Regulation of Maize Kernel Carbohydrate Metabolism by Abscisic Acid Applied at the Grain-Filling Stage at Low Soil Water Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, Experimental Design, and Sampling
2.2. Measurements of Sucrose, Glucose, and Fructose Content
2.3. Measurement of Sucrose Synthetase Activity
2.4. Measurement of Sucrose Invertase Activity
2.5. Measurement of Yield Index
2.6. RNA Isolation and Real-Time RT-PCR
2.7. Statistical Analysis
3. Results
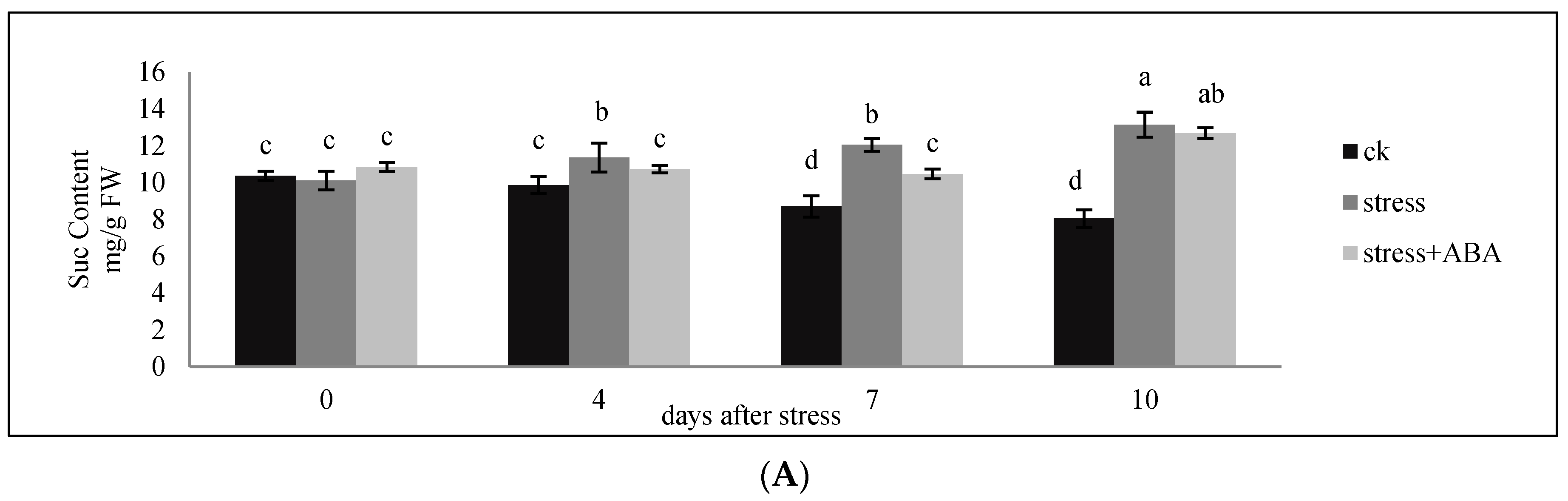
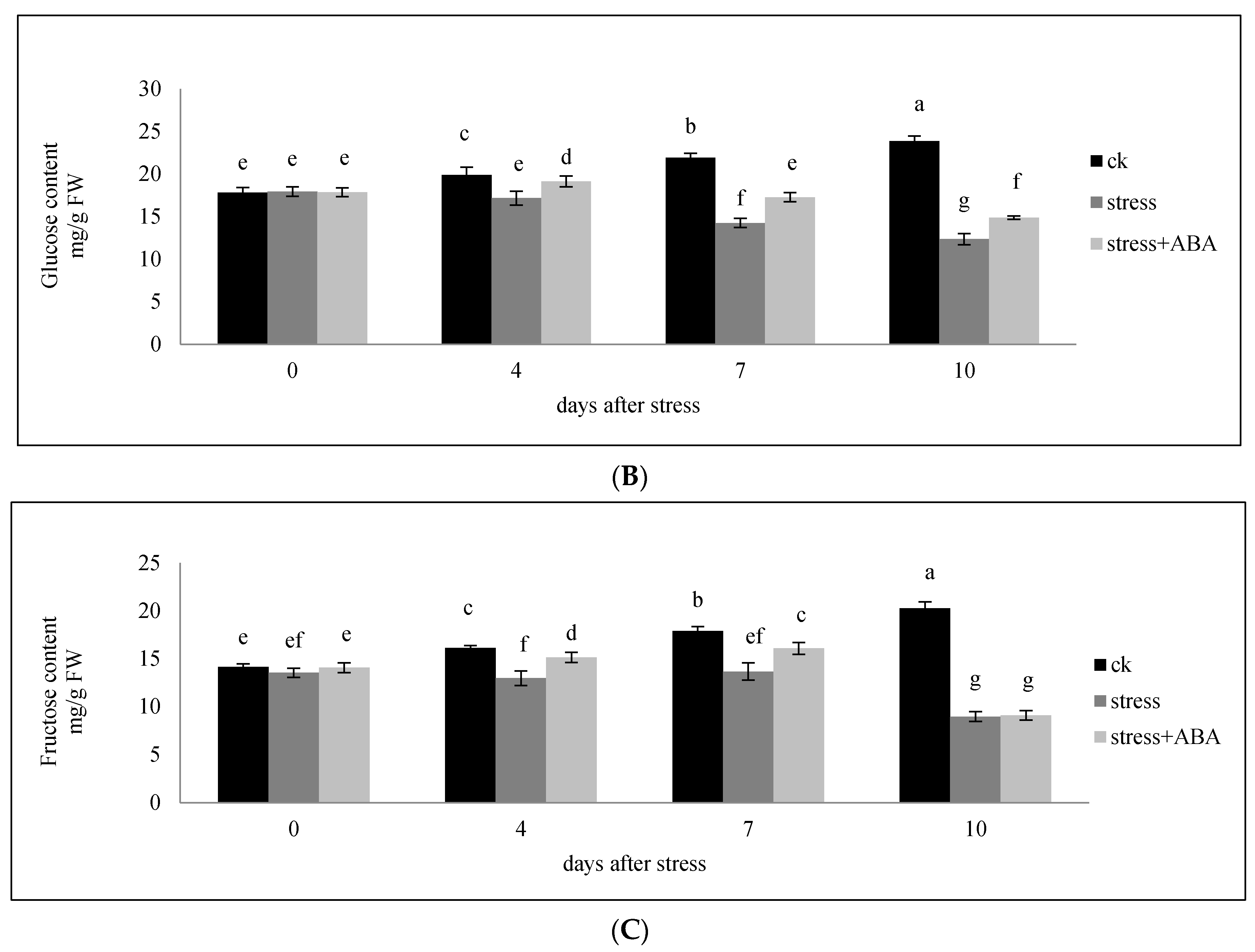
3.1. Sucrose, Glucose, and Fructose Content of Ovaries
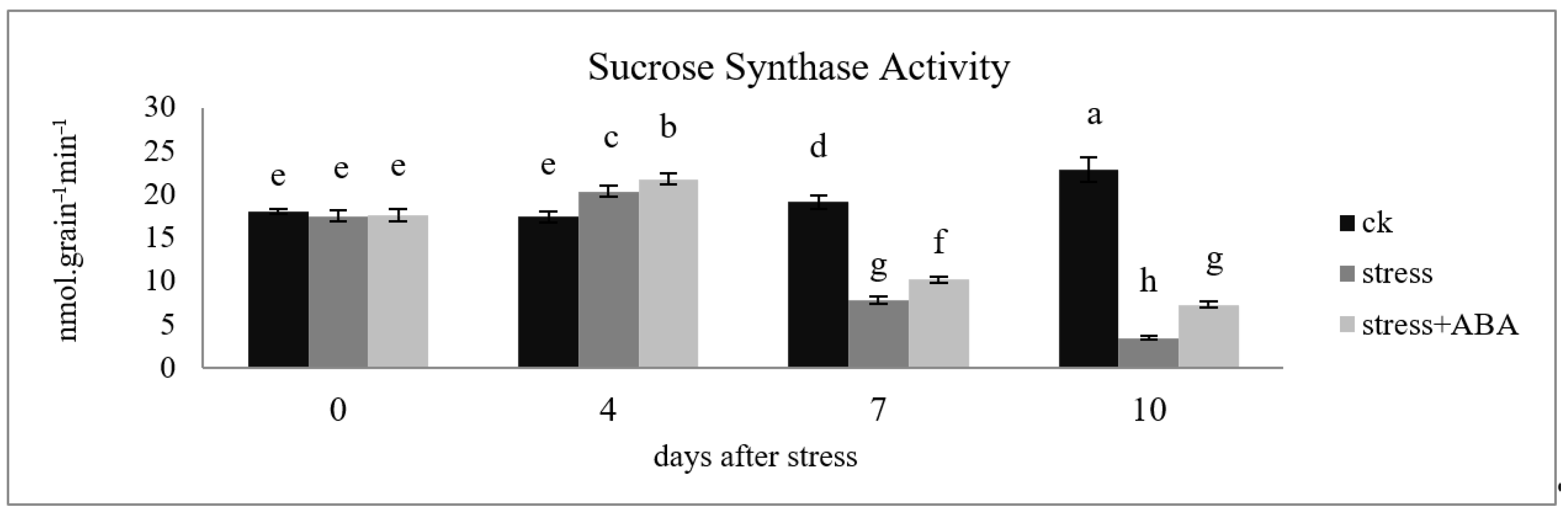
3.2. Effects of Drought Stress and/or ABA Application on Sucrose Synthase Activity
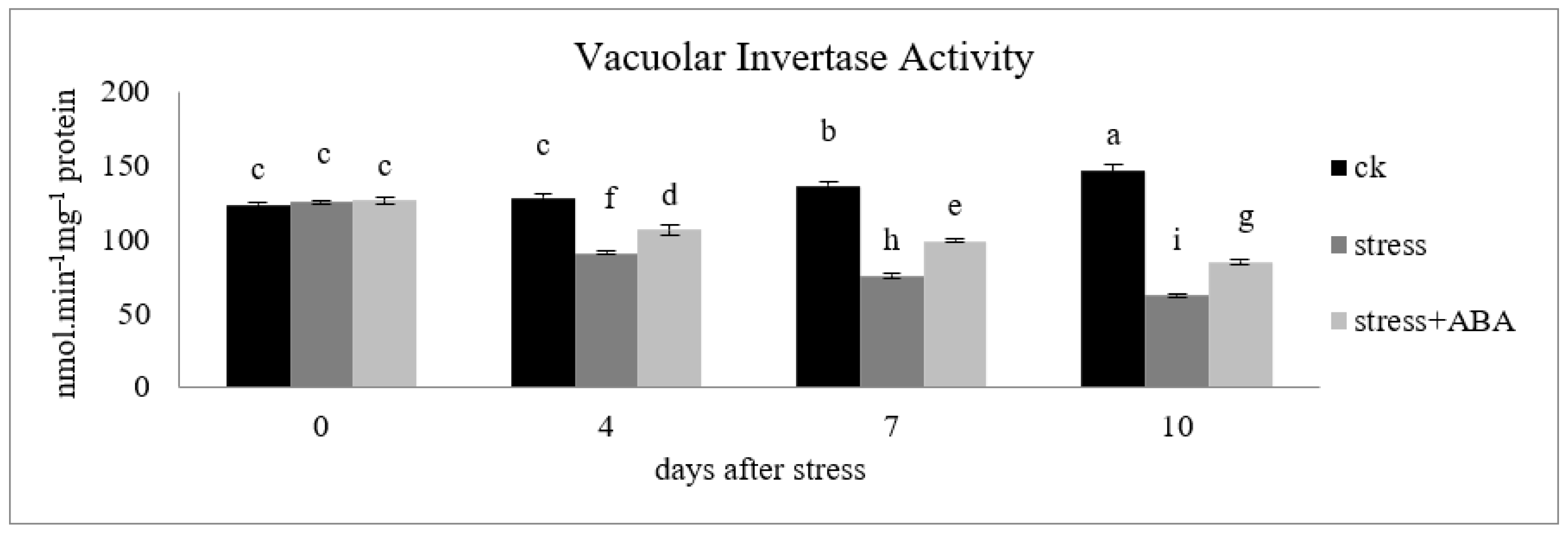
3.3. Effects of Drought Stress and/or ABA Application on Vacuolar Invertase Activity
3.4. Effects of Drought Stress and/or ABA Application on Cell Wall Invertase Activity
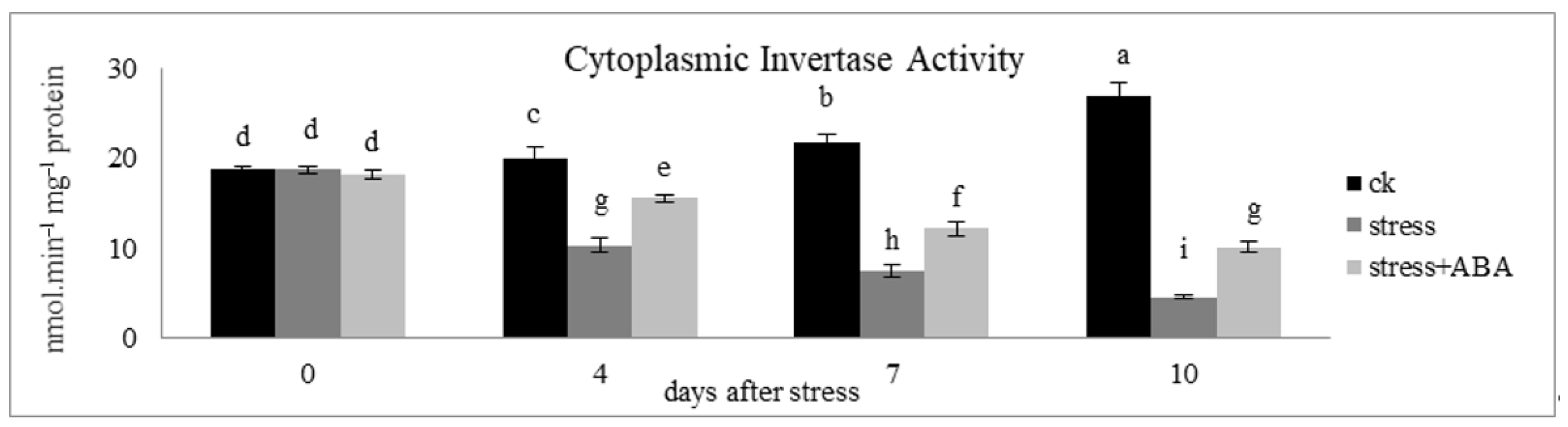
3.5. Effects of Drought Stress and/or ABA Application on Cytoplasmic Invertase Activity
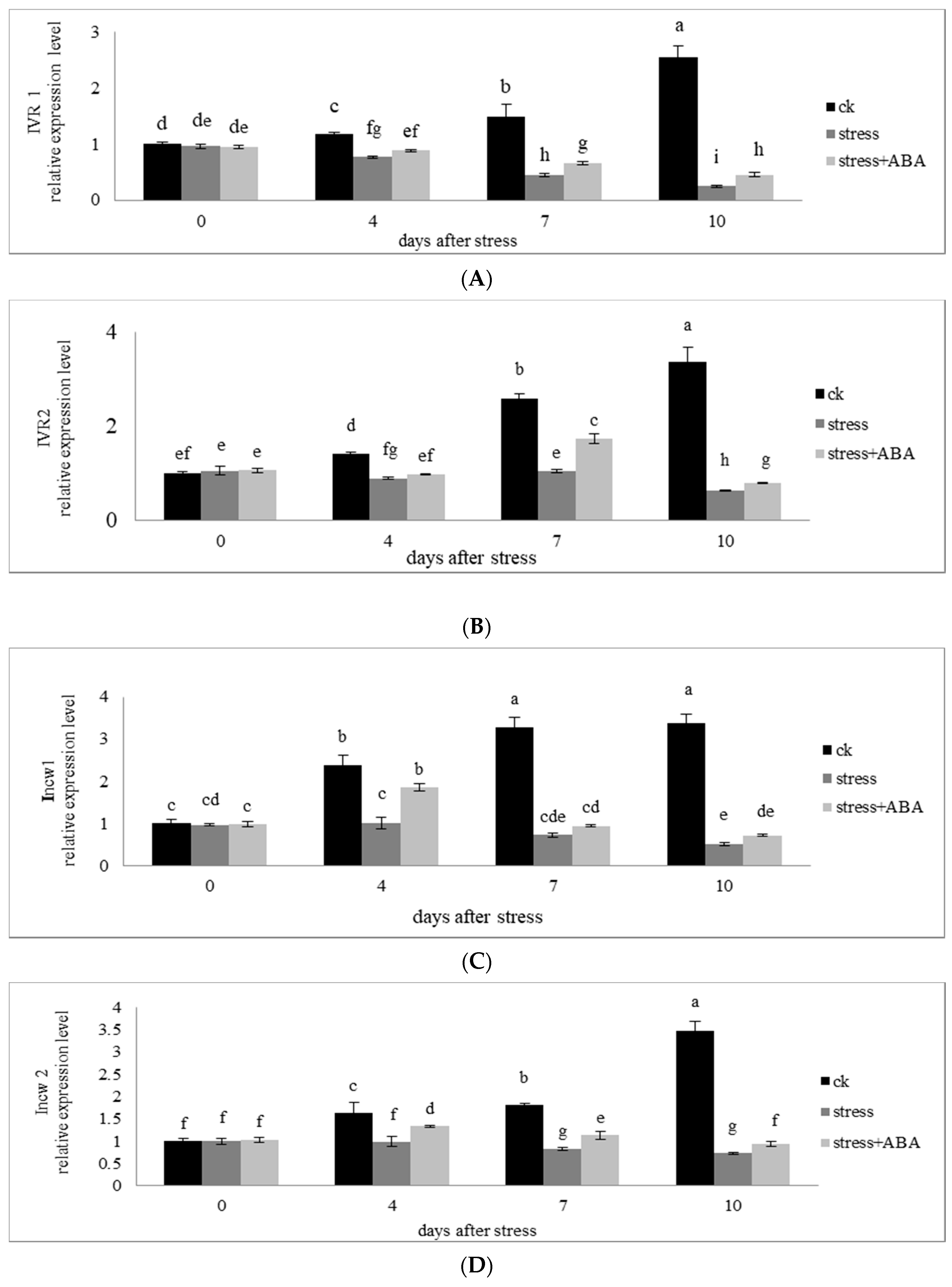
3.6. Effects of Drought Stress and/or ABA Application on the Expression of Genes Encoding Invertase Enzymes
3.7. Effects of Drought Stress and/or ABA Application on the Maize Yield Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans, PsJN and enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Nuccio, M.L.; Wu, J.; Mowers, R.; Zhou, H.P.; Meghji, M.; Primavesi, L.F.; Paul, M.J.; Chen, X.; Gao, Y.; Haque, E.; et al. Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in non-stressed and drought conditions. Nat. Biotechnol. 2015, 33, 862–869. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.G.; Sun, S. Constraints on maize yield and yield stability in the main cropping regions in China. Eur. J. Agron. 2018, 99, 106–115. [Google Scholar] [CrossRef]
- Li, Y.B.; Tao, H.B.; Zhang, B.C.; Huang, S.B.; Wang, P. Timing of water deficit limits maize kernel setting in association with changes in the source-flow-sink relationship. Front Plant Sci. 2018, 9, 1326. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zhang, H.; Zeng, R.; Wang, X.; Huang, L.; Wang, L.; Wang, X.; Zhang, L. Shade effects on peanut yield associate with physiological and expressional regulation on photosynthesis and sucrose metabolism. Inter. J. Mol. Sci. 2020, 21, 5284. [Google Scholar] [CrossRef]
- Lu, M.Z.; Snyder, R.; Grant, J.; Tegeder, M. Manipulation of sucrose phloem and embryo loading affects pea leaf metabolism, carbon and nitrogen partitioning to sinks as well as seed storage pools. Plant J. 2020, 101, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.M.; Wang, L.; Ruan, Y.L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, T.; Shuai, L.; Liao, L.; Li, J.; Duan, Z.; Guo, X.; Xue, X.; Han, D.; Wu, Z. Soluble Acid Invertases Act as Key Factors Influencing the Sucrose/Hexose Ratio and Sugar Receding in Longan (Dimocarpus longan L.) Pulp. J. agric. Food Chem. 2019, 67, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Belhassine, F.; Martinez, S.; Bluy, S.; Fumey, D.; Kelner, J.J.; Costes, E.; Pallas, B. Fruit growth, photosynthesis and starch accumulation are differentially affected by local variation in source/sink ratios. Acta Hortic. 2020, 1281, 455–462. [Google Scholar] [CrossRef]
- Antonietta, M.; Fanello, D.D.; Acciaresi, H.A.; Guiamet, J.J. Senescence and yield responses to plant density in stay green and earlier-senescing maize hybrids from Argentina. Field Crops Res. 2014, 155, 111–119. [Google Scholar] [CrossRef]
- Khan, N.A.; Murayama, S.; Ishimine, Y.; Tsuzuki, E.; Nakamura, I. Physio-morphological Studies of F1 Hybrids in Rice (Oryza sativa L.): Photosynthetic ability and yield. Plant Prod. Sci. 1998, 1, 233–239. [Google Scholar] [CrossRef]
- Milne, R.J.; Perroux, J.M.; Rae, A.L.; Reinders, A.; Ward, J.M.; Offler, C.E.; Patrick, J.W.; Grof, C.P.L. Sucrose transporter localization and function in phloem loading and unloading. Plant Physiol. 2016, 173, 1330–1341. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Raei, N.; Raei, Y. Effects of water deficit and nitrogen levels on grain yield and oil and protein contents of maize. Azarian J. Agric. 2015, 2, 46–50. [Google Scholar]
- Wu, X.; Cai, K.; Zhang, G.; Zeng, F. Metabolite profiling of barley grains subjected to water stress: To explain the genotypic difference in drought-induced impacts on malting quality. Front. Plant Sci. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Barratt, D.H.P.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. 2009, 106, 13124–13129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.M.; Brill, E.; Llewellyn, D.J.; Furbank, R.T.; Ruan, Y.-L. Overexpression of a potato sucrose synthase gene in cotton accelerates leaf expansion, reduces seed abortion, and enhances fiber production. Mol. Plant. 2012, 5, 430–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranwala, A.P.; Baird, W.V.; Miller, W.B. Organ-specific localization and molecular properties of three soluble invertases from Lilium longiflorum flower beds. Physiol. Plant. 1998, 103, 551–559. [Google Scholar] [CrossRef]
- Bieniawska, Z.; Barratt, D.H.P.; Garlick, A.P.; Thole, V.; Kruger, N.J.; Martin, C.; Zrenner, R.; Smith, A.M. Analysis of the sucrose synthase gene family in Arabidopsis. Plant J. 2007, 49, 810–2827. [Google Scholar] [CrossRef]
- Chourey, P.S.; Taliercio, E.W.; Carlson, S.J.; Ruan, Y.-L. Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol. Gen. Genet. 1998, 259, 88–96. [Google Scholar] [CrossRef]
- Wang, F.; Sanz, A.; Brenner, M.L.; Smith, A. Sucrose synthase, starch accumulation, and tomato fruit sink strength. Plant Physiol. 1993, 101, 321–327. [Google Scholar] [CrossRef]
- Zrenner, R.; Salanoubat, M.; Willmitzer, L.; Sonnewald, U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 2010, 7, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Travaglia, C.; Cohen, A.C.; Reinoso, H.; Castillo, C.; Bottini, R. Exogenous abscisic acid increases carbohydrate accumulation and redistribution to the grains in wheat grown under field conditions of soil water restriction. J. Plant Growth Regul. 2007, 26, 285–289. [Google Scholar] [CrossRef]
- Bray, E.A. Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant Cell Environ. 2010, 25, 153–161. [Google Scholar] [CrossRef]
- Souza, T.C.; Magalhães, P.C.; Castro, E.M.; Albuquerque, P.E.P.; Marabesi, M.A. The influence of ABA on water relation, photosynthesis parameters, and chlorophyll fluorescence under drought conditions in two maize hybrids with contrasting drought resistance. Acta Physiol. Plant. 2013, 35, 515–527. [Google Scholar] [CrossRef]
- Nieves, N.; Martínez, M.E.; Castillo, R.; Blanco, M.A. Effect of abscisic acid and jasmonic acid on partial desiccation of encapsulated somatic embryos of sugarcane. Plant Cell Tiss. Org. Cult. 2001, 65, 15–21. [Google Scholar] [CrossRef]
- Prabu, G.; Kawar, P.G.; Pagariya, M.C.; Prassad, D.T. Identification of water deficit stress upregulated genes in sugarcane. Plant Mol. Biol. Rep. 2010, 29, 291–304. [Google Scholar] [CrossRef]
- Mittal, A.; Gampala, S.S.L.; Ritchie, G.L.; Payton, P.; Burke, J.J.; Rock, C.D. Related to ABA-Insensitive3 (ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotechnol J. 2014, 12, 578–589. [Google Scholar] [CrossRef] [Green Version]
- Liu, F. Loss of pod set caused by drought stress is associated with water status and ABA content of reproductive structures in soybean. Funct. Plant Biol. 2003, 30, 271–280. [Google Scholar] [CrossRef]
- Lim, C.W.; Yang, S.H.; Shin, K.H.; Lee, S.C.; Kim, S.H. The AtLRK10L1.2, Arabidopsis ortholog of wheat LRK10, is involved in ABA-mediated signaling and drought resistance. Plant Cell Rep. 2015, 34, 447–455. [Google Scholar] [CrossRef]
- Liu, H.; Searle, I.R.; Mather, D.E.; Able, A.; Able, J. Morphological, physiological and yield responses of durum wheat to pre-anthesis water deficit stress are genotype-dependent. Crop Past. Sci. 2015, 66, 1024–1038. [Google Scholar] [CrossRef]
- Souza, T.C.; Magalhães, P.C.; Castro, E.M.; Carneiro, N.P.; Padiha, F.A.; Gomes, C.C. ABA application to maize hybrids contrasting for drought tolerance: Changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul. 2014, 73, 205–217. [Google Scholar] [CrossRef]
- Marx, D.H.; Hatch, A.B.; Mendicino, J.F. High soil fertility decreases sucrose content and susceptibility of loblolly pine roots to ectomycorrhizal infection by Pisolithus tinctorius. Canadian J. Bot. 1977, 55, 1569–1574. [Google Scholar] [CrossRef]
- Abdellatif, B.; Jun, L.; Miroslav, O.; Ezquer, I.; Muñoz, F.I.; Baroja-Fernández, E.; Romero, J.M.; Almagro, G.; Montero, M.; Hidalgo, M.; et al. Arabidopsis thaliana mutants lacking ADP-glucose pyrophosphorylase accumulate starch and wild-type ADP-glucose content: Further evidence for the occurrence of important sources, other than ADP-glucose pyrophosphorylase, of ADP-glucose linked to leaf starch biosynthesis. J Plant Cell Physiol. 2011, 7, 1162–1176. [Google Scholar]
- Suzuki, Y.; Odanaka, S.; Kanayama, Y. Fructose content and fructose-related enzyme activity during the fruit development of apple and japanese pear. J. Jpn. Soc. Hortic. Sci. 2001, 70, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Douglas, C.D.; Tsung, M.K.; Frederick, C.F. Enzymes of sucrose and hexose metabolism in development of kernels of two inbreds of maize. Plant Physiol. 1988, 86, 1013–1019. [Google Scholar]
- Tsai-Mei, O.L.; Lloyd, S.T. Effect of increased temperature in apical regions of maize ears on starch-synthesis enzymes and accumulation of sugars and starch 1. Plant Physiol. 1985, 79, 852–855. [Google Scholar]
- Yong-Jian, S.; Yuan-Yuan, S.; Kai, L. Effects of water-nitrogen interaction on rice senescence and material transport and yield during grain filling. Plant Nutr. Fert. Sci. 2009, 15, 1339–1349. [Google Scholar]
- Liu, Y.J.; Yuan, Y.; Liu, Y.Y.; Liu, Y.; Fu, J.J.; Zheng, J.; Wang, G.Y. Gene families of maize glutathione–ascorbate redox cycle respond differently to abiotic stresses. J.Plant Physiol. 2012, 169, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. J. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yuan, B.; Leng, P. Cloning of 9-cis-epoxycarotenoid dioxygenase (NCED) gene and the role of ABA on fruit ripening. Plant Signal. Behav. 2009, 4, 460–463. [Google Scholar] [CrossRef] [Green Version]
- Li, C.N.; Manoj, K.S.; Nong, Q.; Li, Y.R. Mechanism of tolerance to drought in sugarcane plant enhanced by foliage dressing of abscisic acid under water stress. Acta Agron. Sin. 2010, 36, 863–870. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, A.K.; Zhawar, V.K. ABA-dependent sucrose regulation of antioxidant metabolism in wheat cultivars varying in ABA-sensitivity. Biologia 2015, 70, 165–173. [Google Scholar] [CrossRef]
- Farrokhi, Z.; Alizadeh, H.; Alizadeh, H. Developmental patterns of enzyme activity, gene expression, and sugar content in sucrose metabolism of two broomrape species. Plant Physiol. Biochem. 2019, 142, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A. Sucrose synthase – an enzyme with a central role in the source–sink coordination and carbon flow in trees. New Phytol. 2021, 229, 186–198. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sánchez-Díaz, M. Alfalfa leaf senescence induced by drought stress: Photosynthesis, hydrogen peroxide metabolism, lipid peroxidation and ethylene evolution. Physiol. Plant. 1992, 84, 67–72. [Google Scholar] [CrossRef]
- Jiang, Q.; Hou, J.; Hao, C.; Wang, L.; Ge, H.; Dong, Y.; Zhang, X. The wheat (T. aestivum) sucrose synthase 2 gene (TaSus2) active in endosperm development is associated with yield traits. Funct Integr. Genom. 2011, 11, 49–61. [Google Scholar] [CrossRef]
- Chengappa, S.; Guilleroux, M.; Phillips, W.; Shields, R. Transgenic tomato plants with decreased sucrose synthase are unaltered in starch and sugar accumulation in the fruit. Plant Mol. Biol. 1999, 40, 213–221. [Google Scholar] [CrossRef]
- Schaffer, M.A.A. Sucrose phosphate synthase, sucrose synthase, and invertase activities in developing fruit of Lycopersicon esculentum, Mill and the sucrose accumulating Lycopersicon hirsutum Humb and Bonpl. Plant Physiol. 1991, 95, 623–627. [Google Scholar]
- Counce, P.A.; Gravois, K.A. Sucrose synthase activity as a potential indicator of high rice grain yield. Crop Sci. 2013, 46, 1501–1507. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M.N. Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Physiol. 2002, 130, 591–604. [Google Scholar] [CrossRef] [Green Version]
- Verma, I.; Roopendra, K.; Sharma, A.; Chndra, A.; Kamal, A. Expression analysis of genes associated with sucrose accumulation and its effect on source–sink relationship in high sucrose accumulating early maturing sugarcane variety. Physiol. Mol. Biol. Plants. 2019, 25, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Shi, Z.; Zhang, Z.; Zhang, Y. Effects of exogenous glucose on seed germination and antioxidant capacity in wheat seedlings under salt stress. J. Plant Growth Regul. 2012, 68, 177–188. [Google Scholar] [CrossRef]
- Chourey, C.P.S. A re-evaluation of the relative roles of two invertases, INCW2 and IVR1, in developing maize kernels and other tissues. Plant Physiol. 1999, 121, 1025–1035. [Google Scholar]
- Hu, Y.P.; Liu, J.; Zhou, Y.; Xia, W.R.; Li, R.M.; Duan, R.J.; Fu, S.P.; Hu, X.W.; Guo, J.C.; Hu, Y.P. Cloning of MeCWINV3 Promoter from Cassava and Transient Expression Analysis in Tobacco. Appl. Mech. Mat. 2014, 522–524, 326–331. [Google Scholar] [CrossRef]
- Hirche, J.; García, J.M.; Sabentheiner, E.; Grobkinsky, D.K.; Roitsch, T. Differential effects of carbohydrates on Arabidopsis pollen germination. Plant Cell Physiol. 2017, 58, 691–701. [Google Scholar] [CrossRef]
- Jameson, P.E.; Dhandapani, P.; Novak, O.; Song, J. Cytokinins and expression of SWEET, SUT, CWINV and AAP Genes Increase as pea seeds germinate. Inter. J. Mol. Sci. 2016, 17, 2013. [Google Scholar] [CrossRef] [PubMed]
- Shao, X. Differences in sucrose metabolism in peach fruit stored at chilling stress versus nonchilling stress temperatures. Hort. Sci. 2015, 50, 1542–1548. [Google Scholar]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Carlson, S.J.; Shanker, S.; Chourey, P.S. A point mutation at the Miniature1 seed locus reduces levels of the encoded protein, but not its mRNA, in maize. Mol. Gen. Genet. 2000, 263, 367–373. [Google Scholar] [CrossRef]
- Chourey, P.S.; Jain, M.; Li, Q.B.; Carlson, S.J. Genetic control of cell wall invertase in developing endosperm of maize. Planta 2006, 223, 159–167. [Google Scholar] [CrossRef]
- Zinselmeier, C.; Sun, Y.; Helentjaris, T.; Beatty, M. The use of gene expression profiling to dissect the stress sensitivity of reproductive development in maize. Field Crops Res. 2002, 75, 111–121. [Google Scholar] [CrossRef]
- Barbosa, E.G.G.; Leite, J.P.; Rockenbach, S.R.; Marinho, J.P.; Correa, J.F.; Fuganti, R.; Boucas, J.R.; Neumaier, N.; Marcelino, F.C.; De Oliveira, M.C.N.; et al. Overexpression of the ABA-dependent AREB1 transcription factor from Arabidopsis thaliana improves soybean tolerance to water deficit. Plant Mol. Biol. Rep. 2013, 31, 719–730. [Google Scholar] [CrossRef]
- Bazrafshan, A.; Shorafa, M.; Mohammadi, M.; Zolfaghari, A.A.; van de Craats, D.; van der Zee, S.E.A.T.M. Comparison of the individual salinity and water deficit stress using water use, yield, and plant parameters in maize. Environ. Monit. Assess. 2020, 192, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, C.; Zhang, D.; He, C.; Zhang, J.; Li, Z. Effects of maize organ-specific drought stress response on yields from transcriptome analysis. BMC Plant Biol. 2019, 19, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliaspour, S.; Sharifi, R.S.; Shirkhani, A.; Farzaneh, S. Effects of biofertilizers and iron nano-oxide on maize yield and physiological properties under optimal irrigation and drought stress conditions. Food Sci. Nutr. 2020, 11, 5985–5998. [Google Scholar] [CrossRef] [PubMed]
- Rezende, W.S.; Beyene, Y.; Mugo, S.; Ndou, E.; Gowda, M.; Sserumaga, J.P.; Asea, G.; Ngolinda, I.; Jumbo, M.; Oikeh, S.O.; et al. Performance and yield stability of maize hybrids in stress-prone environments in eastern Africa. Crop J. 2020, 8, 107–118. [Google Scholar] [CrossRef]


| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| GAPDH IVR1 | 5′-CCCTTCATCACCACGGACTA-3′ 5′-GACGGCAGCCTCCAAACT-3′ | 5′-CTCACCCCACGGGATTTC-3′ 5′-TTCTCCCCATCTAGCACAGG-3′ |
| IVR2 | 5′-ATGGGTTGGCGAGACAGAC-3′ | 5′ –GATGGTGATGCCGCTGAG-3′ |
| Incw1 | X TCCAGCCGCCAATGAACT | X ACCAGCAGCCGTACTTGT |
| Incw2 | 5′-CGGGATACAAACGGCACA-3′ | 5′-GCTTGAACACCCTGAAGAACA-3′ |
| Incw3 | 5′-CCTCATGTGCAACGACCCTA-3′ | 5′-CGTGGTTGAAGACGAAGAGGT-3′ |
| Incw4 | 5′-ACACCGCTGTCTTCTTCCG-3′ | 5′-GATGGTCTCGTGCTCCTCAA-3′ |
| Source of Variation | df | Suc Content | Glu Content | Fru Content | SS Activity | VIN Activity | CIN Activity | CWIN Activity |
|---|---|---|---|---|---|---|---|---|
| T | 2 | 116.061 *** | 425.243 *** | 386.947 *** | 637.950 *** | 1606.622 *** | 1531.118 *** | 1089.682 *** |
| S | 3 | 9.480 *** | 21.461 *** | 80.236 *** | 614.557 *** | 322.431 *** | 165.650 *** | 432.196 *** |
| T × S | 6 | 31.659 *** | 100.743 *** | 125.635 *** | 359.418 *** | 287.110 *** | 264.002 *** | 194.042 *** |
| Treatment | Bald Tip (cm) | Compare with CK (%) | Row Number (Row) | Compare with CK (%) | Grain Number Per Row (Grain) | Compare with CK (%) | 1000 Grain Weight (g) | Compare with CK (%) | |
|---|---|---|---|---|---|---|---|---|---|
| 4th day | CK | 1.0 | 18 | 40 | 434.0 | ||||
| Stress | 3.0 | +200.0 | 18 | 0 | 31 | −22.5 | 369.8 | −14.8 | |
| Stress + ABA | 2.2 | +120.0 | 18 | 0 | 37 | −7.5 | 390.2 | −10.1 | |
| 7th day | CK | 1.1 | 18 | 40 | 402.0 | ||||
| stress | 4.5 | +309.1 | 14 | −22.2 | 25 | −37.5 | 337.8 | −16.0 | |
| stress + ABA | 3.2 | +190.9 | 16 | −11.1 | 32 | −20 | 353.0 | −12.2 | |
| 10th day | CK | 1.2 | 18 | 42 | 451.8 | ||||
| stress | 6.1 | +408.3 | 12 | −33.3 | 17 | −59.5 | 238.2 | −47.3 | |
| stress + ABA | 5.2 | +333.3 | 14 | −22.2 | 23 | −50.0 | 292.6 | −35.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Piao, L.; Guo, D.; Zhu, H.; Wang, S.; Zhu, H.; Yang, Z.; Tao, Y.; Li, M.; Liu, C. Regulation of Maize Kernel Carbohydrate Metabolism by Abscisic Acid Applied at the Grain-Filling Stage at Low Soil Water Potential. Sustainability 2021, 13, 3125. https://doi.org/10.3390/su13063125
Jiang Z, Piao L, Guo D, Zhu H, Wang S, Zhu H, Yang Z, Tao Y, Li M, Liu C. Regulation of Maize Kernel Carbohydrate Metabolism by Abscisic Acid Applied at the Grain-Filling Stage at Low Soil Water Potential. Sustainability. 2021; 13(6):3125. https://doi.org/10.3390/su13063125
Chicago/Turabian StyleJiang, Zizhu, Lin Piao, Dong Guo, Hengguang Zhu, Shuai Wang, Hanyu Zhu, Zhanhui Yang, Yuzhao Tao, Ming Li, and Changzhuang Liu. 2021. "Regulation of Maize Kernel Carbohydrate Metabolism by Abscisic Acid Applied at the Grain-Filling Stage at Low Soil Water Potential" Sustainability 13, no. 6: 3125. https://doi.org/10.3390/su13063125





