Functional Diversity Can Predict Ecosystem Functions Better Than Dominant Species: The Case of Desert Plants in the Ebinur Lake Basin
Abstract
1. Introduction
2. Materials and Methods
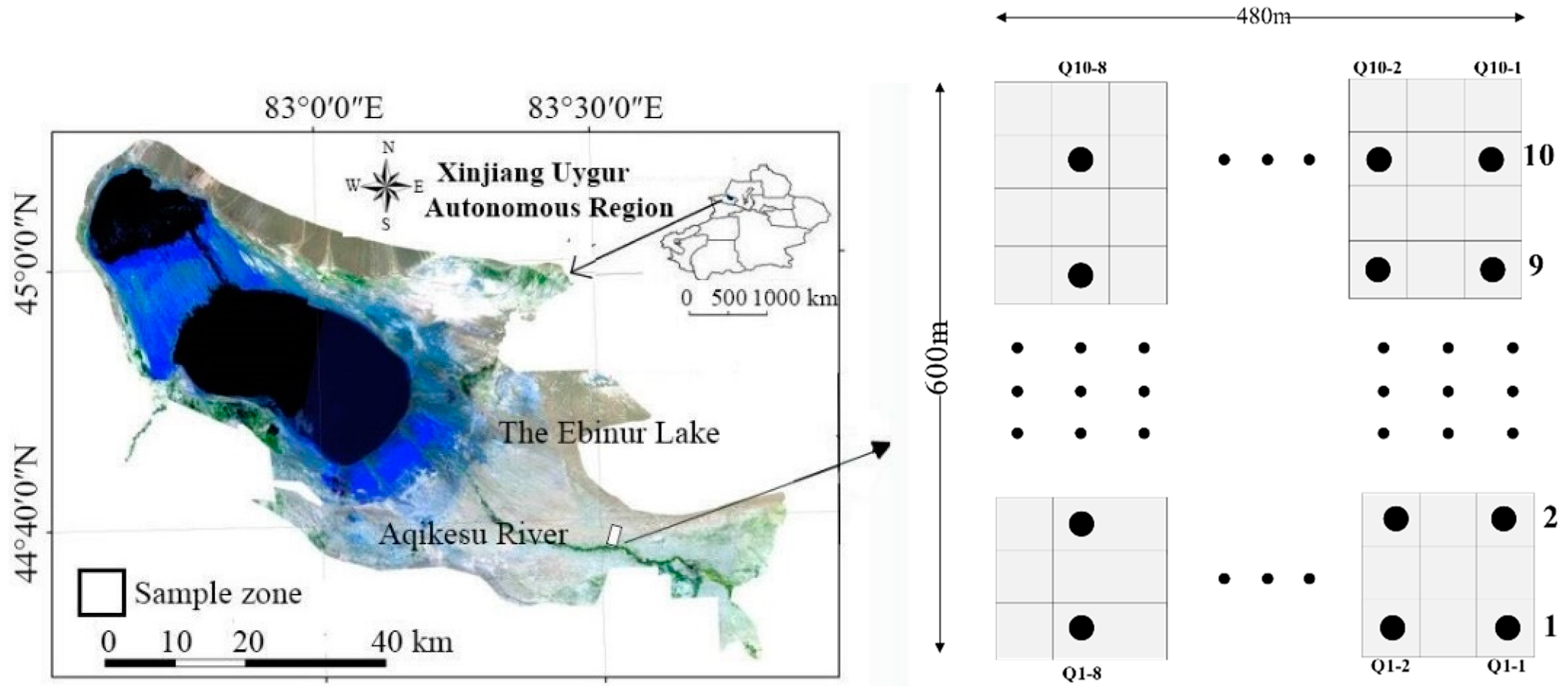
2.1. Overview of the Study Area
2.2. Field Community Survey
2.2.1. Soil Sampling and Determination of Soil Factors
2.2.2. Sampling and Determination of Plants
2.3. Data Processing and Statistics
3. Results and Analysis
3.1. Response of Soil Nutrients to Abundance and Functional Diversity of Dominant Species
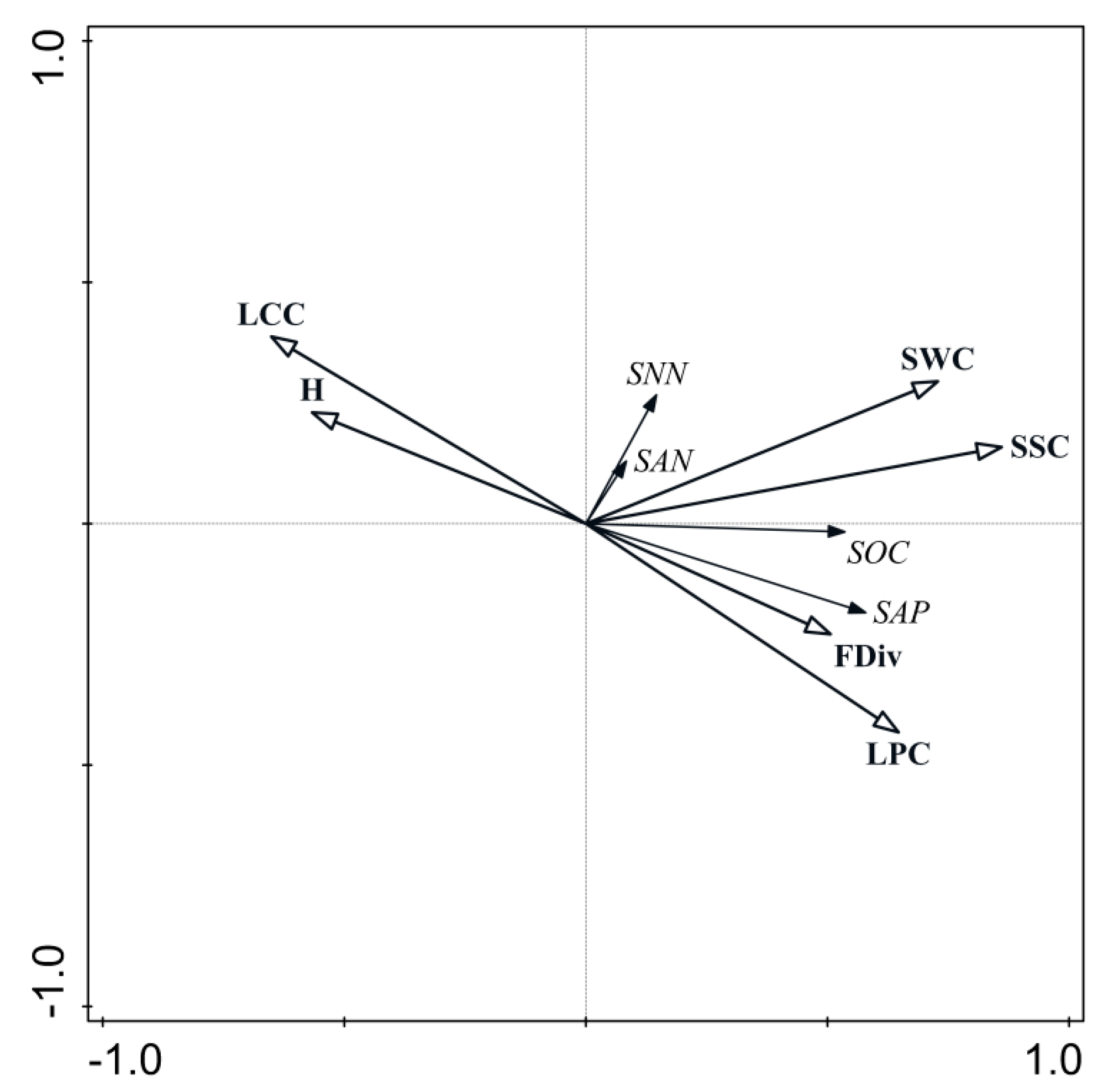
3.2. Relationship between Functional Diversity and Plant Characters and Soil Environment
3.3. The Relationship between Ecosystem Functions and the Richness and Functional Diversity of Dominant Species
3.4. The Action of Dominant Species Richness and Functional Diversity on Ecosystem Functions
4. Discussion
4.1. Relationship between Functional Diversity, Plant Characters, and the Soil Environment
4.2. Functions of Functional Diversity and Dominant Species on Ecosystem Functions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, I.M. Species Loss in Fragments of Tropical Rain Forest: A Review of the Evidence. J. Appl. Ecol. 1996, 33, 200. [Google Scholar] [CrossRef]
- Achard, F. Determination of Deforestation Rates of the World’s Humid Tropical Forests. Science 2002, 297, 999–1002. [Google Scholar] [CrossRef]
- Foley, J.A.; Asner, G.P.; Costa, M.H.; Coe, M.T.; DeFries, R.; Gibbs, H.K.; Howard, E.A.; Olson, S.; Patz, J.; Ramankutty, N.; et al. Amazonia Revealed: Forest Degradation and Loss of Ecosystem Goods and Services in the Amazon Basin. Front. Ecol. Environ. 2007, 5, 25–32. [Google Scholar] [CrossRef]
- De Groot, R.S.; Wilson, M.A.; Boumans, R.M.J. A Typology for the Classification, Description and Valuation of Ecosystem Functions, Goods and Services. Ecol. Econ. 2002, 41, 393–408. [Google Scholar] [CrossRef]
- McMichael, A.; Scholes, R.; Hefny, M.; Pereira, E.; Palm, C.; Foale, S. Linking Ecosystem Services and Human Well-Being; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Zarandian, A.; Baral, H.; Yavari, A.; Jafari, H.; Stork, N.; Ling, M.; Amirnejad, H. Anthropogenic Decline of Ecosystem Services Threatens the Integrity of the Unique Hyrcanian (Caspian) Forests in Northern Iran. Forests 2016, 7, 51. [Google Scholar] [CrossRef]
- Kremen, C. Managing Ecosystem Services: What Do We Need to Know about Their Ecology? Ecology of Ecosystem Services. Ecol. Lett. 2005, 8, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.D.; Okabe, K.; Tylianakis, J.M.; Kumar, P.; Brockerhoff, E.G.; Schellhorn, N.A.; Parrotta, J.A.; Nasi, R. Forest Biodiversity and the Delivery of Ecosystem Goods and Services: Translating Science into Policy. BioScience 2011, 61, 972–981. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.-S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the Evidence for Biodiversity Effects on Ecosystem Functioning and Services: Biodiversity and Ecosystem Functioning/Services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; David, A.W.; et al. Erratum: Corrigendum: Biodiversity Loss and Its Impact on Humanity. Nature 2012, 489, 326. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Fröberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher Levels of Multiple Ecosystem Services Are Found in Forests with More Tree Species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.; Messier, C. The Effect of Biodiversity on Tree Productivity: From Temperate to Boreal Forests: The Effect of Biodiversity on the Productivity. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef]
- Vilà, M.; Carrillo-Gavilán, A.; Vayreda, J.; Bugmann, H.; Fridman, J.; Grodzki, W.; Haase, J.; Kunstler, G.; Schelhaas, M.; Trasobares, A. Disentangling Biodiversity and Climatic Determinants of Wood Production. PLoS ONE 2013, 8, e53530. [Google Scholar] [CrossRef]
- Huston, M.A. Hidden Treatments in Ecological Experiments: Re-Evaluating the Ecosystem Function of Biodiversity. Oecologia 1997, 110, 449–460. [Google Scholar] [CrossRef]
- Hector, A.; Schmid, B.; Beierkuhnlein, C.; Caldeira, M.C.; Diemer, M.; Dimitrakopoulos, P.G.; Finn, J.A.; Freitas, H.; Giller, P.S.; Good, J.; et al. Plant Diversity and Productivity Experiments in European Grasslands. Sci. New Ser. 1999, 286, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and Productivity in a Long-Term Grassland Experiment. Sci. New Ser. 2001, 294, 843–845. [Google Scholar] [CrossRef]
- Gross, K.; Cardinale, B.J.; Fox, J.W.; Gonzalez, A.; Loreau, M.; Wayne Polley, H.; Reich, P.B.; van Ruijven, J. Species Richness and the Temporal Stability of Biomass Production: A New Analysis of Recent Biodiversity Experiments. Am. Nat. 2014, 183, 1–12. [Google Scholar] [CrossRef]
- Sasaki, T.; Lauenroth, W.K. Dominant Species, Rather than Diversity, Regulates Temporal Stability of Plant Communities. Oecologia 2011, 166, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Bonner, K.I.; Barker, G.M. Stability of Ecosystem Properties in Response to Above-Ground Functional Group Richness and Composition. Oikos 2000, 89, 11–23. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. CONSEQUENCES OF DOMINANCE: A REVIEW OF EVENNESS EFFECTS ON LOCAL AND REGIONAL ECOSYSTEM PROCESSES. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef]
- Roscher, C.; Weigelt, A.; Proulx, R.; Marquard, E.; Schumacher, J.; Weisser, W.W.; Schmid, B. Identifying Population- and Community-Level Mechanisms of Diversity-Stability Relationships in Experimental Grasslands: Diversity-Stability Relationships. J. Ecol. 2011, 99, 1460–1469. [Google Scholar] [CrossRef]
- Grman, E.; Lau, J.A.; Schoolmaster, D.R.; Gross, K.L. Mechanisms Contributing to Stability in Ecosystem Function Depend on the Environmental Context: Stabilizing Mechanisms in Grasslands. Ecol. Lett. 2010, 13, 1400–1410. [Google Scholar] [CrossRef]
- Deutschman, D.H. Design and Analysis of Biodiversity Field Experiments. Ecol. Res. 2001, 16, 833–843. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.M.H. Biodiversity and Ecosystem Stability in a Decade-Long Grassland Experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Byrnes, J.E.K.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity Enhances Ecosystem Multifunctionality across Trophic Levels and Habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef]
- Ratcliffe, S.; Wirth, C.; Jucker, T.; van der Plas, F.; Scherer-Lorenzen, M.; Verheyen, K.; Allan, E.; Benavides, R.; Bruelheide, H.; Ohse, B.; et al. Biodiversity and Ecosystem Functioning Relations in European Forests Depend on Environmental Context. Ecol. Lett. 2017, 20, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Li, Z.A.; Shen, W.J.; Yu, Z.Y.; Peng, S.L.; Liao, C.H.; Wu, J.G. Changes of biodiversity and ecosystem function during tropical forest restoration in southern China. Sci. China Ser. C Life Sci. 2006, 563–569. [Google Scholar] [CrossRef]
- Cai, Y. Relationship between Desert Plant Diversity and Ecosystem Multifunctionality along Water and Salt Gradients. Master’s Thesis, Xinjiang University, Ürümqi, China, 2019. [Google Scholar]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; Garcia-Gomez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Bowker, M.A.; Maestre, F.T.; Mau, R.L. Diversity and Patch-Size Distributions of Biological Soil Crusts Regulate Dryland Ecosystem Multifunctionality. Ecosystems 2013, 16, 923–933. [Google Scholar] [CrossRef]
- Li, J.P.; Zhen, Z.R.; Zhao, N.X.; Gao, Y.B. Relationship between ecosystem multifuntionality and species diversity in grassland ecosystems under land-use types of clipping, enclosure and grazing. Chin. J. Plant Ecol. 2016, 40, 735–747. [Google Scholar] [CrossRef]
- Xiong, D.P.; Zhao, G.S.; Wu, J.S.; Shi, P.L.; Zhang, X.Z. The relationship between species diversity and ecosystem multifunctionality in alpine grasslands on the Tibetan Changtang Plateau. Acta Ecol. Sin. 2016, 36, 3362–3371. [Google Scholar] [CrossRef]
- Lei, L.J.; Kong, D.L.; Li, X.M.; Zhou, Z.X.; Li, G.Y. Plant functional traits, functional diversity, and ecosystem functioning: Current knowledge and perspectives. Biodivers. Sci. 2016, 24, 922–931. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.T.; Wang, P.; Zhou, D.W. Calculation method of plant community functional diversity. Chin. J. Ecol. 2011, 30, 2053–2059. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional Diversity (FD), Species Richness and Community Composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Mason, N.W.H.; MacGillivray, K.; Steel, J.B.; Wilson, J.B. An Index of Functional Diversity. J. Veg. Sci. 2003, 14, 571–578. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B.; Setälä, H. Functional Richness, Functional Evenness and Functional Divergence: The Primary Components of Functional Diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Richardson, S.J.; Peltzer, D.A.; de Bello, F.; Wardle, D.A.; Allen, R.B. Changes in Coexistence Mechanisms along a Long-Term Soil Chronosequence Revealed by Functional Trait Diversity: Functional Diversity along Ecological Gradients. J. Ecol. 2012, 100, 678–689. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of Plant Diversity to Ecosystems: Immediate, Filter and Founder Effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Yang, X.H.; He, Q.; Huo, W.; Liu, X.C. Study on the movement of wind-blown sand jump in the southern edge of Taklimakan Desert—A case study of Qira. J. Desert Res. 2012, 32, 910–914. [Google Scholar]
- Li, X.R.; Zhao, Y.; Hui, R.; Su, J.Q.; Gao, Y.H. Review on the research progress and trend of restoration ecology in arid areas of China. Prog. Geogr. 2014, 33, 1435–1443. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Zhao, Q.; Zhang, Q.D. Response of grassland community functional traits to soil water in a typical the Loess Plateau watershed. Acta Ecol. Sin. 2020, 40, 2691–2697. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, H.; Yuan, H.; Chen, J.Y.; Chai, J.W. Distribution of soil moisture, nitrogen and phosphorus in winter wheat field of shallow groundwater-covered area of Hebei Province―Take Anxin County as an example. J. Hebei Agric. Univ. 2020, 43, 103–110. [Google Scholar] [CrossRef]
- Fang, L.; LI, Y.; LI, F.; Zhu, H. Analysis of spatial variation of soil moisture–salinity–nutrient in Ebinur Lake wetlands, China. J. Agro-Environ. Sci. 2019, 38, 157–167. [Google Scholar] [CrossRef]
- Gong, S.H.; Wen, Z.M.; Shi, Y. The response of community-weighted mean plant functional traits to environmental gradients in Yanhe river catchment. Acta Ecol. Sin. 2011, 31, 6088–6097. [Google Scholar]
- Li, Y.L.; Cui, J.H.; Su, Y.Z. Specific leaf area and leaf dry matter content of some plants in different du ne habitats. Acta Ecol. Sin. 2005, 25, 304–311. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, X.A.; Lv, P.; Yue, X.Y.; Zhang, J. Functional Traits and Interrelations of Dominant Plant Species on Typical Grassland in the Horqin Sandy. Arid Zone Res. 2018, 35, 137–143. [Google Scholar]
- Li, X.L.; Liu, Z.Y.; Hou, X.Y.; Wu, X.H.; Wang, Z.; Hu, J.; Wu, Z.N. Plant Functional Traits and Their Trade-offs in Response to Grazing. Chin. Bull. Bot. 2015, 50, 159–170. [Google Scholar] [CrossRef]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J.-S. The Links between Ecosystem Multifunctionality and Above- and Belowground Biodiversity Are Mediated by Climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.K.; Tang, L.; Zhang, X.F.; Liu, S.L.; Liu, Q.R.; Su, X.K.; Zhang, Y.; Wu, X.Y.; Li, Y.; Zhao, Z.Z. Relationship between plant species diversity and functional diversity in alpine grasslands. Acta Ecol. Sin. 2017, 37, 1472–1483. [Google Scholar] [CrossRef]
- Xu, Y. Study on Plant Functional Diversity of Tropical Rainforest in Xishuangbann. Master’s Thesis, Yunnan University, Kunming, China, 2017. [Google Scholar]
- Mensah, S.; Salako, K.V.; Assogbadjo, A.; Glèlè Kakaï, R.; Sinsin, B.; Seifert, T. Functional Trait Diversity Is a Stronger Predictor of Multifunctionality than Dominance: Evidence from an Afromontane Forest in South Africa. Ecol. Indic. 2020, 115, 106415. [Google Scholar] [CrossRef]
- Lv, T.T. Study on the Relationship between Functional Diversity and Ecosystem Function of Herbaceous Plant Community. Master’s Thesis, Northeast Normal University, Changchun, China, 2014. [Google Scholar]


| Functional Diversity Indices | Ecosystem Function | Fitting Equation | R2 | p |
|---|---|---|---|---|
| SR | SAP | y = −0.1x + 17.134 | 0.004 | 0.569 |
| SOC | y = 0.279x + 14.258 | 0.015 | 0.289 | |
| SNN | y = −0.181x + 15.742 | 0.109 | 0.340 | |
| SAN | y = 0.671 + 10.979 | 0.181 | 0.110 | |
| FRic | SAP | y = −0.013x2 + 0.562x + 8.017 | 0.085 | 0.012 |
| SOC | y = 0.057x + 13.335 | 0.080 | 0.481 | |
| SNN | y = 0.045 + 13.451 | 0.088 | 0.441 | |
| SAN | y = −0.122x + 14.343 | 0.108 | 0.344 | |
| FEve | SAP | y = 0.004x + 0.505 | 0.022 | 0.192 |
| SOC | y = 0.008x + 0.0536 | 0.238 | 0.034 | |
| SNN | y = 0.0002x + 0.568 | 0.0001 | 0.932 | |
| SAN | y = −4.45x + 0.569 | 0.0001 | 0.995 | |
| FDiv | SAP | y = 0.012x + 0.613 | 0.181 | 0.0001 |
| SOC | y = 0.004x + 0.805 | 0.012 | 0.337 | |
| SNN | y = 0.003x + 0.815 | 0.01 | 0.372 | |
| SAN | y = −0.002x + 0.837 | 0.001 | 0.751 | |
| FDis | SAP | y = −0.015x + 1.503 | 0.02 | 0.211 |
| SOC | y = 0.009x + 1.2 | 0.003 | 0.626 | |
| SNN | y = 0.011x + 1.209 | 0.009 | 0.401 | |
| SAN | y = 0.04x + 0.972 | 0.026 | 0.158 |
| Impact Factor | Explanatory Quantity% | p |
|---|---|---|
| SSC | 47.5 | 0.002 |
| SWC | 35.1 | 0.004 |
| LPC | 30.0 | 0.004 |
| LCC | 29.7 | 0.004 |
| H | 21.8 | 0.014 |
| FDiv | 19.1 | 0.006 |
| Impact Factor | Explanatory Quantity% | p |
|---|---|---|
| FDiv | 41.6 | 0.02 |
| FEve | 24.3 | 0.094 |
| FDis | 20.7 | 0.174 |
| SR | 16.3 | 0.228 |
| FRic | 11.1 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Lv, G.; Jiang, L. Functional Diversity Can Predict Ecosystem Functions Better Than Dominant Species: The Case of Desert Plants in the Ebinur Lake Basin. Sustainability 2021, 13, 2858. https://doi.org/10.3390/su13052858
Hou Z, Lv G, Jiang L. Functional Diversity Can Predict Ecosystem Functions Better Than Dominant Species: The Case of Desert Plants in the Ebinur Lake Basin. Sustainability. 2021; 13(5):2858. https://doi.org/10.3390/su13052858
Chicago/Turabian StyleHou, Zhufeng, Guanghui Lv, and Lamei Jiang. 2021. "Functional Diversity Can Predict Ecosystem Functions Better Than Dominant Species: The Case of Desert Plants in the Ebinur Lake Basin" Sustainability 13, no. 5: 2858. https://doi.org/10.3390/su13052858
APA StyleHou, Z., Lv, G., & Jiang, L. (2021). Functional Diversity Can Predict Ecosystem Functions Better Than Dominant Species: The Case of Desert Plants in the Ebinur Lake Basin. Sustainability, 13(5), 2858. https://doi.org/10.3390/su13052858






