Abstract
Autonomic nervous system function is an important predictor of physical fitness. The objective of this study was to find out the associations of autonomic activity parameters, lipid profile, insulin concentrations, and insulin resistance in overweight men with the level of physical activity. A descriptive and correlational study was carried out in 28 overweight men: 14 physically active (PA) and 14 physically inactive (PI). The following variables were assessed: Level of physical activity, HRV (heart rate variability), basal insulin, HOMA-IR index (Homeostasis Model Assessment Insulin-Resistance), and lipid profile. The main results show a positive correlation between the spectral parameters of the HRV and total cholesterol (r = 0.24), LDL (r = 0.59), VLDL (r = 0.86), and insulin (r = 0.88) of sedentary people, evidencing a directly proportional correlation with BMI. We conclude that weight gain and a sedentary lifestyle are associated with an increase in sympathetic discharge, which, in turn, is associated with an increase in lipid profile and insulin levels.
1. Introduction
Obesity and overweight pose one of the greatest challenges to public health. Physical inactivity and obesity are a global pandemic with enormous consequences for health and the economy. The rising medical costs of obesity cannot be managed by any healthcare system [1,2]. Despite many advances in our understanding of the health benefits of physical activity and behavioral, environmental, and policy strategies to increase physical activity, global levels of physical inactivity, obesity, or overweight remain high [3,4]. Based on a strong independent identity and a greater evidence base, integrating actions within existing systems in both the health and non-health sectors can greatly increase the impact and sustainability of policies to combat this pandemic. Whole solutions that apply to the environment and affect the entire population have strengths compared to programs focused solely on education and health promotion programs [1,4]. Several risk factors are involved in the evolution of this condition, including a lifestyle of low physical activity [5,6,7], which is often associated with high urban densities, smoking, and excessive use of technology. The latter, in turn, is becoming increasingly common in contemporary societies and generates an increase in morbidity [8]. According to the WHO, around 1.9 billion people were affected by overweight in 2016, which represents 13% of the world population [9]. In this context, obesity and overweight are defined as an excessive accumulation of body fat that induces a state of chronic inflammation and insulin resistance (IR) [10,11].
The WHO defines body mass index (BMI) as a simple indicator of the relationship between body weight and size, and it is frequently used to identify overweight and obesity in adult people. In this line, the increase in adipose tissue initially accumulates in the subcutaneous adipose tissue. However, when the subcutaneous tissue exceeds its capacity to store additional lipids, visceral fat begins to accumulate. This induces an increase of inflammatory mediators from the adipose tissue, resulting in structural and functional remodeling, thus promoting an inflammatory process [12]. One of the most frequently used methods to assess the visceral adipose tissue is waist circumference (WC) [13], which is a cardiovascular risk factor [14].
The risk factors associated with the increase of adiposity in overweight and obesity are abnormal levels of plasma lipids (dyslipidemias) [15], which are defined as any imbalance in the normal concentrations of total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG) [16]. These alterations are induced by atherosclerosis, which causes sequestration of oxidized LDL-Cho particles by macrophages in the subendothelial matrix. This, in turn, is linked to an inflammatory process that involves connective tissue and smooth muscle, thus forming the atheroma plaque, thereby contributing to an endothelial lesion [17,18]. Studies conducted in the last decades have shown that several components of the metabolic syndrome, such as dyslipidemias, are associated with adrenergic markers that generate an increase in the activity of the Sympathetic Nervous System (SNS) [19].
Consequently, overweight has been linked to the functional alteration of the autonomic nervous system (ANS), due to the imbalance in autonomic discharges on the sinus node [20,21]. Therefore, the autonomic imbalance is often associated with an unhealthy lifestyle, energy imbalance, sedentariness, and metabolic anomalies [22,23]. In this context, the increase in body fat is correlated with greater sympathetic activity at rest, with up to 50% higher levels with respect to healthy individuals [24], and with lower parasympathetic activity associated with a decrease in baroreflex sensitivity [10,11,25,26]. It has been demonstrated that, in people with overweight and obesity, there is a sympathetic predominance over the autonomic activity balance, which implies a decrease in vagal activity associated with a greater risk of acute coronary disease [27,28]. Therefore, it is important to assess the autonomic nervous system function, and a simple and non-invasive way of achieving this is to analyze the heart rate variability (HRV) [29], as well as ANS diseases [30]. The analysis of HRV encompasses different methods for the determination of this parameter, which include linear and nonlinear components. Linear methods provide information on the time and frequency domains of HRV [31] and nonlinear methods, which report on the fractal behavior of HRV on their randomness of frequency patterns between R-R interval time series [32]. Both methods relate HRV with the autonomic nervous system function showing their rebalance in people with overweight and obesity with the sympathetic predominance over vagal function.
Furthermore, dyslipidemias are associated with a decrease in HRV, which suggests a greater risk of cardiac death. Thus, it is necessary to consider and monitor the lipid parameters based on the cardiovascular system and its neural control [33].
Among the strategies to control overweight and obesity, physical activity programs stand out, especially those focused on weight loss. The current guidelines of physical activity in this population, according to the available evidence, recommend 150–300 min of moderate-intensity physical activity every week and 75–150 min of vigorous-intensity, which captures the maximal risk reduction for health outcomes [34]. However, the effects on body weight are limited, although this exercise regime has been associated with a significant decrease of the cardiovascular risk factors and a substantial improvement in the quality of life [35].
The aim of this study was to analyze the lipid profile, insulin resistance, and the response of the autonomic nervous system in people with obesity, as well as to compare and examine their relationships according to the level of physical activity.
2. Materials and Methods
2.1. Design
This study followed a cross-sectional, descriptive, and correlational design.
2.2. Participants
The sample consisted of 28 men with overweight (BMI, 26.7 ± 1.5 kg·m−2) without a history of cardiorespiratory disease and with an average age of 24.1 ± 2.17 years. The participants were classified as physically active (n = 14) (PA group) and physically inactive (n = 14) (PI group) according to a self-guided survey. The participants were recruited through an announcement published in the University of Santo Tomás, to which they replied voluntarily in Santiago de Chile. The recruitment was conducted by convenience sampling, with the following inclusion criteria: (i) Male, (ii) no consumption of drugs known to affect the autonomic nervous system function, (iii) BMI between 25 and 29.9, (iv) fasting for at least 12 h prior to the assessment, and (v) signed informed consent.
2.3. Procedure
The study was carried out in the physiology laboratory of the University of Santo Tomás (Santiago de Chile). Firstly, the level of physical activity of the participants was categorized by applying the International Physical Activity Questionnaire (IPAQ). The IPAQ is a self-guided questionnaire that measures the level of physical activity: If someone practices physical activity three or more days per week or expends between 1.500 and 3.000 Mets-minutes/week is considered physically active, and someone who does not fulfill any of those two categories is considered physically inactive. Though the IPAQ could have some limitations to evaluate physical fitness, it has been validated to assess the level of physical activity [36]. Then, BMI and WC were measured.
In the next stage, the autonomic nervous system function of the participants was assessed through HRV, using a Polar H7® heart rate monitor, which records the RR interval variations and the Cardiomood® software, which expresses the linear and nonlinear methods. This software analyses the linear component LF/HF (Low Frequency/High Frequency) Ratio, which is an indirect index of the vagal vs. sympathetic balance. Regarding nonlinear components, the software records DFA-α1 (Detrended Fluctuation analysis), which describes short-term fluctuations, and SD1, which is the Standard Deviation of Poincaré plot perpendicular to the line of identity. Both nonlinear components are correlated with the LF/HF ratio. In this sense, a low LF/HF ratio reflects parasympathetic dominance, while a high LF/HF ratio indicates sympathetic dominance (Shaffer and Ginsberg, 2017). All HRV measurements were conducted every day between 8 and 10 a.m. HRV was measured with the participants in the supine position, and the RR intervals were recorded for 15 min, with the last 5 min at rest. During this time, the breathing frequency was recorded, for which the participants were previously asked to breathe normally, ideally 12 cycles per minute, avoiding Valsalva maneuvers. Then the blood samples were taken. Endovenous blood was extracted by percutaneous puncture, using appropriate needles and syringes, and was then collected in tubes (5 mL) with anticoagulants. A Siemens Advia 2400® clinical chemistry analyzer was used to determine the lipid profile, baseline insulin, and glycemia. For the calculation of HOMA-IR, the following equation was used: HOMA-IR = (fasting insulinemia (µU/mL) × fasting glycemia (mg/dL))/405.
2.4. Statistical Analysis
A descriptive statistical analysis was conducted, with the mean value and standard deviation, using GraphPad Prism 5 software for Windows®. Additionally, an inferential analysis was also carried out, using the Shapiro–Wilk test to verify whether the data followed a normal distribution, and the Levene test to determine the homoscedasticity. The comparison between groups was performed using Student’s T-test or Mann–Whitney U-test, depending on the normality and homoscedasticity tests. Pearson’s or Spearman’s correlation test was conducted, depending on normality and homoscedasticity. Moreover, a simple linear regression analysis was also carried out to determine the relationship between the study variables. The statistical level accepted as significant was 5% (p < 0.05).
3. Results
Table 1 shows the results obtained in the analyzed variables, comparing the responses between participants based on their level of physical activity. There are significant differences in all variables, except in HDL and the autonomic NS function markers SD1 and DFA α1. The PA Group shows better values in comparison to the PI Group on anthropometric variables (BMI: 22.5 ± 0.55 vs. 26.7 ± 1.54 and WC 97 ± 9.11 vs. 112 ± 5.41). Regarding the lipid profile, PI Group is significantly worse in all variables except in HDL. Lastly, LF/HF ratio is significantly lower in PA Group.

Table 1.
Intergroup comparisons. Anthropometric parameters, lipid profile and autonomic nervous system function (Means ± SD).
Lipid Profile
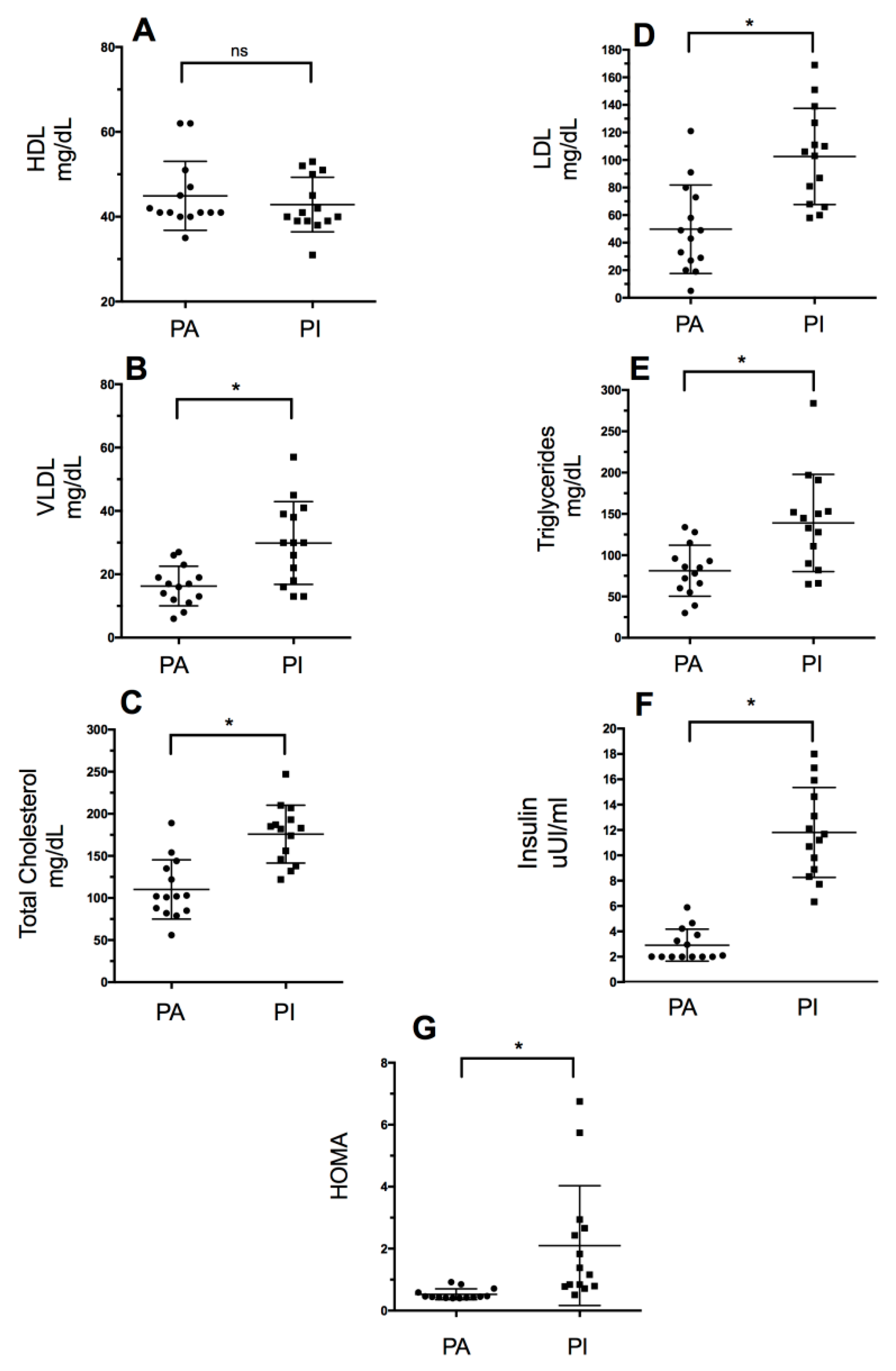
Figure 1 shows the lipid profile values (intergroup comparisons): High-density lipoproteins (HDL), low-density lipoproteins (LDL), very-low-density lipoproteins (VLDL), triglycerides (TG), and total cholesterol. Significant differences were observed in the serum values of total cholesterol (p = 0.0001), triglycerides (p = 0.004), LDL (p = 0.0004), and VLDL (p = 0.0033) (Table 1). Moreover, there were statistically significant differences in HOMA-IR and insulin level (p = 0.0001) between groups, with the PI group showing higher values in both variables.
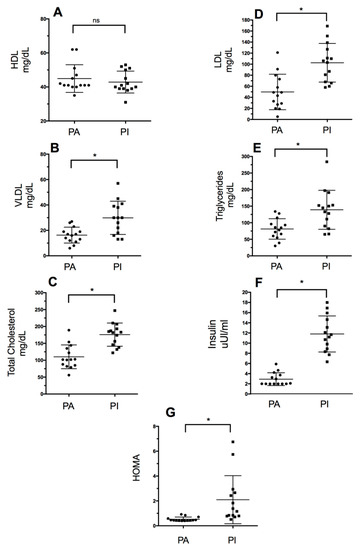
Figure 1.
Lipid profile values based on the level of physical activity. PA: Physically Active, PI: Physically Inactive (A): High-Density Lipoproteins, (B): Very-Low-Density Lipoproteins, (C): Total Cholesterol, (D): High-Density Lipoproteins, (E): Triglycerides, (F): Insulin, and (G): HOMA-IR. * Significant differences between groups (p < 0.05); ns: no significant differences (p > 0.05).
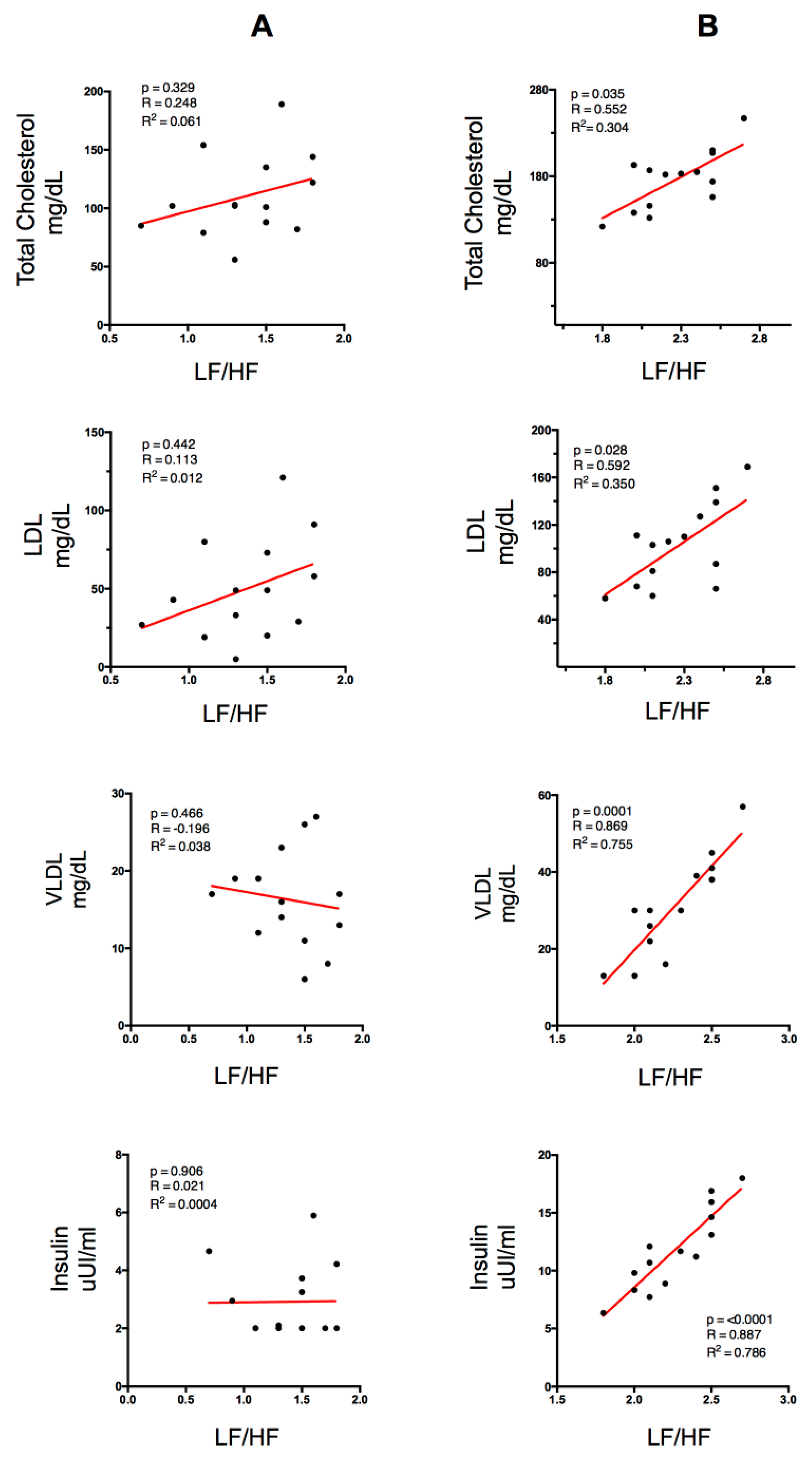
Figure 2 shows the correlations between lipid profile (Total Cholesterol, LDL, VLDL), insulin plasma level and the spectral variable LF/HF ratio. Column A represents the physically active group (PA) and column B represents the physically inactive group (PI). In the PI group, a significant positive correlation with total cholesterol and a significant negative correlation with VLDL were found. However, in the PA group no significant correlations were found.
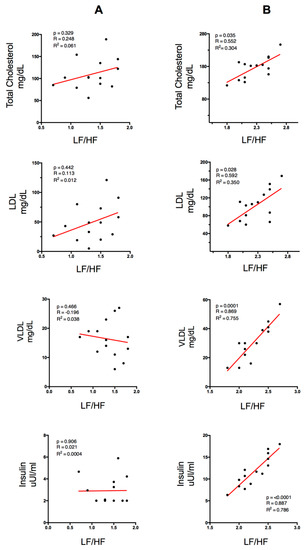
Figure 2.
Correlation between lipid profile and insulin with the spectral variable LF/HF ratio for the PA group (column A) and the PI group (column B). LDL (Low-Density Lipoproteins), VLDL (Very-Low-Density Lipoproteins). p < 0.05 (significant differences), R (correlation coefficient), R2 (coefficient of determination).
4. Discussion
This study correlates parameters of autonomic nervous system function with lipid profile and insulin in individuals with overweight based on their level of physical activity. The physically inactive group was correlated with indicators of sympathetic activity at rest, as well as with an increase in the lipid profile and insulin levels.
Apart from measuring cardiovascular function, HRV allows estimating indicators of autonomic nervous system (ANS) function. Thus, the LF/HF ratio is considered a reliable indicator of the balance between vagal and sympathetic ANS activity, which is altered in people with overweight [10,37]. Moreover, the increase in blood lipid concentration is strongly correlated with an increase in cardiovascular risk, e.g., due to an increase in the atherogenic index [38,39,40]. The results show a significant correlation between the LF/HF ratio and the lipid profile in the PI group (Figure 2). This association is statistically significant in total cholesterol (p = 0.035, R2 = 0.304) and LDL (p = 0.028, R2 = 0.35), although there was high direct correlation between VLDL and the LF/HF ratio (p = 0.0001, R2 = 0.755), showing that high dyslipidemia is an indicator of greater sympathetic activity and lower levels of parasympathetic activity (Figure 2). These results are in line with those reported by Thayer et al. 2013, who analyzed the relationship between the parasympathetic parameters of HRV for 24 h and the levels of plasma cholesterol in order to measure the ANS function in 611 healthy individuals [41]. The results showed a negative correlation between the temporal components related to vagal activity (SDNN, pNN50, and RMSSD) and the components of the plasma lipid profile (TC, LDL, and LDL/HDL ratio). On the other hand, there was a positive correlation between SDNN/RMSSD (as an indicator of the sympathetic/vagal balance) and TC, LDL, and LDL/HDL. However, in the PA group, the levels of LF/HF ratio did not increase significantly with the increases of lipid levels, which could be due to a modulatory effect of physical activity practice on the excess of sympathetic function. In that sense, it could indicate that in the analysis of physical activity, there is a direct relationship between the parasympathetic component and the level of physical activity and a reverse response for sedentary people [42].
The increase in insulin levels is a usual consequence of obesity and overweight since the secretion of large amounts of adipokines, especially TNF alpha, increases the phosphorylation of tyrosine residues in insulin receptor substrate 1 (IRS-1), which is responsible for the intracellular signal of this hormone, and decreases the gene expression of insulin-responsive glucose transport protein GLUT-4 [43]. This correlation is established by various studies, which reveal that, among cardiovascular risk factors, insulin resistance and central adiposity were independently associated with cardiac autonomic modulation in obese individuals [39,44]. In this study, it was observed that the PA group obtained better results in both insulin concentrations and HOMA-IR levels than the PI group (Table 1 and Figure 1). Numerous studies have established a direct relationship between high values of lipid profile and high levels of plasma insulin measured through Steady-state Plasma Glucose (SSPG) and Quantitative Insulin Sensitivity Check Index (QUICKI), respectively [22,23,45,46]. Similarly, the PI group obtained a strong correlation between insulin levels and the HL/HF ratio (p = 0.0001, R2 = 0.786) (Figure 2). IR would cause an alteration in the stimulation of the ANS on the activity in the sinus node, modifying its sensitivity. Thus, it has been demonstrated that there is a correlation between hyperinsulinemia and high sympathetic activation [47]. There are two mechanisms that could explain the sympathetic stimulation linked to hyperinsulinemia. At the central level, there would be an activation from the arcuate nucleus of the hypothalamus, which is the route that hyperpolarizes the neurons of the dorsal motor nucleus of the vagus and of the nucleus of the solitary tract, associated with an increase of plasma insulin levels [48,49]. On the other hand, at the peripheral level, these compensatory mechanisms would be associated with specific ionic currents in the mechanoreceptors of the carotid bodies, such as the desensitization of an afferent vagal pathway, stimulated by gastric cholecystokinin and leptin [50]. Different studies have demonstrated that overweight and IR are associated with an increase of HR at rest due to adrenergic stimulation [38,42,51,52]. The described findings are in line with those reported in a study that associated the autonomic activation degree with IR, also reporting a negative correlation between HOMA-IR and the levels of parasympathetic modulation [42]. This could be due to the chronic low-level inflammation usually found in people with obesity, which is characterized by a high production of pro-inflammatory adipokines, such as TNF-α and interleukin 1 (IL-1). In this sense, TNF-α would be the precursor of IR, since, firstly, it promotes a decrease in the phosphorylation capacity of tyrosine residues in insulin receptor substrate 1 (IRS-1) (necessary for the intracellular signaling of insulin) and, secondly, it reduces the gene expression of insulin-sensitive glucose transport protein GLUT-4 [53]. As was previously indicated, these findings are in agreement with the results obtained in this study, which show a statistically significant tendency between the spectral power determined by the LF/HF ratio (an indicator of greater sympathetic activity) and the level of insulin in people with overweight who are also sedentary (Figure 2).
Regarding the lipid profile, it is known that people with overweight tend to show atherogenic dyslipidemia, which is characterized by an increase of TG and LDL and a decrease in HDL levels [40]. Thus, the results of the present study show that the PI group had greater levels of blood lipids, with differences between TC, TG, LDL, VLDL, and WC based on the level of physical activity (Table 1, Figure 1). The PA group showed significantly lower levels in all the indicators of dyslipidemia. Therefore, it could be asserted that having a physically active lifestyle protects against this cardiovascular risk factor even in people with overweight. This result is confirmed by a study in which a group of people with obesity, with type 2 diabetes and nonalcoholic fatty liver disease (NAFLD), performed an eight-week HIIT training protocol. These individuals obtained greater decreases in lipid levels (TC pre 191.4 ± 9.5 vs. post 176.5 ± 8.2; LDL pre 96.3 ± 5.4 vs. post 90.4 ± 4.7; VLDL pre 86.2 ± 4.3 vs. post 81.4 ± 3.9), suggesting that people with obesity with high lipid profile levels could greatly improve their dyslipidemia levels through intervention programs based on physical exercise (in this case HIIT training) [54].
Being physically active, increasing the daily minutes of vigorous and/or moderate physical activity, and reducing sedentary habits play a fundamental role in the metabolic and autonomic response. A physically active lifestyle can be related to better indicators of body composition (BMI, WC), which are associated with lower cardiovascular risk factors and better quality of life [7,55,56]. This assertion is in line with the results obtained in this work (Table 1). In the present study, the level of physical activity was evaluated through the application of the IPAQ questionnaire. Regardless of the intensity of the physical exercise declared, the PA group showed better values in both the lipid profile and the indicators of ANS function (Table 1). In agreement with these results, an intervention of physical exercise, in which different training intensities were compared (high and moderate intensity) to determine the intensity that produced the best response in the cardiovagal parameters, reported that both training protocols showed changes in SDNN, HF, and LF in favor of a greater modulation of the vagal function on the sympathetic function [57]. The recruitment process to achieve many volunteer participants among people with overweight or obesity for this type of study is not easy, however, it must be recognized that the small sample size is a limitation to generalize the results with guarantees.
5. Conclusions
The results show that there is a significant direct correlation between sympathetic activity, insulin levels, and the indicators of dyslipidemia in the group of people who reported a sedentary lifestyle. There were also better values of lipid profile and body composition in the group that declared to be physically active. A physically active lifestyle could prevent harmful alterations in the lipid profile, even in people with overweight, and lead to greater parasympathetic activity. Considering that the size of the analyzed sample is not representative, more investigations with larger samples and physical fitness tests to know the physical activity level are necessary.
Author Contributions
Conceptualization A.E.-S., and J.A.G.-J. Data curation J.C.-M. and E.M.-S. Formal analysis A.E.-S. and J.A.G.-J. Investigation J.C.-M., E.M.-S. and A.E.-S. Methodology A.E.-S., and J.A.G.-J. Resources J.C.-M., E.M.-S. Supervision E.M.-S., and J.A.G.-J., Visualization J.C.-M., and A.E.-S. Writing—original draft E.M.-S., J.C.-M., A.E.-S., and J.A.G.-J. Writing, reviewing, and editing A.E.-S. and J.A.G.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of University of Santo Tomás de Santiago de Chile (ID: CEC UST Nº 11/2020, data of approval: 16 November 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kohl, H.W.; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380. [Google Scholar] [CrossRef]
- James, W.P. Obesity—A modern pandemic: The burden of disease. Endocrinol. Nutr. 2013, 60 (Suppl. S1). [Google Scholar] [CrossRef]
- Pratt, M.; Ramirez Varela, A.; Salvo, D.; Hw, K.I.; Ding, D. Attacking the pandemic of physical inactivity: What is holding us back? Br. J. Sports Med. 2020, 54. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378. [Google Scholar] [CrossRef]
- Da Cuña Carrera, I.; Lantarón Caeiro, E.M.; González González, Y.; Gutiérrez Nieto, M. Repercusión del sedentarismo en la respuesta cardiorrespiratoria en estudiantes universitarios / Sedentarism Impact on Cardio-Respiratory Response in College Students. RIMCAFD 2017. [Google Scholar] [CrossRef]
- Díaz-Martínez, X.; Petermann, F.; Leiva, A.M.; Garrido-Méndez, A.; Salas-Bravo, C.; Martínez, M.A.; Labraña, A.M.; Duran, E.; Valdivia-Moral, P.; Zagalaz, M.L.; et al. Association of physical inactivity with obesity, diabetes, hypertension and metabolic syndrome in the chilean population. Rev. Med. Chil. 2018, 146, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Pazzianotto-Forti, E.M.; Moreno, M.A.; Plater, E.; Baruki, S.B.S.; Rasera-Junior, I.; Reid, W.D. Impact of Physical Training Programs on Physical Fitness in People with Class II and III Obesity: A Systematic Review and Meta-Analysis. Phys. Ther. 2020, 100, 963–978. [Google Scholar] [CrossRef]
- Biadgilign, S.; Mgutshini, T.; Haile, D.; Gebremichael, B.; Moges, Y.; Tilahun, K. Epidemiology of obesity and overweight in sub-Saharan Africa: A protocol for a systematic review and meta-analysis. BMJ Open 2017, 7, e017666. [Google Scholar] [CrossRef]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 December 2020).
- Liao, C.-D.; Tsauo, J.-Y.; Hsiao, D.-J.; Liou, T.-H.; Huang, S.-W.; Lin, L.-F. Association of physical capacity with heart rate variability based on a short-duration measurement of resting pulse rate in older adults with obesity. PLoS ONE 2017, 12, e0189150. [Google Scholar] [CrossRef]
- Voulgari, C.; Pagoni, S.; Vinik, A.; Poirier, P. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism 2013, 62, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Ayer, J.; Charakida, M.; Deanfield, J.E.; Celermajer, D.S. Lifetime risk: Childhood obesity and cardiovascular risk. Eur. Heart J. 2015, 36, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.A.; Thom, J.M.; Rye, K.-A.; Parmenter, B.J. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis 2018, 274, 162–171. [Google Scholar] [CrossRef]
- Guarino, D.; Nannipieri, M.; Iervasi, G.; Taddei, S.; Bruno, R.M. The Role of the Autonomic Nervous System in the Pathophysiology of Obesity. Front. Physiol. 2017, 8, 665. [Google Scholar] [CrossRef]
- Jokinen, E. Obesity and cardiovascular disease. Minerva Pediatr. 2015, 67, 25–32. [Google Scholar] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Paschoal, M.A.; Trevizan, P.F.; Scodeler, N.F. Heart rate variability, blood lipids and physical capacity of obese and non-obese children. Arq. Bras. Cardiol. 2009, 93, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Salinas, A.; Zafra-Santos, E.; Pavez-Von Martens, G.; Cofré-Bolados, C.; Lemus-Zúñiga, J.; Sánchez-Aguilera, P. Heart rate variability and insulin resistance among obese males. Rev. Med. Chil. 2015, 143, 1129–1135. [Google Scholar] [CrossRef]
- Costa, J.; Moreira, A.; Moreira, P.; Delgado, L.; Silva, D. Effects of weight changes in the autonomic nervous system: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.M.; Solomon, S.D. Influence of Physical Activity on Hypertension and Cardiac Structure and Function. Curr. Hypertens. Rep. 2015, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Rabbone, I.; Bobbio, A.; Rabbia, F.; Bertello, M.C.; Ignaccoldo, M.G.; Saglio, E.; Morello, F.; Veglio, F.; Pacini, G.; Cerutti, F. Early cardiovascular autonomic dysfunction, beta cell function and insulin resistance in obese adolescents. Acta Biomed. 2009, 80, 29–35. [Google Scholar]
- Alvarez, G.E.; Beske, S.D.; Ballard, T.P.; Davy, K.P. Sympathetic neural activation in visceral obesity. Circulation 2002, 106, 2533–2536. [Google Scholar] [CrossRef] [PubMed]
- Farah, B.Q.; Andrade-Lima, A.; Germano-Soares, A.H.; Christofaro, D.G.D.; de Barros, M.V.G.; do Prado, W.L.; Ritti-Dias, R.M. Physical Activity and Heart Rate Variability in Adolescents with Abdominal Obesity. Pediatr. Cardiol. 2018, 39, 466–472. [Google Scholar] [CrossRef]
- Chen, W.; Leo, S.; Weng, C.; Yang, X.; Wu, Y.; Tang, X. Mechanisms mediating renal sympathetic nerve activation in obesity-related hypertension. Herz 2015, 40 (Suppl. S2), 190–196. [Google Scholar] [CrossRef]
- Chintala, K.K.; Krishna, B.H.; N, M.R. Heart rate variability in overweight health care students: Correlation with visceral fat. J. Clin. Diagn. Res. 2015, 9, CC06-8. [Google Scholar] [CrossRef]
- Mehta, R.K. Impacts of obesity and stress on neuromuscular fatigue development and associated heart rate variability. Int. J. Obes. 2015, 39, 208–213. [Google Scholar] [CrossRef]
- Böhm, M.; Reil, J.-C.; Deedwania, P.; Kim, J.B.; Borer, J.S. Resting heart rate: Risk indicator and emerging risk factor in cardiovascular disease. Am. J. Med. 2015, 128, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Badrov, M.B.; Bartol, C.L.; DiBartolomeo, M.A.; Millar, P.J.; McNevin, N.H.; McGowan, C.L. Effects of isometric handgrip training dose on resting blood pressure and resistance vessel endothelial function in normotensive women. Eur. J. Appl. Physiol. 2013, 113, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Poddar, M.G.; Kumar, V.; Sharma, Y.P. Automated diagnosis of coronary artery diseased patients by heart rate variability analysis using linear and non-linear methods. J. Med. Eng. Technol. 2015, 39, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Williams, S.M.; Eleftheriadou, A.; Alam, U.; Cuthbertson, D.J.; Wilding, J.P.H. Cardiac Autonomic Neuropathy in Obesity, the Metabolic Syndrome and Prediabetes: A Narrative Review. Diabetes Ther. 2019, 10, 1995–2021. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T. Effectiveness of lifestyle intervention in overweight children. Proc. Nutr. Soc. 2011, 70, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Mantilla Toloza, S.C.; Gómez-Conesa, A. El Cuestionario Internacional de Actividad Física. Un instrumento adecuado en el seguimiento de la actividad física poblacional. Rev. Iberoam. Fisioter. Kinesiol 2007, 10, 48–52. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Christensen, J.H. The autonomic nervous system and cardiovascular disease: Role of n-3 PUFAs. Vascul. Pharmacol. 2015, 71, 1–10. [Google Scholar] [CrossRef]
- Contreras-Leal, É.A.; Santiago-García, J. Obesidad, síndrome metabólico y su impacto en las enfermedades cardiovasculares. Rev. Ordem Med. 2011, 22, 103–115. [Google Scholar]
- Saxton, S.N.; Clark, B.J.; Withers, S.B.; Eringa, E.C.; Heagerty, A.M. Mechanistic Links Between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue. Physiol. Rev. 2019, 99, 1701–1763. [Google Scholar] [CrossRef]
- Hamjane, N.; Benyahya, F.; Nourouti, N.G.; Mechita, M.B.; Barakat, A. Cardiovascular diseases and metabolic abnormalities associated with obesity: What is the role of inflammatory responses? A systematic review. Microvasc. Res. 2020, 131, 104023. [Google Scholar] [CrossRef]
- Thayer, J.F.; Fischer, J.E. Heart rate variability, overnight urinary norepinephrine, and plasma cholesterol in apparently healthy human adults. Int. J. Cardiol. 2013, 162, 240–244. [Google Scholar] [CrossRef]
- Oliveira, C.; Silveira, E.A.; Rosa, L.; Santos, A.; Rodrigues, A.P.; Mendonça, C.; Silva, L.; Gentil, P.; Rebelo, A.C. Risk Factors Associated with Cardiac Autonomic Modulation in Obese Individuals. J. Obes. 2020, 2020, 7185249. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, S.; Swenne, C.A.; Gast, K.B.; Maan, A.C.; le Cessie, S.; Jukema, J.W.; Rosendaal, F.R.; den Heijer, M.; de Mutsert, R. The role of insulin resistance in the association between body fat and autonomic function. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 93–99. [Google Scholar] [CrossRef]
- Silva, L.R.B.E.; Zamunér, A.R.; Gentil, P.; Alves, F.M.; Leal, A.G.F.; Soares, V.; Silva, M.S.; Vieira, M.F.; Simões, K.; Pedrino, G.R.; et al. Cardiac Autonomic Modulation and the Kinetics of Heart Rate Responses in the On- and Off-Transient during Exercise in Women with Metabolic Syndrome. Front. Physiol. 2017, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Poon, A.K.; Whitsel, E.A.; Heiss, G.; Soliman, E.Z.; Wagenknecht, L.E.; Suzuki, T.; Loehr, L. Insulin resistance and reduced cardiac autonomic function in older adults: The Atherosclerosis Risk in Communities study. BMC Cardiovasc. Disord. 2020, 20, 217. [Google Scholar] [CrossRef]
- Zhao, K.; Ao, Y.; Harper, R.M.; Go, V.L.W.; Yang, H. Food-intake dysregulation in type 2 diabetic Goto-Kakizaki rats: Hypothesized role of dysfunctional brainstem thyrotropin-releasing hormone and impaired vagal output. Neuroscience 2013, 247, 43–54. [Google Scholar] [CrossRef][Green Version]
- Williams, K.W.; Smith, B.N. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: Implications for ingestive behaviour. J. Physiol. 2006, 573, 395–412. [Google Scholar] [CrossRef]
- Lambert, E.A.; Teede, H.; Sari, C.I.; Jona, E.; Shorakae, S.; Woodington, K.; Hemmes, R.; Eikelis, N.; Straznicky, N.E.; De Courten, B.; et al. Sympathetic activation and endothelial dysfunction in polycystic ovary syndrome are not explained by either obesity or insulin resistance. Clin. Endocrinol. 2015, 83, 812–819. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.J.; Genest, J., Jr. High-density lipoproteins and endothelial function. Circulation 2001, 104, 1978–1983. [Google Scholar] [CrossRef]
- Katsogiannos, P.; Kamble, P.G.; Wiklund, U.; Sundbom, M.; Espes, D.; Hammar, U.; Karlsson, F.A.; Pereira, M.J.; Eriksson, J.W. Rapid changes in neuroendocrine regulation may contribute to reversal of type 2 diabetes after gastric bypass surgery. Endocrine 2020, 67, 344–353. [Google Scholar] [CrossRef]
- Windham, B.G.; Fumagalli, S.; Ble, A.; Sollers, J.J.; Thayer, J.F.; Najjar, S.S.; Griswold, M.E.; Ferrucci, L. The Relationship between Heart Rate Variability and Adiposity Differs for Central and Overall Adiposity. J. Obes. 2012, 2012, 149516. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.; Tantawy, S.A.; Kamel, D.M.; Alqahtani, B.A.; Soliman, G.S. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine 2019, 98, e14918. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.R.L.; de Souza, K.A.; Dos Santos, K.M.; Peçanha, T.; Ferreira, J.C.; Cambri, L.T.; Arsa, G. Acute Exercise Increases the Ambulatory Cardiac Modulation of Young Men with Overweight/Obesity. Res. Q. Exerc. Sport 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Ben Abderrahman, A.; Khodamoradi, A.; Saeidi, A.; Jayavel, A.; Hackney, A.C.; Laher, I.; Algotar, A.M.; Jabbour, G. Effects of physical training on anthropometrics, physical and physiological capacities in individuals with obesity: A systematic review. Obes. Rev. 2020, 21, e13039. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.B.; Gentil, P.R.V.; Beltrame, T.; Basso Filho, M.A.; Alves, F.M.; Silva, M.S.; Pedrino, G.R.; Ramirez-Campillo, R.; Coswig, V.; Rebelo, A.C.S. Exponential model for analysis of heart rate responses and autonomic cardiac modulation during different intensities of physical exercise. R. Soc. Open Sci. 2019, 6, 190639. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).