Genotype X Environment Interaction Analysis of Faba Bean (Vicia faba L.) for Biomass and Seed Yield across Different Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Sampling and Measurement
2.3. Statistical Analyses
3. Results
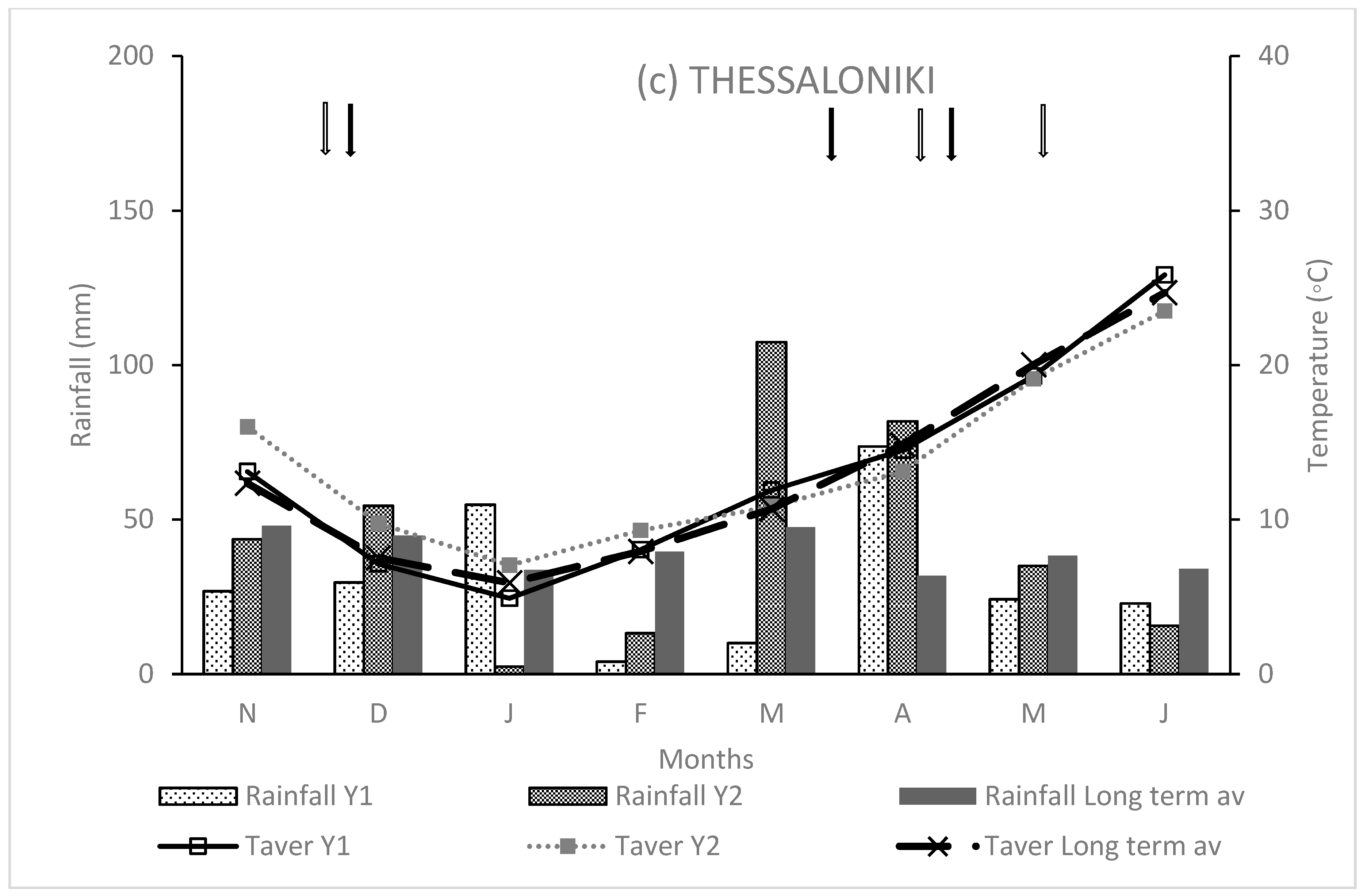
3.1. Weather Conditions
3.2. Agronomic Traits
3.3. Correlation between Traits
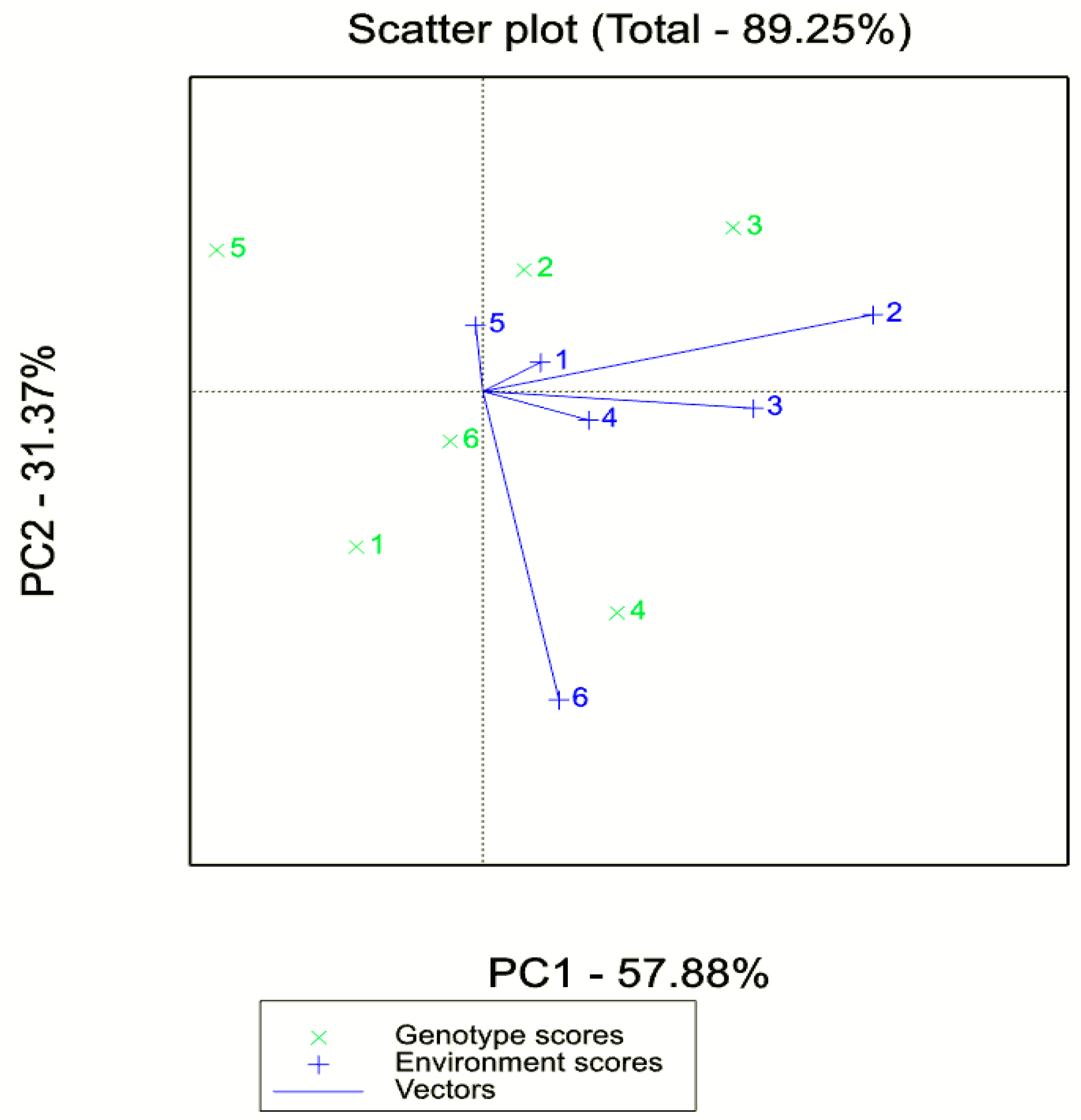
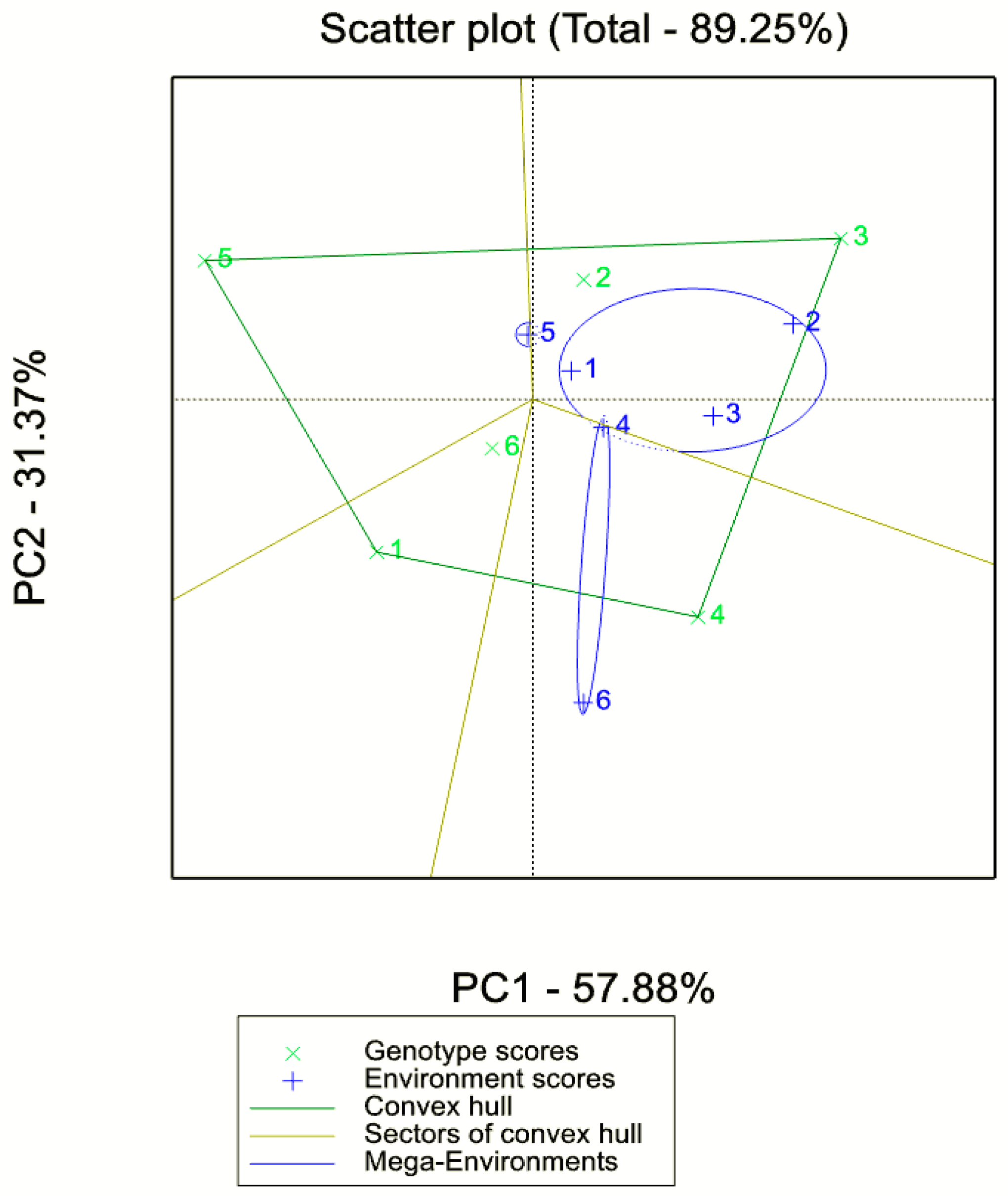
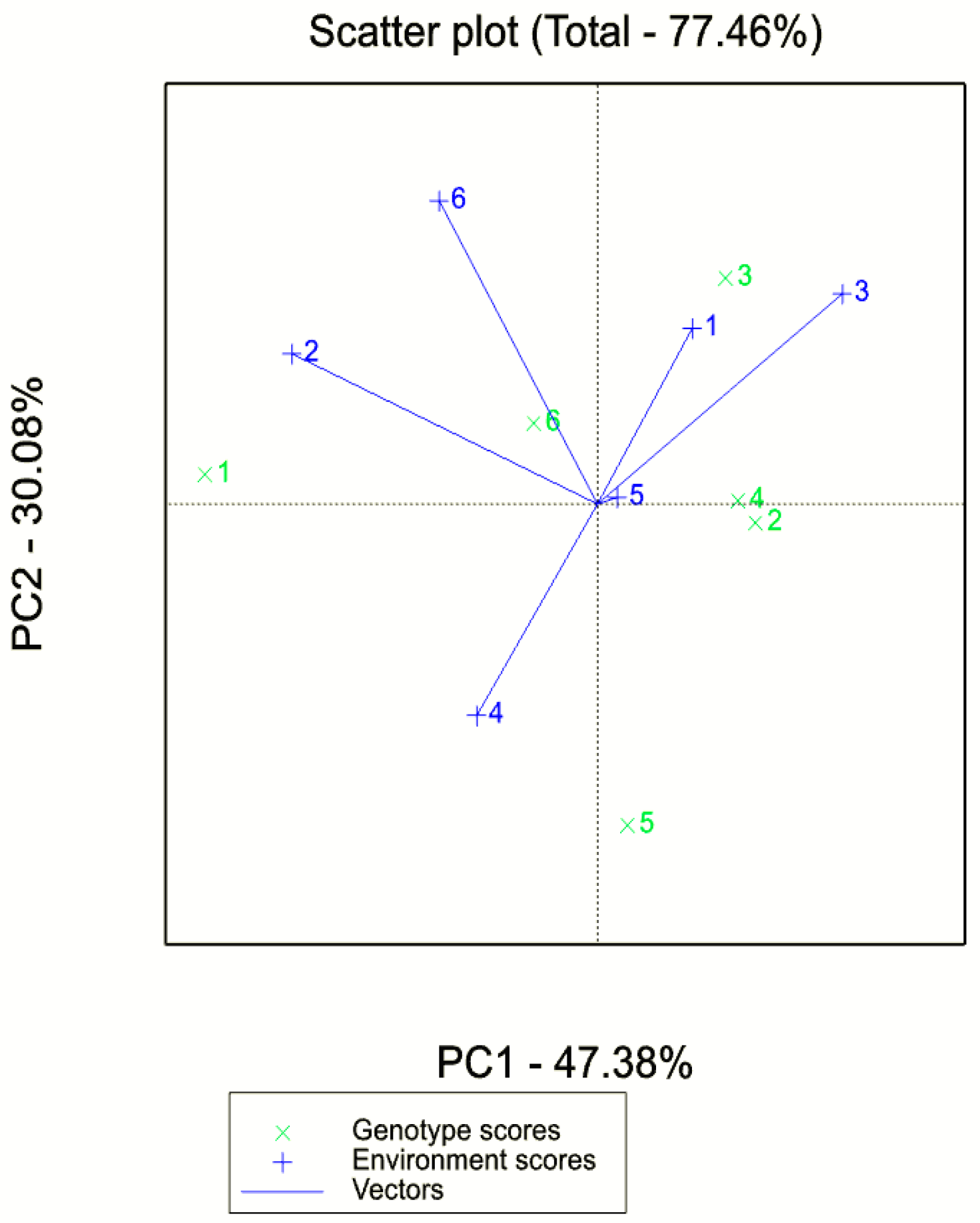
3.4. Adaptability and Stability Analysis (GGE Biplot AMMI) for Biomass and Seed Yield
4. Discussion
4.1. Weather Conditions
4.2. Agronomic Traits
4.3. Water Use Efficiency
4.4. GxE Plot Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afzal, M.; Shabbir, G.; Ilyas, M.; Jan, S.S.A.; Jan, S.A. Impact of climate change on crop adaptation: Current challenges and future perspectives. Pure Appl. Biol. 2018, 7, 965–972. [Google Scholar] [CrossRef]
- Kole, C.; Muthamilarasan, M.; Henry, R.; Edwards, D.; Sharma, R.; Abberton, M.; Batley, J.; Bentley, A.; Blakeney, M.; Bryant, J.; et al. Application of genomics-assisted breeding for generation of climate resilient crops: Progress and prospects. Front. Plant Sci. 2015, 6, 563. [Google Scholar] [CrossRef] [PubMed]
- Lammerts van Bueren, E.T.; Struik, P.C.; van Eekeren, N.; Nuijten, E. Towards resilience through systems-based plant breeding. A review. Agron. Sustain. Dev. 2018, 38, 42. [Google Scholar] [CrossRef]
- Duc, G.; Agrama, H.; Bao, S.; Berger, J.; Bourion, V.; de Ron, A.M.; Gowda, C.L.L.; Mikic, A.; Millot, D.; Singh, K.B.; et al. Breeding Annual Grain Legumes for Sustainable Agriculture: New Methods to Approach Complex Traits and Target New Cultivar Ideotypes. Crit. Rev. Plant Sci. 2015, 34, 381–411. [Google Scholar] [CrossRef]
- Cernay, C.; Ben-Ari, T.; Pelzer, E.; Meynard, J.-M.; Makowski, D. Estimating variability in grain legume yields across Europe and the Americas. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.A.; Reckling, M.; Preissel, S.; Bachinger, J.; Bergkvist, G.; Kuhlman, T.; Lindström, K.; Nemecek, T.; Topp, C.F.; Vanhatalo, A.; et al. Grain Legume Production and Use in European Agricultural Systems. Adv. Agron. 2017, 144, 235–303. [Google Scholar]
- Bahl, P.N. Climate Change and Pulses: Approaches to Combat Its Impact. Agric. Res. 2015, 4, 103–108. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World, 3rd ed.; Oxford University Press: Oxford, UK, 2000; p. 316. [Google Scholar]
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouée, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crops Res. 2010, 115, 329–339. [Google Scholar]
- Duc, G.; Aleksić, J.M.; Marget, P.; Mikić, A.; Paull, J.; Redden, R.J.; Sass, O.; Stoddard, F.L.; Vandenberg, A.; Vishnyakova, M.; et al. Faba Bean. In Grain Legumes; de Ron, A.M., Ed.; Springer: New York, NY, USA, 2015; pp. 141–178. [Google Scholar]
- Köpke, U.; Nemecek, T. Ecological services of faba bean. Field Crops Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- Suso, M.J.; del Río, R. A crop—Pollinator inter-play approach to assessing seed production patterns in faba bean under two pollination environments. Euphytica 2015, 201, 231–251. [Google Scholar] [CrossRef]
- Zong, X.; Yang, T.; Liu, R. Faba Bean (Vicia Faba L.) Breeding. In Advances in Plant Breeding Strategies: Legumes; Al-Khayri, J.M., Jain, S.M., Johnson, D.J., Eds.; Springer: Cham, Switzerland, 2019; pp. 245–286. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOSTAT. Available online: www.fao.org/faostat/en/#data/QC (accessed on 14 December 2020).
- Sillero, J.C.; Villegas-Fernández, A.M.; Thomas, J.; Rojas-Molina, M.M.; Emeran, A.A.; Fernández-Aparicio, M.; Rubiales, D. Faba bean breeding for disease resistance. Field Crops Res. 2010, 115, 297–307. [Google Scholar] [CrossRef]
- Link, W.; Balko, C.; Stoddard, F.L. Winter hardiness in faba bean: Physiology and breeding. Field Crops Res. 2010, 115, 287–296. [Google Scholar] [CrossRef]
- Khan, H.R.; Paull, J.G.; Siddique, K.H.M.; Stoddard, F.L. Faba bean breeding for drought-affected environments: A physiological and agronomic perspective. Field Crops Res. 2010, 115, 279–286. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Hauggaard-Nielsen, H. Faba bean in cropping systems. Field Crops Res. 2010, 115, 203–216. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Eizenberg, H.; Grenz, J.H.; Sillero, J.C.; Ávila, C.; Sauerborn, J.; Rubiales, D. Broomrape management in faba bean. Field Crops Res. 2010, 115, 319–328. [Google Scholar] [CrossRef]
- Stoddard, F.L.; Nicholas, A.G.; Rubiales, D.; Thomas, J.; Villegas-Fernández, A.M. Integrated pest management in faba bean. Field Crops Res. 2010, 115, 308–318. [Google Scholar] [CrossRef]
- Singh, A.K.; Bharati, R.C.; Manibhushan, N.C.; Pedpati, A. An assessment of faba bean (Vicia faba L.) current status and future prospect. Afr. J. Agric. Res. 2013, 8, 6634–6641. [Google Scholar]
- Maalouf, F.; Hu, J.; O’Sullivan, D.M.; Zong, X.; Hamwieh, A.; Kumar, S.; Baum, M. Breeding and genomics status in faba bean (Vicia faba). Plant Breed. 2019, 138, 465–473. [Google Scholar] [CrossRef]
- Temesgen, T.; Keneni, G.; Sefera, T.; Jarso, M. Yield stability and relationships among stability parameters in faba bean (Vicia faba L.) genotypes. Crop J. 2015, 3, 258–268. [Google Scholar] [CrossRef]
- Sharifi, P.; Aminpanah, H.; Erfani, R.; Mohaddesi, A.; Abbasian, A. Evaluation of genotype x environment interaction in rice based on AMMI model in Iran. Rice Sci. 2017, 24, 173–180. [Google Scholar] [CrossRef]
- Gurmu, F.; Lire, E.; Asfaw, A.; Alemayehu, F.; Rezene, Y.; Ambachew, D. GGE-biplot analysis of grain yield of faba bean genotypes in southern Ethiopia. Electron. J. Plant Breed. 2012, 3, 898–907. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. BBCH Monograph, 2nd ed.; Blackwell Science: Berlin, Germany, 2001; p. 158. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill: New York, NY, USA, 1980; p. 672. [Google Scholar]
- Yan, W. GGEbiplot—A Windows application for graphical analysis of multi-environment trial data and other types of two-way data. Agron. J. 2001, 93, 1111–1118. [Google Scholar] [CrossRef]
- Yan, W. Singular-value partitioning in biplot analysis of multi-environment trial data. Agron. J. 2002, 94, 990–996. [Google Scholar] [CrossRef]
- Payne, R.W. GenStat. Wiley Interdiscip. Rev. Comput. Stat. 2009, 1, 255–258. [Google Scholar] [CrossRef]
- Mwiinga, B.; Sibiya, J.; Kondwakwenda, A.; Musvosvi, C.; Chigeza, G. Genotype x environment interaction analysis of soybean (Glycine max (L.) Merrill) grain yield across production environments in Southern Africa. Field Crop. Res. 2020, 256, 107922. [Google Scholar]
- Flores, F.; Nadal, S.; Solis, I.; Winkler, J.; Sass, O.; Stoddard, F.L.; Link, W.; Raffiot, B.; Muel, F.; Rubiales, D.; et al. Faba bean adaptation to autumn sowing under European climates. Agron. Sustain. Dev. 2012, 32, 727–734. [Google Scholar] [CrossRef]
- Reckling, M.; Döring, T.F.; Bergkvist, G.; Chemielewski, F.-M.; Stoddard, F.L.; Watson, C.A.; Sedding, S.; Bachinger, J. Grain legume yield instability has increased over 60 years in long-term experiments as measured by a scale-adjusted coefficient of variation. Asp. Appl. Biol. 2018, 138, 15–20. [Google Scholar]
- Stoddard, F.L.; Balko, C.; Erskine, W.; Khan, H.R.; Link, W.; Sarker, A. Screening techniques and sources of resistance to abiotic stresses in cool-season food legumes. Euphytica 2006, 147, 167–186. [Google Scholar] [CrossRef]
- Boote, K.J.; Mínguez, M.I.; Sau, F. Adapting the CROPGR legume model to simulate growth of faba bean. Agron. J. 2002, 94, 743–756. [Google Scholar] [CrossRef]
- Lake, L.; Godoy-Kutchartt, D.E.; Calderini, D.F.; Verrell, A.; Sadras, V.O. Yield determination and the critical period of faba bean (Vicia faba L.). Field Crop. Res. 2019, 241, 107575. [Google Scholar] [CrossRef]
- Link, W.; Abdelmula, A.A.; von Kittlitz, E.; Bruns, S.; Riemer, H.; Stelling, D. Genotypic variation for drought tolerance in Vicia faba. Plant Breed. 1999, 118, 477–483. [Google Scholar] [CrossRef]
- Kazai, P.; Noulas, C.; Khah, E.; Vlachostergios, D. Yield and seed quality parameters of common bean cultivars grown under water and heat stress field conditions. AIMS Agric. Food 2019, 4, 285–302. [Google Scholar] [CrossRef]
- Skovbjerg, C.K.; Knudsen, J.N.; Füchtbauer, W.; Stougaard, J.; Stoddard, F.L.; Janss, L.; Andersen, S.U. Evaluation of yield, yield stability and yield-protein relationship in 17 commercial faba bean cultivars. Legume Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Karimizadeh, R.; Mohammadi, M.; Sabaghni, N.; Mahmoodi, A.A.; Roustami, B.; Seyyedi, F.; Akbari, F. GGE biplot analysis of yield stability in multi-environment trials of lentil genotypes under rainfed conditions. Not. Sci. Biol. 2013, 5, 256–262. [Google Scholar] [CrossRef]
- Galanopoulou, K.; Lithourgidis, A.S.; Dordas, C.A. Intercropping of faba bean with barley at various spatial arrangements affects dry matter and N yield, nitrogen nutrition index, and interspecific competition. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1116–1127. [Google Scholar]
- Etemadi, F.; Hashemi, M.; Zandvakili, O.; Mangan, F.X. Phenology, yield and growth pattern of faba bean varieties. Int. J. Plant Prod. 2018, 12, 243–250. [Google Scholar] [CrossRef]
- Di Paolo, E.; Garofalo, P.; Rinaldi, M. Irrigation and nitrogen fertilization treatments on productive and qualitative traits of broad bean (Vicia faba var. minor L.) in a Mediterranean environment. Legume Res. 2015, 38, 209–218. [Google Scholar]
- Youseif, S.H.; El-Megeed, F.H.A.; Saleh, S.A. Improvement of faba bean yield using Rhizobium/Agrobacterium inoculant in low-fertility sandy soil. Agronomy 2017, 7, 2. [Google Scholar] [CrossRef]
- Bodner, G.; Kronberga, A.; Lepse, L.; Olle, M.; Vågen, I.M.; Rabante, L.; Fernández, J.A.; Ntatsi, G.; Balliu, A.; Rewald, B. Trait identification of faba bean ideotypes for Northern European environments. Eur. J. Agron. 2018, 96, 1–12. [Google Scholar] [CrossRef]
- Olle, M.; Williams, I.H.; Rosa, E. Selecting appropriate faba bean var. minor varieties for production under Northern European environmental conditions. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 432–438. [Google Scholar]
- Toker, C. Estimates of broad-sense heritability for seed yield and yield criteria in faba bean (Vicia faba L.). Hereditas 2004, 140, 222–225. [Google Scholar] [CrossRef]
- Alan, O.; Geren, H. Evaluation of heritability and correlation for seed yield and yield components in faba bean (Vicia faba L.). J. Agron. 2007, 6, 484–487. [Google Scholar]
- Duc, G.; Link, W.; Marget, P.; Redden, R.J.; Stoddard, F.L.; Torres, A.M.; Cubero, J.I. Genetic Adjustment to Changing Climates: Faba Bean. In Crop Adaptation to Climate Change; Yadav, S.S., Redden, R.J., Hatfield, J.L., Lotze-Campen, H., Hall, A.E., Eds.; Wiley: Oxford, UK, 2011; pp. 269–286. [Google Scholar]
- Patrick, J.W.; Stoddard, F.L. Physiology of flowering and grain filling in faba bean. Field Crops Res. 2010, 115, 234–242. [Google Scholar] [CrossRef]
- Catt, S.C.; Paull, J.G. Effects of ambient temperature and photoperiod on flowering time in Faba bean (Vicia faba L.). Crop Pasture Sci. 2017, 68, 893–901. [Google Scholar] [CrossRef]
- Ulukan, H.; Guler, M.; Keskin, S. A path coefficient analysis some yield and yield components in faba bean (Vicia faba L.) genotypes. Pak. J. Biol. Sci. 2003, 6, 1951–1955. [Google Scholar] [CrossRef][Green Version]
- Mwanamwenge, J.; Loss, S.P.; Siddique, K.H.M.; Cocks, P.S. Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 1999, 11, 1–11. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Al-Shameri, A.M.; Migdadi, H.M.; Ammar, M.H.; El-Harty, E.H.; Khan, M.A.; Farooq, M. Physiological and molecular characterization of faba bean (Vicia faba L.) genotypes for adaptation to drought stress. J. Agron. Crop Sci. 2015, 201, 401–409. [Google Scholar]
- Muktadir, M.A.; Adhikari, K.N.; Merchant, A.; Belachew, K.Y.; Vandenberg, A.; Stoddard, F.L.; Khazaei, H. Physiological and Biochemical Basis of faba Bean Breeding for Drought Adaptation—A Review. Agronomy 2020, 10, 1345. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.H.M.; Regan, K.L.; Tennant, D.; Thomson, B.D. Water use and water use efficiency of cool season grain legumes in low rainfall Mediterranean-type environments. Eur. J. Agron. 2001, 15, 267–280. [Google Scholar] [CrossRef]
- Desoky, E.M.; Mansour, E.; Yasin, M.A.T.; El-Sobky, E.E.A.; Rady, M.M. Improvement of drought tolerance in five different cultivars of Vicia faba with foliar application of ascorbic acid or silicon. Span. J. Agric. Res. 2020, 18, e0802. [Google Scholar] [CrossRef]
- Mwanamwenge, J.; Loss, S.P.; Siddique, K.H.M.; Cocks, P.S. Growth, seed yield and water use of faba bean (Vicia faba L.) in a short-season Mediterranean-type environment. Aust. J. Exp. Agric. 1998, 38, 171–180. [Google Scholar]
- Zeleke, K.; Nendel, C. Growth and yield response of faba bean to soil moisture regimes and sowing dates: Field experiment and modelling study. Agric. Water Manag. 2019, 213, 1063–1077. [Google Scholar] [CrossRef]
- Zabawi, A.G.M.; Dennet, M.D.D. Responses of faba bean (Vicia faba L. cv. Maris Bead) to different levels of plant available water. II. Yield, water use and water use efficiency. J. Trop. Agric. Food Sci. 2010, 38, 145–152. [Google Scholar]
- Tukamuhabwa, P.; Asiimwe, M.; Nabasirye, M.; Kabayi, P.; Maphosa, M. Genotype by environment interaction of advanced generation soybean lines for grain yield in Uganda. Afr. Crop Sci. J. 2012, 20, 107–115. [Google Scholar]
- Annicchiarico, P. Genotype × Environment Interactions: Challenges and Opportunities for Plant Breeding and Cultivar Recommendations; Food and Agriculture Organization: Rome, Italy, 2002; pp. 5–12. [Google Scholar]




| Environment | Sand% | Clay % | Loam % | pH | CaCO3 (%) | EC (mS/cm) | Organic Matter (%) |
|---|---|---|---|---|---|---|---|
| AUA, Southern Greece Env1 + Εnv2 | 50 | 26 | 24 | 7.9 | 11.6 | 3.0 | 1.43 |
| IIFC, Central Greece Env3 + Env4 | 20 | 57 | 23 | 8.0 | 1.5 | 4.9 | 1.30 |
| AUTH, Northern Greece Env5 + Env6 | 25 | 27 | 48 | 7.7 | 11.3 | 1.1 | 1.24 |
| EC: electrical conductivity | |||||||
| Env1 | Env2 | 30-Year Average Values | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nov. | Dec. | Jan. | Feb. | N-F | Nov. | Dec. | Jan. | Feb. | N-F | Nov. | Dec. | Jan. | Feb. | N-F | |
| Abs Min T | 7.0 | 3.0 | −1.0 | 2.6 | 2.9 | 11.5 | 3.4 | 2.4 | 1.3 | 4.7 | 4.8 | 0.8 | −0.3 | −0.1 | 1.3 |
| Av Min T | 12.3 | 6.8 | 5.5 | 6.5 | 7.8 | 13.9 | 8.9 | 5.9 | 6.9 | 8.9 | 10.4 | 7.1 | 5.4 | 5.6 | 7.1 |
| NFD | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | |||||
| NDER | 4 | 5 | 9 | 7 | 25 | 7 | 7 | 3 | 3 | 20 | |||||
| Env3 | Env4 | 30-Year Average Values | |||||||||||||
| Abs Min T | 6.2 | −1.8 | −7.2 | −0.9 | −0.9 | 7.5 | 0.8 | −3.6 | −1.2 | 0.9 | 0.7 | −3.4 | −5.6 | −2.7 | −2.8 |
| Av Min T | 9.7 | 2.8 | 1.1 | 4.1 | 4.4 | 11.6 | 6.4 | 1.1 | 4.4 | 5.9 | 5.8 | 1.9 | 0.7 | 1.3 | 2.4 |
| NFD | 0 | 4 | 12 | 2 | 18 | 0 | 0 | 14 | 3 | 17 | |||||
| NDER | 4 | 2 | 5 | 1 | 12 | 6 | 4 | 0 | 2 | 12 | |||||
| Env5 | Env6 | 30-Year Average Values | |||||||||||||
| Abs Min T | 4.1 | 1.3 | −3.5 | −0.3 | 0.4 | 10.2 | 2.4 | −0.1 | 1.5 | 3.5 | 1.7 | −1.8 | −4.4 | −1.9 | −1.6 |
| Av Min T | 10.6 | 4.1 | 2.4 | 4.6 | 5.4 | 13.4 | 7.6 | 3.7 | 5.8 | 7.6 | 6.9 | 3 | 1.4 | 2.3 | 3.4 |
| NFD | 0 | 0 | 7 | 1 | 8 | 0 | 0 | 1 | 0 | 1 | |||||
| NDER | 3 | 2 | 3 | 0 | 8 | 2 | 4 | 0 | 1 | 7 | |||||
| Env1 | Env2 | 30-Year Average Values | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mar. | Apr. | May | Jun. | M-J | Mar. | Apr. | May | Jun. | M-J | Mar. | Apr. | May | Jun. | M-J | |
| Abs Max T | 23.3 | 26.3 | 31.7 | 35.6 | 29.2 | 22.4 | 25.6 | 36.7 | 33.8 | 29.6 | 21.9 | 25.7 | 31.5 | 36.8 | 29 |
| Av Max T | 17.6 | 19.1 | 25.2 | 30.6 | 23.1 | 16.8 | 19.2 | 26.7 | 29.2 | 23.0 | 15.6 | 19.6 | 24.8 | 29.7 | 22.4 |
| NDMAX | 0 | 0 | 6 | 11 | 17 | 0 | 0 | 11 | 10 | 21 | |||||
| NDER | 2 | 5 | 0 | 0 | 7 | 4 | 3 | 2 | 0 | 9 | |||||
| Env3 | Env4 | 30-Year Average Values | |||||||||||||
| Abs Max T | 25.9 | 30.8 | 33.1 | 38.7 | 32.1 | 25.7 | 30.6 | 39.4 | 37.3 | 33.3 | 24.1 | 28.0 | 33.5 | 38.8 | 31.1 |
| Av Max T | 19.9 | 21.4 | 27.5 | 33.2 | 25.5 | 18.8 | 21.4 | 29.1 | 31.1 | 25.1 | 14.9 | 19.8 | 25.8 | 31.1 | 22.9 |
| NDMAX | 0 | 4 | 14 | 14 | 32 | 0 | 5 | 17 | 11 | 33 | |||||
| NDER | 1 | 1 | 3 | 0 | 5 | 5 | 3 | 1 | 1 | 10 | |||||
| Env5 | Env6 | 30-Year Average Values | |||||||||||||
| Abs Max T | 22.8 | 24.8 | 29.0 | 35.2 | 28.0 | 23.2 | 24.7 | 31.9 | 35.3 | 28.8 | 21.5 | 26.1 | 30.4 | 35.5 | 28.4 |
| Av Max T | 17.1 | 18.9 | 24.6 | 30.4 | 22.8 | 15.3 | 18.0 | 24.3 | 27.1 | 21.2 | 14.3 | 19.2 | 24.5 | 29.3 | 21.8 |
| NDMAX | 0 | 0 | 4 | 11 | 15 | 0 | 0 | 3 | 4 | 7 | |||||
| NDER | 1 | 4 | 2 | 1 | 8 | 8 | 5 | 1 | 1 | 15 | |||||
| BY (t ha−1) | SY (t ha−1) | PP | SP | TSW (g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| df | MS | EV% | MS | EV% | MS | EV% | MS | EV% | MS | EV% | |
| E | 5 | 186 *** | 88.4 | 31.7 *** | 81 | 1803 *** | 88.3 | 21,217 *** | 88 | 121,632 *** | 49.4 |
| G | 5 | 8.28 *** | 3.9 | 0.96 * | 2.4 | 48.5 ** | 2.4 | 647 ** | 2.7 | 99,365 *** | 40.4 |
| GxE | 25 | 3.25 * | 7.7 | 1.31 *** | 16.6 | 37.9 *** | 9.3 | 448 ** | 9.3 | 5037 *** | 10.2 |
| PH (cm) | Earliness (DAS) | WUEBY (kg ha−1 m−3) | WUESY (kg ha−1 m−3) | ||||||||
| E | 5 | 11,520 *** | 93 | 14,157 *** | 98.2 | 28.2 *** | 88.7 | 4.05 *** | 80.7 | ||
| G | 5 | 152.4 ns | 1.2 | 188 *** | 1.3 | 1.13 ** | 3.6 | 0.12 * | 2.4 | ||
| GxE | 25 | 142.4 * | 5.8 | 13.0 *** | 0.5 | 0.49 * | 7.7 | 0.17 *** | 16.9 | ||
| Genotype | BY (t ha−1) | SY (t ha−1) | PH (cm) | PP | SP | TSW (g) | Earliness (DAS) | WUEBY (kg ha−1 m−3) | WUESY (kg ha−1 m−3) |
|---|---|---|---|---|---|---|---|---|---|
| G1 | 8.44 bc | 3.01 a | 89.2 a | 15.5 ab | 48.8 ab | 542.6 a | 116.6 e | 3.49 ab | 1.08 ab |
| G2 | 8.85 abc | 2.85 ab | 88.2 a | 16.5 ab | 50.2 ab | 534.2 ab | 121.3 b | 3.66 ab | 1.06 ab |
| G3 | 9.46 ab | 2.90 ab | 94.0 a | 17.9 a | 56.6 a | 497.2 c | 121.5 b | 3.87 a | 1.06 ab |
| G4 | 9.53 a | 2.83 ab | 91.3 a | 14.4 b | 43.6 b | 448.8 d | 120.7 c | 3.93 a | 1.05 ab |
| G5 | 8.00 c | 2.50 b | 88.8 a | 18.1 a | 57.6 a | 371.0 e | 124.6 a | 3.36 b | 0.92 b |
| G6 | 8.86 abc | 3.07 a | 87.0 a | 16.1 ab | 52.2 ab | 507.9 bc | 118.1 d | 3.68 ab | 1.13 a |
| Tukey (0.05) | 1.08 | 0.475 | 7.35 | 3.08 | 11.4 | 27.3 | 0.505 | 0.172 | 0.454 |
| Environment | BY (t ha−1) | SY (t ha−1) | PH (cm) | PP | SP | TSW (g) | Earliness (DAS) | WUEBY (kg ha−1 m−3) | WUESY (kg ha−1 m−3) |
|---|---|---|---|---|---|---|---|---|---|
| Env1 | 4.70 d | 2.99 bc | 71.2 d | 16.6 c | 48.8 c | 395.7 d | 81.9 f | 2.09 e | 1.32 ab |
| Env2 | 9.45 b | 2.64 c | 90.8 bc | 28.9 a | 97.6 a | 516.1 b | 113.1 d | 2.83 d | 0.74 c |
| Env3 | 10.5 a | 3.35 b | 93.7 b | 12.5 d | 36.7 d | 463.7 c | 152.5 a | 4.25 b | 1.23 b |
| Env4 | 11.4 a | 3.31 b | 129.6 a | 13.2 d | 39.4 cd | 418.0 d | 129.3 c | 4.95 a | 1.21 b |
| Env5 | 6.13 c | 0.74 d | 69.4 d | 4.17 e | 13.4 e | 523.3 b | 135.0 b | 3.41 c | 0.37 d |
| Env6 | 10.9 a | 4.12 a | 83.9 c | 23.0 b | 73.2 b | 584.9 a | 111.0 e | 4.47 b | 1.43 a |
| Tukey (0.05) | 1.08 | 0.475 | 7.35 | 3.08 | 11.4 | 27.3 | 0.505 | 0.172 | 0.454 |
| BY | SY | PH | PP | SP | TSW | |
|---|---|---|---|---|---|---|
| SY | 0.455 ** | |||||
| PH | 0.674 ** | 0.380 ** | ||||
| PP | 0.263 ** | 0.491 ** | 0.068ns | |||
| SP | 0.237 ** | 0.439 ** | 0.050ns | 0.977 ** | ||
| TSW | 0.202 * | 0.126ns | −0.158ns | 0.141ns | 0.176 * | |
| EAR | 0.440 ** | −0.173 * | 0.320 ** | −0.404 ** | −0.374 ** | 0.071ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papastylianou, P.; Vlachostergios, D.N.; Dordas, C.; Tigka, E.; Papakaloudis, P.; Kargiotidou, A.; Pratsinakis, E.; Koskosidis, A.; Pankou, C.; Kousta, A.; et al. Genotype X Environment Interaction Analysis of Faba Bean (Vicia faba L.) for Biomass and Seed Yield across Different Environments. Sustainability 2021, 13, 2586. https://doi.org/10.3390/su13052586
Papastylianou P, Vlachostergios DN, Dordas C, Tigka E, Papakaloudis P, Kargiotidou A, Pratsinakis E, Koskosidis A, Pankou C, Kousta A, et al. Genotype X Environment Interaction Analysis of Faba Bean (Vicia faba L.) for Biomass and Seed Yield across Different Environments. Sustainability. 2021; 13(5):2586. https://doi.org/10.3390/su13052586
Chicago/Turabian StylePapastylianou, Panayiota, Dimitrios N. Vlachostergios, Christos Dordas, Evangelia Tigka, Paschalis Papakaloudis, Anastasia Kargiotidou, Emmanouil Pratsinakis, Avraam Koskosidis, Chrysanthi Pankou, Angeliki Kousta, and et al. 2021. "Genotype X Environment Interaction Analysis of Faba Bean (Vicia faba L.) for Biomass and Seed Yield across Different Environments" Sustainability 13, no. 5: 2586. https://doi.org/10.3390/su13052586
APA StylePapastylianou, P., Vlachostergios, D. N., Dordas, C., Tigka, E., Papakaloudis, P., Kargiotidou, A., Pratsinakis, E., Koskosidis, A., Pankou, C., Kousta, A., Mylonas, I., Tani, E., Abraham, E. M., Karatassiou, M., & Kostoula, S. (2021). Genotype X Environment Interaction Analysis of Faba Bean (Vicia faba L.) for Biomass and Seed Yield across Different Environments. Sustainability, 13(5), 2586. https://doi.org/10.3390/su13052586








