultural Use of Sewage. In Waste Water Treatment and Use in Agriculture, FAO Irrigation and Drawing Paper 47; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992. [Google Scholar]
- Xu, P.; Liu, A.; Li, F.; Tinkov, A.A.; Liu, L.; Zhou, J.C. Associations between Metabolic Syndrome and Four Heavy Metals: A Systematic Review and Meta-Analysis. Environ. Pollut. 2021, 273, 116480. [Google Scholar] [CrossRef]
- Veeken, A.H.M.; Hamelers, H.V.M. Removal of heavy metals from sewage sludge by extraction with organic acids. Water Sci. Technol. 1999, 40, 129–136. [Google Scholar] [CrossRef]
- Geng, H.; Xu, Y.; Zheng, L.; Gong, H.; Dai, L.; Dai, X. An overview of removing heavy metals from sewage sludge: Achievements and perspectives. Environ. Pollut. 2020, 266, 115375. [Google Scholar] [CrossRef]
- Casado-Vela, J.; Sellés, S.; Díaz-Crespo, C.; Navarro-Pedreño, J.; Mataix-Beneyto, J.; Gómez, I. Effect of composted sewage sludge application to soil on sweet pepper crop (Capsicum annuum var. annuum) grown under two exploitation regimes. Waste Manag. 2007, 27, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S. Pilot-scale vermireactors for sewage sludge stabilization and metal remediation process: Comparison with small-scale vermireactors. Ecol. Eng. 2010, 36, 703–712. [Google Scholar] [CrossRef]
- Kong, L.; Liu, J.; Han, Q.; Zhou, Q.; He, J. Integrating metabolomics and physiological analysis to investigate the toxicological mechanisms of sewage sludge-derived biochars to wheat. Ecotoxicol. Environ. Saf. 2019, 185, 109664. [Google Scholar] [CrossRef]
- Samaras, P.; Papadimitriou, C.A.; Haritou, I.; Zouboulis, A.I. Investigation of sewage sludge stabilization potential by the addition of fly ash and lime. J. Hazard. Mater. 2008, 154, 1052–1059. [Google Scholar] [CrossRef]
- Slaný M, Jankovič L, Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay. Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Pharmaceutically active compounds in sludge stabilization treatments: Anaerobic and aerobic digestion, wastewater stabilization ponds and composting. Sci. Total Environ. 2015, 503–504, 97–104. [Google Scholar] [CrossRef]
- Samara, E.; Matsi, T.; Balidakis, A. Soil application of sewage sludge stabilized with steelmaking slag and its effect on soil properties and wheat growth. Waste Manag. 2017, 68, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cheng, M.; Li, Z.; Ren, J.; Shen, L.; Wang, S.; Luo, Y.; Christie, P. Major nutrients, heavy metals and PBDEs in soils after long-term sewage sludge application. J. Soils Sediments 2012, 12, 531–541. [Google Scholar] [CrossRef]
- Elmi, A.; Al-Khaldy, A.; AlOlayan, M. Sewage sludge land application: Balancing act between agronomic benefits and environmental concerns. J. Clean. Prod. 2020, 250, 119512. [Google Scholar] [CrossRef]
- Guoqing, X.; Xiuqin, C.; Liping, B.; Hongtao, Q.; Haibo, L. Absorption, accumulation and distribution of metals and nutrient elements in poplars planted in land amended with composted sewage sludge: A field trial. Ecotoxicol. Environ. Saf. 2019, 182, 109360. [Google Scholar] [CrossRef] [PubMed]
- Lag-Brotons, A.; Gómez, I.; Navarro-Pedreño, J.; Mayoral, A.M.; Curt, M.D. Sewage sludge compost use in bioenergy production—A case study on the effects on Cynara cardunculus L energy crop. J. Clean. Prod. 2014, 79, 32–40. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rosikon, K.; Grobelak, A.; Hutchison, D.; Kacprzak, M.J. Modification of properties of energy crops under Polish condition as an effect of sewage sludge application onto degraded soil. J. Environ. Manage. 2018, 217, 509–519. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Baredar, P.; Shukla, A. A review on biomass energy resources, potential, conversion and policy in India. Renew. Sustain. Energy Rev. 2015, 45, 530–539. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ. Int. 2009, 35, 142–156. [Google Scholar] [CrossRef]
- Liu, H. Achilles heel of environmental risk from recycling of sludge to soil as amendment: A summary in recent ten years (2007–2016). Waste Manag. 2016, 56, 575–583. [Google Scholar] [CrossRef]
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P. Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Lamastra, L.; Suciu, N.A.; Trevisan, M. Sewage sludge for sustainable agriculture: Contaminants’ contents and potential use as fertilizer. Chem. Biol. Technol. Agric. 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Chang, A.C., Page. Developing Human Health-Related Chemical Guidelines for Reclaimed Waster and Sewage Sludge Applications in Agriculture; World Health Organization: Geneva, Switzerland, 2002; p. 105. [Google Scholar]
- European Commission Environment—Sewage sludge. Available online: https://ec.europa.eu/environment/waste/sludge/ (accessed on 13 April 2020).
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. In Text with EEA relevance; European Commission: Bruxelles, Belgium, 2006.
- EuroStat. Available online: https://ec.europa.eu/eurostat/ (accessed on 13 April 2020).
- European Union. Directive 2008/98/EC of the European Parliament and the Council of 19 November 2008 on Waste and Repealing Certain Directives. Off. J. Eur. Union 2008, 34, 99–126. [Google Scholar]
- European Commission. Closing the Loop: An Eu Action Plan for the Circular Economy. In Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, COM (2015) 614/2; European Commission: Brussels, Belguim, 2015. [Google Scholar]
- Singh, R.P.; Agrawal, M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere 2007, 67, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Santanen, A.; Stoddard, F.L.; Mäkelä, P. Feedstock quality and growth of bioenergy crops fertilized with sewage sludge. Chemosphere 2012, 89, 1211–1217. [Google Scholar] [CrossRef]
- Lavado, R.S. Effects of sewage-sludge application on soils and sunflower yield: Quality and toxic element accumulation. J. Plant. Nutr. 2006, 29, 975–984. [Google Scholar] [CrossRef]
- Farrell, M.; Rangott, G.; Krull, E. Difficulties in using soil-based methods to assess plant availability of potentially toxic elements in biochars and their feedstocks. J. Hazard. Mater. 2013, 250–251, 29–36. [Google Scholar] [CrossRef]
- de Melo, W.J.; de Stéfani Aguiar, P.; Maurício Peruca de Melo, G.; Peruca de Melo, V. Nickel in a tropical soil treated with sewage sludge and cropped with maize in a long-term field study. Soil Biol. Biochem. 2007, 39, 1341–1347. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y. Effects of sewage sludge compost application on crops and cropland in a 3-year field study. Chemosphere 2005, 59, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Lemming, C.; Oberson, A.; Hund, A.; Jensen, L.S.; Magid, J. Opportunity costs for maize associated with localised application of sewage sludge derived fertilisers, as indicated by early root and phosphorus uptake responses. Plant. Soil 2016, 406, 201–217. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.D.; Patra, A.K.; Dwivedi, B.S. Changes in soil quality in response to short-term application of municipal sewage sludge in a typic haplustept under cowpea-wheat cropping system. Environ. Nanotechnology, Monit. Manag. 2015, 4, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Chopra, A.K. Accumulation and translocation of metals in soil and different parts of french bean (Phaseolus vulgaris L.) amended with sewage sludge. Bull. Environ. Contam. Toxicol. 2014, 92, 103–108. [Google Scholar] [CrossRef]
- Rehman, R.A.; Rizwan, M.; Qayyum, M.F.; Ali, S.; Zia-ur-Rehman, M.; Zafar-ul-Hye, M.; Hafeez, F.; Iqbal, M.F. Efficiency of various sewage sludges and their biochars in improving selected soil properties and growth of wheat (Triticum aestivum). J. Environ. Manage. 2018, 223, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.A.; Qayyum, M.F. Co-composts of sewage sludge, farm manure and rock phosphate can substitute phosphorus fertilizers in rice-wheat cropping system. J. Environ. Manage. 2020, 259, 109700. [Google Scholar] [CrossRef] [PubMed]
- Mazen, A.; Faheed, F.A.; Ahmed, A.F. Study of potential impacts of using sewage sludge in the amendment of desert reclaimed soil on wheat and jews mallow plants. Brazilian Arch. Biol. Technol. 2010, 53, 917–930. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.L.; García, C.; Hernández, T.; Ayuso, M. Application of composted sewage sludges contaminated with heavy metals to an agricultural soil: Effect on lettuce growth. Soil Sci. Plant. Nutr. 1997, 43, 565–573. [Google Scholar] [CrossRef]
- Kidd, P.S.; Domínguez-Rodríguez, M.J.; Díez, J.; Monterroso, C. Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere 2007, 66, 1458–1467. [Google Scholar] [CrossRef]
- Lakhdar, A.; Iannelli, M.A.; Debez, A.; Massacci, A.; Jedidi, N.; Abdelly, C. Effect of municipal solid waste compost and sewage sludge use on wheat (Triticum durum): Growth, heavy metal accumulation, and antioxidant activity. J. Sci. Food Agric. 2010, 90, 965–971. [Google Scholar] [CrossRef]
- Moffat, A.J.; Armstrong, A.T.; Ockleston, J. The optimization of sewage sludge and effluent disposal on energy crops of short rotation hybrid poplar. Biomass Bioenergy 2001, 20, 161–169. [Google Scholar] [CrossRef]
- Delibacak, S.; Voronina, L.; Morachevskaya, E.; Ongun, A.R. Use of sewage sludge in agricultural soils: Useful or harmful. Eurasian J. Soil Sci. 2020, 9, 126–139. [Google Scholar] [CrossRef]
- Aoshima, K.; Fan, J.; Cai, Y.; Katoh, T.; Teranishi, H.; Kasuya, M. Assessment of bone metabolism in cadmium-induced renal tubular dysfunction by measurements of biochemical markers. Toxicol. Lett. 2003, 136, 183–192. [Google Scholar] [CrossRef]
- FAO/WHO. Information and use in discussion related to contaminants and toxins in the GSCTFF. Jt. FAO/WHO Food Stand. Program. Codex Comm. Contam. Foods 2011, 1–90. [Google Scholar]
- Singh, R.P.; Agrawal, M. Effect of different sewage sludge applications on growth and yield of Vigna radiata L. field crop: Metal uptake by plant. Ecol. Eng. 2010, 36, 969–972. [Google Scholar] [CrossRef]
- Düring, R.A.; Hoß, T.; Gäth, S. Depth distribution and bioavailability of pollutants in long-term differently tilled soils. Soil Tillage Res. 2002, 66, 183–195. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Use of sewage sludge as fertiliser supplement for Abelmoschus esculentus plants: Physiological, biochemical and growth responses. Int. J. Environ. Waste Manag. 2009, 3, 91–106. [Google Scholar] [CrossRef]
- Latare, A.M.; Kumar, O.; Singh, S.K.; Gupta, A. Direct and residual effect of sewage sludge on yield, heavy metals content and soil fertility under rice-wheat system. Ecol. Eng. 2014, 69, 17–24. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.T.; Zhou, X.; Liu, L.; Gu, J.F.; Wang, W.L.; Zou, J.L.; Tian, T.; Peng, P.Q.; Liao, B.H. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Yu, H.; Wang, J.; Fang, W.; Yuan, J.; Yang, Z. Heavy metal accumulations of 24 asparagus bean cultivars grown in soil contaminated with Cd alone and with multiple metals (Cd, Pb, and Zn). J. Agric. Food Chem. 2007, 55, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Krzesłowska, M. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Probst, A.; Liu, H.; Fanjul, M.; Liao, B.; Hollande, E. Response of Vicia faba L. to metal toxicity on mine tailing substrate: Geochemical and morphological changes in leaf and root. Environ. Exp. Bot. 2009, 66, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Loutfy, N.; Fuerhacker, M.; Tundo, P.; Raccanelli, S.; El Dien, A.G.; Ahmed, M.T. Dietary intake of dioxins and dioxin-like PCBs, due to the consumption of dairy products, fish/seafood and meat from Ismailia city, Egypt. Sci. Total Environ. 2006, 370, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Real, M.I.H.; Azam, H.M.; Majed, N. Consumption of heavy metal contaminated foods and associated risks in Bangladesh. Environ. Monit. Assess. 2017, 189. [Google Scholar] [CrossRef] [PubMed]
- Türkdogan, M.K.; Fevzi, K.; Kazim, K.; Ilyas, T.; Ismail, U. Heavy metals in soil, vegetables and fruits in the endemic upper gastrointestinal cancer region of Turkey. Environ. Toxicol. Pharmacol. 2002, 13, 175–179. [Google Scholar] [CrossRef]
- Lentini, P.; Zanoli, L.; de Cal, M.; Granata, A.; Dell’Aquila, R. Lead and Heavy Metals and the Kidney, 3rd ed.2019; ISBN 9780323449427. [Google Scholar]
- Al-Saleh, I.; Al-Rouqi, R.; Elkhatib, R.; Abduljabbar, M.; Al-Rajudi, T. Risk assessment of environmental exposure to heavy metals in mothers and their respective infants. Int. J. Hyg. Environ. Health 2017, 220, 1252–1278. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead exposure and cardiovascular disease—A systematic review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisskopf, M.G.; Weuve, J.; Nie, H.; Saint-Hilaire, M.H.; Sudarsky, L.; Simon, D.K.; Hersh, B.; Schwartz, J.; Wright, R.O.; Hu, H. Association of cumulative lead exposure with Parkinson’s disease. Environ. Health Perspect. 2010, 118, 1609–1613. [Google Scholar] [CrossRef]
- Mossa, A.W.; Bailey, E.H.; Usman, A.; Young, S.D.; Crout, N.M.J. The impact of long-term biosolids application (>100 years) on soil metal dynamics. Sci. Total Environ. 2020, 720. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Koupaie, E.; Eskicioglu, C. Health risk assessment of heavy metals through the consumption of food crops fertilized by biosolids: A probabilistic-based analysis. J. Hazard. Mater. 2015, 300, 855–865. [Google Scholar] [CrossRef]
- Moreno, J.L.; García, C.; Hernández, T.; Pascual, J.A. Transference of heavy metals from a calcareous soil amended with sewage-sludge compost to barley plants. Bioresour. Technol. 1996, 55, 251–258. [Google Scholar] [CrossRef]
- Antonious, G.F.; Kochhar, T.S.; Coolong, T. Yield, quality, and concentration of seven heavy metals in cabbage and broccoli grown in sewage sludge and chicken manure amended soil. J. Environ. Sci. Heal. Part. A Toxic/Hazardous Subst. Environ. Eng. 2012, 47, 1955–1965. [Google Scholar] [CrossRef]
- Salem, H.M.; Abdel-Salam, A.; Abdel-Salam, M.A.; Seleiman, M.F. Phytoremediation of metal and metalloids from contaminated soil. In Plants Under Metal and Metalloid Stress: Responses, Tolerance and Remediation; Springer Nature Singapure: Berlin/Heidelberg, Germany, 2018; ISBN 9789811322426. [Google Scholar]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O. Phytoremediation: From Theory Toward Practice; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128139134. [Google Scholar]
- Polechońska, L.; Klink, A. Trace metal bioindication and phytoremediation potentialities of Phalaris arundinacea L. (reed canary grass). J. Geochemical Explor. 2014, 146, 27–33. [Google Scholar] [CrossRef]
- Placek, A.; Grobelak, A.; Kacprzak, M. Improving the phytoremediation of heavy metals contaminated soil by use of sewage sludge. Int. J. Phytoremediation. 2016, 18, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Landberg, T. Use of willow in phytoextraction. Int. J. Phytoremediation 1999, 1, 115–123. [Google Scholar] [CrossRef]
- Schwartz, C.; Echevarria, G.; Morel, J.L. Phytoextraction of cadmium with Thlaspi caerulescens. Plant. Soil 2003, 249, 27–35. [Google Scholar] [CrossRef]
- Fernando, A.L.; Barbosa, B.; Costa, J.; Papazoglou, E.G. Giant Reed (Arundo Donax l.): A Multipurpose Crop Bridging Phytoremediation with Sustainable Bioeconomy; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128028728. [Google Scholar]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant. Sci. 2018, 871, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Guerinot, M. Lou The ZIP family of metal transporters. Biochim. Biophys. Acta 2000, 1465, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavas, I.; Ünay, A.; Abdel-Daim, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef] [Green Version]
- Bais, S.S.; Lawrence, K.; Pandey, A.K. Phytoremediation Potential of Eichhornia crassipes (Mart.) Solms. Int. J. Environ. Agric. Biotechnol. 2016, 1, 210–217. [Google Scholar] [CrossRef]
- Das, S.; Goswami, S.; Das Talukdar, A. Physiological responses of water hyacinth, eichhornia crassipes (Mart.) solms, to cadmium and its phytoremediation potential. Turkish J. Biol. 2016, 40, 84–94. [Google Scholar] [CrossRef]
- Gunathilakae, N.; Yapa, N.; Hettiarachchi, R. Effect of arbuscular mycorrhizal fungi on the cadmium phytoremediation potential of Eichhornia crassipes (Mart.) Solms. Groundw. Sustain. Dev. 2018, 7, 477–482. [Google Scholar] [CrossRef]
- Çelebi, Ş.Z.; Ekin, Z.; Zorer, Ö.S. Accumulation and tolerance of Pb in some bioenergy crops. Polish J. Environ. Stud. 2018, 27, 591–596. [Google Scholar] [CrossRef]
- Klang-Westin, E.; Eriksson, J. Potential of Salix as phytoextractor for Cd on moderately contaminated soils. Plant. Soil 2003, 249, 127–137. [Google Scholar] [CrossRef]
- da Silva, P.H.M.; Poggiani, F.; Laclau, J.P. Applying Sewage Sludge to Eucalyptus grandis Plantations: Effects on Biomass Production and Nutrient Cycling through Litterfall. Appl. Environ. Soil Sci. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Leila, S.; Mhamed, M.; Heilmeier, H.; Kharytonov, M.; Wiche, O.; Moschner, C.; Onyshchenko, E.; Nadia, B. Fertilization value of municipal sewage sludge for Eucalyptus camaldulensis plants. Biotechnol. Reports 2017, 13, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Unterbrunner, R.; Puschenreiter, M.; Sommer, P.; Wieshammer, G.; Tlustoš, P.; Zupan, M.; Wenzel, W.W. Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ. Pollut. 2007, 148, 107–114. [Google Scholar] [CrossRef] [PubMed]
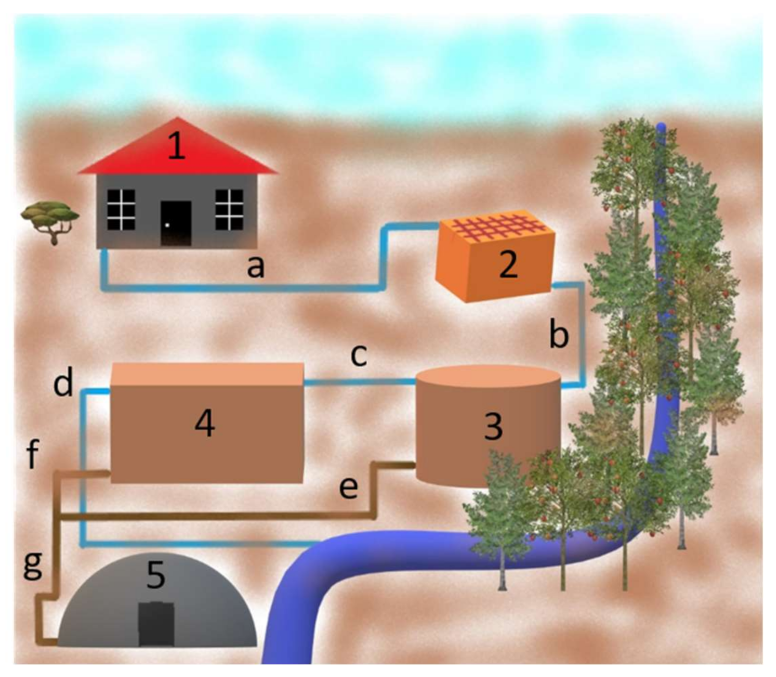
| WHO—World Health Organization | * FAO—Food and Agriculture Organization | # Directive 86/278/EEC | |
|---|---|---|---|
| Heavy Metal Limits | Maximum Permissible Pollutant Concentrations in the Receiving Soils (mg kg−1) | Maximum Permissible Concentration of Potentially Toxic Elements in Soil (mg kg−1 Dry Solids) | Limit Values for Concentrations of Heavy Metals in Soil (mg kg−1 of Dry Matter of Soil) |
| Arsenic (As) | 8 | 50 | - |
| Cadmium (Cd) | 4 | 35 | 1 to 3 |
| Chromium (Cr) | - | 400 (prov.) | - |
| Copper (Cu) | - | 80 | 50 to 140 |
| Fluoride (F) | 635 | 500 | - |
| Lead (Pb) | 84 | 300 | 50 to 300 |
| Mercury (Hg) | 7 | 1 | 1 to 1.5 |
| Molybdenum (Mo) | 0.6 | 4 | - |
| Nickel (Ni) | 107 | 50 | 30 to 75 |
| Zinc (Zn) | - | 200 | 150 to 300 |
| Silver (Ag) | 3 | - | - |
| Boron (B) | 1.7 | - | - |
| Beryllium (Be) | 0.2 | - | - |
| Barium (Ba) | 302 | - | - |
| Selenium (Se) | 6 | - | - |
| Antimony (Sb) | 36 | - | - |
| Titanium (Ti) | 0.3 | - | - |
| Vanadium (V) | 47 | - | - |
| pH | N | P | K | Cd | Cr | Cu | Hg | Ni | Pb | Zn | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country/City | g kg−1 DW | mg kg−1 DW | ||||||||||
| Argentina/Buenos Aires | - | 2.7 ± 0.47 | 7.7 ± 1.4 | 1.4 ± 0.3 | 3.9 ± 1.9 | 155.6 ± 51.8 | 360.3 ± 80.9 | - | 85.5 ± 76.1 | 322.7± 151.6 | 1526 ± 523 | [47] |
| Australia/Brisbane | - | - | - | - | 1.8 | 16.7 ± 0.3 | 447.7 ± 1.5 | - | 19.8 ± 0.2 | 41.7 ± 0.3 | 830.6 ± 4.2 | [48] |
| Brazil/Jaboticabal | - | 34.08 | 21.62 | 1.9 | 11 | 808 | 722 | - | 231 | 186 | 2159 | [49] |
| China/Hebei | 5.8 | 56.6 | 9.8 | - | 0.3 | 83.51 | 221.9 | - | 32.62 | 73.31 | [22] | |
| China/Taiyuan | - | - | - | - | 22.1 | 245.8 | 1122 | 20.6 * | - | 118.5 | 3059 | [50] |
| Denmark/Copenhagen | 7.7 | 47 | 33 | - | 1.4 | 98 | 244 | - | 31 | 178 | 1041 | [51] |
| Finland/Helsinki | 7.2 | 0.031 | 0.026 | 0.002 | 0.4 | 30 | 270 | - | 20 | 20 | 470 | [46] |
| India/New Delhi | 6.4 | 18 | 16.1 | 1.83 | - | - | 173 | - | - | 78 | 1853 | [52] |
| India/Uttarakhand | 9.0 | - | 0.216 ± 0.002# | - | 10.24 ± 0.14 | 8.63 ± 1.06 | 18.96 ± 1.09 | - | - | 9.33 ± 1.01 | 11.25 ± 1.00 | [53] |
| India/Varanasi | 7.0 | 17.3 ± 0.2 | 0.717 ± 0.06 | 0.209 ± 0.002 | 154.5 ± 2.52 | 35.5 ± 0.76 | 317.7 ± 1.92 | - | 18.9 ± 0.09 | 60 ± 5.77 | 785.3 ± 16.69 | [45] |
| Morocco/Meknès-Saïs | 6.1 | 52.2 ± 2 | 0.586 ± 0.018 # | 0.920 ± 0.021 | 1.15 ± 0.2 | 32.8 ± 2.4 | 17.9 ± 1.2 | 0.44 ± 0.1 | 20.9 ± 1.7 | 81 ± 4.5 | 215 ± 12.4 | [6] |
| Pakistan/Multan | 6.9 | 14.6 | 13.38 | - | 5.5 | - | 145 | - | 35 | 20 | - | [54] |
| Pakistan/Multan | 7.6 | 6 | 13 | 13 | 26 | - | - | - | 160 | 13 | - | [55] |
| Spain/Alicante | 6.5 | 2.48 | 5.62 | 7.89 | 1.6 | 16.6 | 157 | n.d. | n.d. | 40.8 | 470 | [20] |
| pH | EC | Organic Matter | N | P | K | Cd | Cr | Cu | Hg | Ni | Pb | Zn | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country/City | dS m−1 | g kg−1 DW | mg kg−1 DW | |||||||||||
| China/Taiyuan | 7.2 | - | - | 1.2 | - | - | 38 | 112.5 | 34.6 | - | - | 87.7 | 81.2 | [50] |
| Egypt/Sohag | 7.9 | 0.61 | 2 | 0.053 | 0.003 | 0.077 | 0.5 | - | 11 | - | - | 7 | 32 | [56] |
| India/New Delhi | 8.4 | 0.19 | - | 0.0116 | 2.65 | 141.5 | - | - | 2.07 | - | - | 0.056 | 3.76 | [52] |
| India/Uttarakhand | 7.4 | 2.63 | - | - | - | - | 0.09 | 0.18 | 2.42 | - | - | 0.12 | 0.88 | [53] |
| India/Varanasi | 8.2 | 0.24 | - | 1.8 | 0.054 | - | 1.51 | 0.34 | 3.51 | - | 4.95 | 2.83 | 2.11 | [45] |
| Morocco/Meknès-Saïs | 8.2 | 0.1 | 9.8 | 0.7 | - | 0.271 | 0.22 | 57.5 | 1.6 | <0.1 | 21.5 | 16.2 | 3.1 | [6] |
| Poland/Silesia | 7.9 | - | - | 0.481 | 0.015 | - | 2.3 | 26.32 | 29.44 | - | 28.99 | 46.57 | 112 | [31] |
| Spain/Alicante | 7.9 | 1.64 | 1.29 | 0.0125 | 0.007 | 0.3 | 0.15 | 14.7 | 0.94 | n.d. | n.d. | 0.21 | 0.59 | [20] |
| Spain/Murcia | 8.5 | 0.16 | 6.71 | 0.66 | 0.32 | 4.76 | 0.1 | - | 7.3 | - | 14.3 | - | 24.3 | [57] |
| Spain/Santiago de Compostela | 4.8 | - | - | 5 | - | - | - | 30 | 12 | - | 20 | 36 | 78 | [58] |
| Tunisia/Tunes | 8.0 | 0.263 | - | 1.1 | - | - | - | - | 32 | - | 50 | 22 | 70 | [59] |
| U.K./Reading | 5.6 | - | 77 | - | 1.208 | - | 1.2 | 35 | 38 | 0.2 | 12 | 34 | 71 | [60] |
| Specie/Plant Organ * | Cd | Cr | Cu | Ni | Pb | Zn | Sewage Sludge/Compost Application | City/Country | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 DW | |||||||||
| Hordeum vulgare/Seeds | - | - | 5.4 ± 0.5 | 0.060 ± 0.010 | - | 20 ± 3 | 22.3 ton ha−1 year−1 | Girona/Spain | [1] |
| Hordeum vulgare/Seeds | - | - | 2.8 | - | - | 15.3 | 150 ton ha−1 compost (2:1, wood waste:SS) | Taiyuan/China | [50] |
| Hordeum vulgare/Seeds | 0.038 | - | 11.2 | 1.29 | - | 61.7 | 80 ton ha−1 | Murcia/Spain | [83] |
| Phaseolus vulgaris/Fruit | 2a | 0.4a | 0.8a | - | 0.02a | 5a | 100% | Haridwar/India | [53] |
| Vigna radiata/Fruit | 1.62 ± 0.13 | 1.47 ± 0.15 | 2.22 ± 0.22 | 5.67 ± 0.51 | 3.47 ± 0.35 | 22.07 ± 1.08 | 120 ton ha−1 | Varanasi/India | [64] |
| Brassica rapa/Leaves | - | - | 21.9 | - | - | 23.8 | 150 ton ha−1 compost (2:1, wood waste:SS) | Taiyuan/China | [50] |
| Lactuca sativa/ Leaves | 1.2 | - | 12.3 | 28 | - | 202 | 80 ton ha−1 | Murcia/Spain | [58] |
| Zea mays/Seeds | - | - | 1.8 ± 0.6 | 280 ± 50 | - | 17.5 ± 0.8 | 25.4 ton ha−1 year−1 | Girona/Spain | [1] |
| Zea mays/Seeds | 0.06 | 10.55 | 5.6 | 0.98 | - | 85.8 | Pots with 2.66 kg of SS on top + 13.33 kg of Soil | Helsinki/Finland | [46] |
| Zea mays/Seeds | - | - | - | 2.65 | - | - | 67.5 ton ha−1 | Jaboticabal/Brazil | [49] |
| Brassica oleracea cv. Blue Vantage/Leaves | 0 | 0.04 | 2 | 0.36 | 0.06 | 7.5 | 2.69 ton ha−1 | Kentucky/USA | [84] |
| Brassica oleracea cv. Packman/heads | 0 | 0.05 | 3 | 0.475 | 0.1 | 18 | 0.67 ton ha−1 | Kentucky/USA | [84] |
| Abelmoschus esculentus/Fruit | 21 | 1.1 | 9.4 | 7.3 | 4.3 | 34.3 | 40% (w/w) | Varanasi/India | [66] |
| Brassica napus/Seeds | 0.05 | 0.12 | 2.5 | 0.22 | - | 19.5 | Pots with 2.66 kg of SS on top + 13.33 kg of Soil | Helsinki/Finland | [46] |
| Oryza sativa/Seeds | 2.28 ± 0.03 | 6.37 ± 0.13 | - | 11.68 ± 0.49 | 0.85 ± 0.08 | 22.59 ± 2.73 | 40 ton ha−1 | Varanasi/India | [67] |
| Beta vulgaris/Leaves | 25a | 3a | 22a | 5a | 2a | 75a | 40% (w/w) | Varanasi/India | [45] |
| Triticum aestivum/Seeds | 1.09 ± 0.02 | 0.49 ± 0.03 | - | 2.32 ± 0.23 | n.d. | 52.44 ± 0.84 | 40 ton ha−1 | Varanasi/India | [67] |
| Triticum vulgare/Seeds | 0.15 ± 0.05 | - | 8.333 ± 0.775 | - | 4.800 ± 0.087 | 16.202 ± 0.368 | 75% (w/w) | Sohag/Egypt | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, N.; Ragonezi, C.; Gouveia, C.S.S.; Pinheiro de Carvalho, M.Â.A. Review of Sewage Sludge as a Soil Amendment in Relation to Current International Guidelines: A Heavy Metal Perspective. Sustainability 2021, 13, 2317. https://doi.org/10.3390/su13042317
Nunes N, Ragonezi C, Gouveia CSS, Pinheiro de Carvalho MÂA. Review of Sewage Sludge as a Soil Amendment in Relation to Current International Guidelines: A Heavy Metal Perspective. Sustainability. 2021; 13(4):2317. https://doi.org/10.3390/su13042317
Chicago/Turabian StyleNunes, Nuno, Carla Ragonezi, Carla S.S. Gouveia, and Miguel Â.A. Pinheiro de Carvalho. 2021. "Review of Sewage Sludge as a Soil Amendment in Relation to Current International Guidelines: A Heavy Metal Perspective" Sustainability 13, no. 4: 2317. https://doi.org/10.3390/su13042317
APA StyleNunes, N., Ragonezi, C., Gouveia, C. S. S., & Pinheiro de Carvalho, M. Â. A. (2021). Review of Sewage Sludge as a Soil Amendment in Relation to Current International Guidelines: A Heavy Metal Perspective. Sustainability, 13(4), 2317. https://doi.org/10.3390/su13042317









