Comparing Wild and Cultivated Arnica montana L. from the Italian Alps to Explore the Possibility of Sustainable Production Using Local Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Germination Trials
- addition of 5 mL of distilled water (control);
- addition of 5 mL of a solution containing 50 mg L−1 of gibberellic acid (GA3);
- addition of 5 mL of a solution containing 100 mg L−1 of GA3;
- addition of 5 mL of a solution containing 200 mg L−1 of GA3.
2.3. Phytochemical Analysis
2.3.1. Chemicals and Reagents
2.3.2. Preparation and Analysis through HPLC and NMR of Crude Lactone Extracts
2.3.3. Volatiles Analysis through SPME GC-MS
3. Results
3.1. Seeds Germination
3.2. Phytochemical Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maguire, B. A monograph of the genus arnica. Brittonia 1943, 4, 386–510. [Google Scholar] [CrossRef]
- Hultén, E.; Fries, M. Atlas of North European Vascular Plants North of the Tropic of Cancer; Koeltz Scientific Books: Koenigstein, Germany, 1986; pp. 1894–1981. [Google Scholar]
- Kahmen, S.; Poschlod, P. Population size, plant performance, and genetic variation in the rare plant Arnica montana L. in the Rhön, Germany. BAAE 2000, 1, 43–51. [Google Scholar] [CrossRef]
- Parolo, G.; Rossi, G.; Ferrarini, A. Toward improved species niche modelling: Arnica montana. In: The Alps as a case study. J. Appl. Ecol. 2008, 45, 1410–1418. [Google Scholar] [CrossRef]
- Cislaghi, A.; Giupponi, L.; Tamburini, A.; Giorgi, A.; Bischetti, G.B. The effects of mountain grazing abandonment on plant community, forage value and soil properties: Observations and field measurements in an Alpine area. Catena 2019, 181, 104086. [Google Scholar] [CrossRef]
- Ellenberger, A. Assuming responsibility for a protected plant: WELEDA’s endeavour to secure the firm’s supply of Arnica montana. In Medicinal Plant Trade in Europe: Conservation and Supply, Proceedings of the First International Symposium on the Conservation of Medicinal Plants in Trade in Europe, Kew, UK, 22–23 June 1998; Traffic Europe: Kew, UK, 1999; pp. 127–130. [Google Scholar]
- Giupponi, L.; Leoni, V. VegeT: An easy tool to classify and facilitate the management of seminatural grasslands and dynamically connected vegetation of the Alps. Land 2020, 9, 473. [Google Scholar] [CrossRef]
- Falniowski, A.; Bazos, I.; Hodálová, I.; Lansdown, R.; Petrova, A. “Arnica montana”. IUCN 2012. IUCN Red List of Threatened Species, Version 2012.2; IUCN.
- Korneck, D.; Schnittler, M.; Vollmer, I. Red list of pteridophyta and spermatophyta in Germany. In Red List of Endangered Plants in Germany; Ludwig, G., Schnittler, M., Eds.; Bundesamt für Naturschutz: Bonn, Germany, 1996; Volume 28, pp. 21–187. [Google Scholar]
- Judžentienė, A.; Būdienė, J. Analysis of the chemical composition of flower essential oils from Arnica montana of Lithuanian origin. Chemija 2009, 20, 190–194. [Google Scholar]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Naidu, R.V.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-inflammatory treatments for chronic diseases: A review. Inflamm. Allergy Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef]
- Puhlmann, J.; Zenk, M.H.; Wagner, H. Immunologically active polysaccharides of Arnica montana cell cultures. Phytochemistry 1991, 30, 1141–1145. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. JID 2007, 127, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Klaas, C.A.; Wagner, G.; Laufer, S.; Sosa, S.; Della Loggia, R.; Bomme, U.; Pahl, H.L.; Merfort, I. Studies on the anti-inflammatory activity of phytopharmaceuticals prepared from Arnica flowers. Planta Med. 2002, 68, 385–391. [Google Scholar] [CrossRef]
- Jager, C.; Hrenn, A.; Zwuingmann, J.; Suter, A.; Merfort, I. Phytomedicines prepared from Arnica flowers inhibit the transcription factors AP-1 and NF-kappaB and modulate the activity of MMP1 and MMP13 in human and bovine chondrocytes. Planta Med. 2009, 75, 1319–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, J.A.; Smallfield, B.M.; Burgess, E.J.; Perry, N.B.; Anderson, R.E.; Douglas, M.H.; Glennie, V.L. Sesquiterpene Lactones in Arnica montana: A Rapid Analytical Method and the Effects of Flower Maturity and Simulated Mechanical Harvesting on Quality and Yield. Planta Med. 2004, 70, 166–170. [Google Scholar]
- Kathe, W. Conservation of Eastern-European medicinal plants: Arnica montana in Romania. In Medicinal and Aromatic Plants: Agricultural, Cultivated, Ecological, Legal, Pharmacological and Social Aspects; Bogers, R.J., Craker, L.E., Dagmar, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 17, pp. 203–211. [Google Scholar]
- Bomme, U.; Daniel, G. Erste Untersuchungsergebnisse zur Auslesezuechtung bei Arnica montana L. unter Einbeziehung der in-vitro-Kultur. In Vortraege fuer Pflanzenzuechtung; Bayerischer Landwirschaftsverlag GmbH: München, Germany, 1993; Volume 26, pp. 92–106. [Google Scholar]
- Bomme, U.; Daniel, G. Erste Untersuchungsergebnisse zur Auslesezüchtung bei Arnica montana L. In Gartenbauwissenschaft; Bayerischer Landwirschaftsverlag GmbH: München, Germany, 1994; Volume 59, pp. 67–71. [Google Scholar]
- Belliardo, F.; Bicchi, C.; Cordero, C.; Liberto, E.; Rubiolo, P.; Sgorbini, B. Headspace solid phase microextraction in the analysis of the volatile fraction of aromatic and medicinal plants. J. Chromatogr. Sci. 2006, 44, 416–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeleń, H.H.; Majchera, M.; Dziadas, M. Microextraction techniques in the analysis of food flavour compounds: A review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardencki, W. A headspace solid phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Petri, G.; Lemberkovics, E.; Foldvari, F. Examination of differences between propolis (bee glue) produced from different flora environment. In Flavors and Fragrances: A World Perspective; Lawrence, B.M., Mookherjee, B.D., Willis, B.J., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1988; pp. 439–446. [Google Scholar]
- Miguel, M.G.; Nunes, S.; Cruz, C.; Duarte, J.; Antunes, M.D.; Cavaco, A.M.; Mendes, M.D.; Lima, A.S.; Pedro, L.G.; Barroso, J.G.; et al. Propolis volatiles characterization from acaricide-treated and -untreated beehives maintained at Algarve (Portugal). Nat. Prod. Res. 2013, 27, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Leven, W.; Willuhn, G. Sesquiterpene lactones from Arnica chamissonis Less. VI. Identification and quantitative determination by high-performance liquid and gas chromatography. J. Chromatogr. 1987, 410, 329–342. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Bomme, U.; Alfermann, A.W. Sesquiterpene lactone content in leaves of in vitro and field cultivated Arnica montana. Planta Med. 1998, 64, 268–270. [Google Scholar] [CrossRef]
- Beekman, A.C.; Woerdenbag, H.J.; van Uden, W.; Pras, N.; Konings, A.W.; Wikström, H.V.; Schmidt, T.J. Structure-cytotoxicity relationships of some helenanolide-type sesquiterpene lactones. J. Nat. Prod. 1997, 60, 252–257. [Google Scholar] [CrossRef]
- Rivas-Martinez, S.; Rivas-Saenz, S. Sistema de Clasificacion Bioclimatica Mundial. Espana: Centro de Investigaciones Fitosociologicas. 2009. Available online: http://www.globalbioclimatics.org (accessed on 1 January 2021).
- Blasi, C.; Capotorti, G.; Copiz, R.; Guida, D.; Mollo, B.; Smiraglia, D.; Zavattero, L. Classification and mapping of the ecoregions of Italy. Plant Biosyst. 2014, 148, 1255–1345. [Google Scholar] [CrossRef]
- Kowalski, R.; Sugier, D.; Sugier, P.; Kołodziej, B. Evaluation of the chemical composition of essential oils with respect to the maturity of flower heads of Arnica montana L. and Arnica chamissonis Less. cultivated for industry. Ind. Crops Prod. 2015, 76, 857–865. [Google Scholar] [CrossRef]
- Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Citti, C.; Cattaneo, C.; Cavaletto, M.; Giorgi, A. Phytochemical and ecological analysis of two varieties of hemp (Cannabis sativa L.) grown in a mountain environment of Italian Alps. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Isla, F.; Benites-Alfaro, O.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application “GerminaQuant for R”. Ecol. Res. 2018, 34, 339–346. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment or Statistical Computing. R Foundation for Statistical Computing. 2019. Available online: http://www.r-project.org/ (accessed on 1 January 2021).
- Staneva, J.; Denkova, P.; Todorova, M.; Evstatieva, L. Quantitative analysis of sesquiterpenes lactones in extract of Arnica montana L. by 1H NMR spectroscopy. J. Pharm. Biomed. Anal. 2011, 54, 94–99. [Google Scholar] [CrossRef]
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Giupponi, L.; Leoni, V.; Pavlovic, R.; Giorgi, A. Influence of Altitude on Phytochemical Composition of Hemp Inflorescence: A Metabolomic Approach. Molecules 2020, 25, 1381. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, A.; Panseri, S.; Nanayakkara, N.N.M.C.; Chiesa, L.M. HS-SPME-GC/MS analysis of the volatile compounds of Achillea collina: Evaluation of the emissions fingerprint induced by Myzus persicae infestation. J. Plant Biol. 2012, 55, 251–260. [Google Scholar] [CrossRef]
- Giorgi, A.; Panseri, S.; Mattara, M.S.; Andreis, C.; Chiesa, L.M. Secondary metabolites and antioxidant capacities of Waldheimia glabra (decne.) regel from Nepal. J. Sci. Food Agric. 2013, 93, 1026–1034. [Google Scholar] [CrossRef]
- Giorgi, A.; De Marinis, P.; Granelli, G.; Chiesa, L.M.; Panseri, S. Secondary metabolite profile, antioxidant capacity, and mosquito repellent activity of Bixa orellana from Brazilian Amazon region. J. Chem. 2013, 409826. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, A.; Manzo, A.; Nanayakkara, N.N.; Giupponi, L.; Cocucci, M.; Panseri, S. Effect of biotic and abiotic stresses on volatile emission of Achillea collina Becker ex Rchb. Nat. Prod. Res. 2015, 29, 1695–1702. [Google Scholar] [CrossRef]
- Pavlovic, R.; Borgonovo, G.; Leoni, V.; Giupponi, L.; Ceciliani, G.; Sala, S.; Bassoli, A.; Giorgi, A. Effectiveness of different analytical methods for the characterization of propolis: A case of study in Northern Italy. Molecules 2020, 25, 504. [Google Scholar] [CrossRef] [Green Version]
- Pierce, S.; Spada, A.; Caporali, E.; Ceriani, R.M.; Buffa, G. Enzymatic scarification of Anacamptis morio (Orchidaceae) seed facilitates lignin degradation, water uptake and germination. Plant Biol. 2019, 21, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Giupponi, L.; Leoni, V. Alpine pioneer plants in soil bioengineering for slope stabilization and restoration: Results of a preliminary analysis of seed germination and future perspectives. Sustainability 2020, 12, 7190. [Google Scholar] [CrossRef]
- Pedrini, S.; Lewandrowski, W.; Stevens, J.C.; Dixon, K.W. Optimising seed processing techniques to improve germination and sowability of native grasses for ecological restoration. Plant Biol. 2019, 21, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Schwienbacher, E.; Navarro-Cano, J.A.; Neuner, G.; Erschbamer, B. Correspondence of seed traits with niche position in glacier foreland succession. Plant Ecol. 2012, 213, 371–382. [Google Scholar] [CrossRef]
- Sugier, D.; Sugier, P.; Gawlik-Dziki, U. Propagation and Introduction of Arnica montana L. into Cultivation: A Step to Reduce the Pressure on Endangered and High-Valued Medicinal Plant Species. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Kos, O.; Lindenmeyer, M.T.; Tubaro, A.; Sosa, S.; Merfort, I. New sesquiterpene lactones from Arnica tincture prepared from fresh flowerheads of Arnica montana. Planta Med. 2005, 71, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C. Altitudinal variation of secondary metabolites in flowering heads of the Asteraceae: Trends and causes. Phytochem. Rev. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- López, M.L.; Villatoro, C.; Fuentes, T.; Graell, J.; Lara, I.; Echeverría, G. Volatile compounds, quality parameters and consumer acceptance of ‘Pink Lady®’ apples stored in different conditions. Postharvest Biol. Technol. 2007, 43, 55–66. [Google Scholar] [CrossRef]
- Giorgi, A.; Pentimalli, D.; Giupponi, L.; Panseri, S. Quality traits of saffron (Crocus sativus L.) produced in the Italian Alps. Open Agric. 2017, 2, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Giupponi, L.; Ceciliani, G.; Leoni, V.; Panseri, S.; Pavlovic, R.; Lingua, G.; Di Filippo, A.; Giorgi, A. Quality traits of saffron produced in Italy: Geographical area effect and good practices. J. Appl. Bot. Food Qual. 2019, 92, 336–342. [Google Scholar]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A plant of healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef] [Green Version]
- Harborne, J.B.; Williams, A.C.; Wilson, K.L. Flavonoids in leaves and inflorescences of Australian Cyperus species. Phytochemistry 1982, 21, 2491–2507. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Janković, T.; Jelačić, S.; Novaković, M.; Menković, N.; Beatović, D.; Dajić-Stevanović, Z. Morphological and chemical characterization of Arnica montana L. under different cultivation models. Ind. Crops Prod. 2014, 52, 233–244. [Google Scholar] [CrossRef]
- Daniewski, W.M.; Gumulka, M.; Ptaszynska, K.; Skibicki, P.; Bloszyk, E.; Drozdz, B.; Stromberg, S.; Norin, T.; Holub, M. Antifeedant activity of some sesquiterpenoids of the genus Lactarius (Agaricales: Russulaceae). Eur. J. Entomol. 1993, 90, 65–70. [Google Scholar]
- Giupponi, L.; Leoni, V.; Colombo, F.; Cassani, E.; Hejna, M.; Rossi, L.; Pilu, R. Characterization of “Mais delle Fiorine” (Zea mays L.) and nutritional, morphometric and genetic comparison with other maize landraces of Lombardy region (Northern Italy). Genet. Resour. Crop. Evol. 2021, 1–17. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Ferreira Mendes, A. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, H.D.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; Vieira da Silva, F.; Cunha, F.V.M.; Wanderley, C.W.S.; Pinheiro, G.; Cândido, A.G.F.; Wong, D.V.T.; et al. α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef] [PubMed]
- De Souza Siqueira Quintans, J.; Passos Menezes, P.; Viana Santos, M.R.; Rigoldi Bonjardim, L.; Guedes Silva Almeida, J.R.; Pens Gelain, D.; de Souza Araújo, A.A.; Quintans-Júnior, L.J. Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytomedicine 2013, 20, 436–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrnhöfer-Ressler, M.M.; Fricke, K.; Pignitter, M.; Walker, J.M.; Walker, J.; Rychlik, M.; Somoza, V. Identification of 1,8-Cineole, Borneol, Camphor, and Thujone as Anti-inflammatory Compounds in a Salvia officinalis L. Infusion Using Human Gingival Fibroblasts. J. Agric. Food Chem. 2013, 61, 3451–3459. [Google Scholar] [CrossRef]
- Muroi, H.; Kubo, A.; Kubo, I. Antimicrobial activity of cashew apple flavor compounds. J. Agric. Food Chem. 1993, 41, 1106–1109. [Google Scholar] [CrossRef]
- Amorim, J.L.; Reis Simas, D.L.; Martins Gomes Pinheiro, M.; Sales Alviano Moreno, D.; Sales Alviano, C.; Ribeiro da Silva, A.J.; Dias Fernandes, P. Anti-inflammatory properties and chemical characterization of the essential oils of four citrus species. PLoS ONE 2016, 11, e0153643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bülow, N.; König, W.A. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 2000, 55, 141–168. [Google Scholar] [CrossRef]
- Perry, N.B.; Burgess, E.J.; Rodríguez Guitián, M.A.; Romero Franco, R.; López Mosquera, E.; Smallfield, B.M.; Joyce, N.I.; Littlejohn, R.P. Sesquiterpene Lactones in Arnica montana: Helenalin and Dihydrohelenalin Chemotypes in Spain. Planta Med. 2009, 75, 660–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]





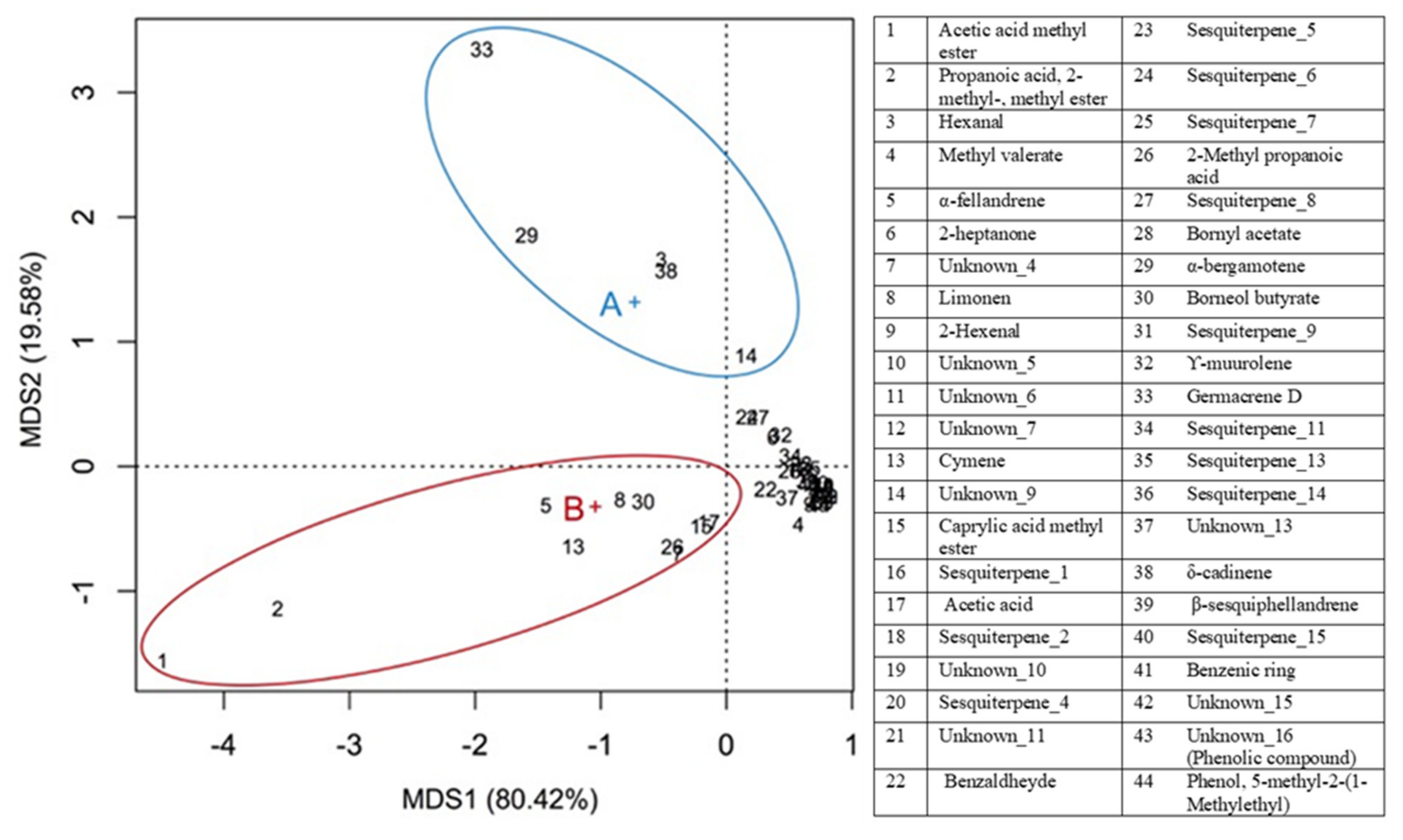
| RT a | Compound | Wild Arnica | Cultivated Arnica | p-Value | Signif. Code | ||
|---|---|---|---|---|---|---|---|
| Mean b | ±SD c | Mean b | ±SD c | ||||
| 25.39 | peak 1 | 13.41 | 1.08 | 23.34 | 2.46 | <0.0001 | *** |
| 27.74 | peak 2 (HM) | 17.50 | 1.11 | 19.35 | 2.66 | 0.1893 | ns |
| 29.04 | peak 3 (n.i.) | 3.28 | 0.24 | 2.65 | 0.23 | 0.0029 | ** |
| 31.44 | peak 4 (n.i.) | 17.21 | 1.31 | 22.60 | 1.65 | 0.0005 | *** |
| 34.79 | peak 5 (HMB) | 7.44 | 1.06 | 16.24 | 2.38 | <0.0001 | *** |
| 35.55 | peak 6 (HIB) | 3.91 | 0.37 | 5.47 | 1.60 | 0.0649 | ns |
| 43.23 | peak 7 (n.i.) | 37.26 | 4.47 | 12.00 | 7.61 | 0.0002 | *** |
| RT a | Compounds | Wild Arnica | Cultivated Arnica | ||||
|---|---|---|---|---|---|---|---|
| Mean b | ±SD c | Mean b | ±SD c | p-Value | Signif. Code | ||
| 2.33 | Acetic acid methyl ester | 11.56 | 1.90 | 38.73 | 4.43 | 0.0006 | ** |
| 2.93 | Unknown_1 | 7.99 | 7.20 | 3.99 | 1.78 | 0.9876 | ns |
| 3.52 | 2-Methyl-propanoic acid 2-methyl-, methyl ester | 10.51 | 4.14 | 31.57 | 9.36 | 0.0235 | * |
| 7.77 | Camphene | 3.00 | 1.18 | 4.92 | 1.37 | 0.1404 | ns |
| 8.98 | Hexanal | 13.68 | 3.14 | 4.44 | 0.37 | 0.0072 | ** |
| 9.45 | Methyl valerate | 0.00 | 0.00 | 2.03 | 0.87 | 0.0155 | * |
| 10.86 | Unknown_2 | 3.09 | 3.64 | 1.20 | 0.74 | 0.4294 | ns |
| 11.07 | Unknown_3 | 4.44 | 5.04 | 1.75 | 0.82 | 0.4131 | ns |
| 11.4 | o-Xylene | 0.84 | 0.37 | 0.89 | 0.11 | 0.8529 | ns |
| 11.72 | p-Xylene | 0.85 | 0.31 | 1.20 | 0.26 | 0.2145 | ns |
| 12.98 | α-Phellandrene | 7.37 | 1.42 | 15.24 | 2.17 | 0.0063 | ** |
| 13.71 | m-Xylene | 0.94 | 0.24 | 1.01 | 0.18 | 0.7035 | ns |
| 14.1 | 2-Heptanone | 3.99 | 1.28 | 1.78 | 0.17 | 0.0422 | * |
| 14.3 | Unknown_4 | 2.05 | 0.54 | 9.05 | 3.84 | 0.0354 | * |
| 14.38 | Caproic acid methyl ester | 5.64 | 1.61 | 13.76 | 5.32 | 0.0647 | ns |
| 14.52 | Limonene | 5.63 | 1.16 | 11.15 | 2.69 | 0.0309 | * |
| 14.8 | Terpene 1 | 0.29 | 0.05 | 0.41 | 0.13 | 0.2245 | ns |
| 15.22 | 2-Hexenal | 0.49 | 0.22 | 1.07 | 0.06 | 0.012 | * |
| 15.32 | Dodecane | 0.45 | 0.36 | 0.00 | 0.00 | 0.0957 | ns |
| 15.61 | Unknown_5 | 0.83 | 0.24 | 0.00 | 0.00 | 0.004 | ** |
| 16.19 | Unknown_6 | 0.76 | 0.20 | 0.00 | 0.00 | 0.0029 | ** |
| 16.23 | Unknown_7 | 0.33 | 0.04 | 0.00 | 0.00 | 0.0002 | ** |
| 17.26 | Cymene | 5.09 | 0.84 | 14.55 | 1.56 | 0.0008 | ** |
| 18.08 | Methyl heptanoate | 4.06 | 1.03 | 9.18 | 3.90 | 0.0929 | ns |
| 19.44 | 6-Methyl-5-hepten-2-one | 2.35 | 0.69 | 2.07 | 0.28 | 0.5473 | ns |
| 20.54 | Unknown_9 | 7.79 | 2.76 | 1.69 | 0.16 | 0.0188 | * |
| 20.98 | Caprylic acid methyl ester | 2.49 | 0.49 | 7.29 | 2.34 | 0.0254 | * |
| 21.69 | Sesquiterpene_1 | 1.60 | 0.46 | 0.66 | 0.16 | 0.0291 | ** |
| 22.23 | Acetic acid | 2.45 | 0.47 | 6.80 | 0.83 | 0.0014 | ** |
| 22.58 | Sesquiterpene_2 | 2.12 | 0.53 | 1.01 | 0.16 | 0.0262 | * |
| 22.73 | Sesquiterpene_3 | 4.13 | 0.74 | 4.62 | 5.34 | 0.8821 | ns |
| 22.94 | Unknown_10 | 0.31 | 0.06 | 0.00 | 0.00 | 0.0009 | ** |
| 23.34 | Sesquiterpene_4 | 1.06 | 0.09 | 0.23 | 0.06 | 0.0002 | ** |
| 23.47 | Nonanoic acid methyl ester | 0.56 | 0.11 | 0.55 | 0.21 | 0.9359 | ns |
| 23.54 | Unknown_11 | 0.55 | 0.07 | 0.29 | 0.04 | 0.0045 | ** |
| 23.85 | Benzaldehyde | 2.21 | 0.28 | 3.13 | 0.48 | 0.0439 | * |
| 23.95 | Sesquiterpene_5 | 2.23 | 0.30 | 0.75 | 0.14 | 0.0015 | ** |
| 24.25 | Sesquiterpene_6 | 5.43 | 0.85 | 2.78 | 0.48 | 0.0095 | ** |
| 24.41 | Sesquiterpene_7 | 2.24 | 0.47 | 0.80 | 0.17 | 0.0075 | ** |
| 24.68 | β-Linalool | 1.04 | 0.12 | 1.04 | 0.13 | 0.9967 | ns |
| 24.93 | 2-Methyl propanoic acid | 2.47 | 0.59 | 9.21 | 0.74 | 0.0002 | ** |
| 25.12 | Sesquiterpene_8 | 5.13 | 0.89 | 2.20 | 0.18 | 0.0051 | ** |
| 25.26 | Bornyl acetate | 2.27 | 0.18 | 1.50 | 0.27 | 0.0151 | * |
| 25.49 | α-Bergamotene | 18.15 | 2.15 | 11.23 | 1.80 | 0.0129 | * |
| 25.61 | Caryophyllene | 70.10 | 8.85 | 59.60 | 3.87 | 0.1327 | ns |
| 26.54 | Borneol butyrate | 4.95 | 0.70 | 9.94 | 2.08 | 0.0171 | * |
| 26.67 | Isocaryophyllene | 1.42 | 0.15 | 1.19 | 0.16 | 0.1362 | ns |
| 26.96 | Sesquiterpene_9 | 0.80 | 0.11 | 0.00 | 0.00 | 0.0002 | ** |
| 27.07 | Humulene | 11.25 | 1.03 | 10.61 | 0.76 | 0.434 | ns |
| 27.14 | Sesquiterpene_10 | 1.53 | 0.35 | 1.27 | 0.10 | 0.2818 | ns |
| 27.31 | Unknown_12 | 3.77 | 0.50 | 3.42 | 0.45 | 0.4215 | ns |
| 27.45 | trans-β-Farnesene | 3.49 | 0.29 | 2.52 | 0.57 | 0.0604 | ns |
| 27.52 | ϒ-muurolene | 3.87 | 0.31 | 1.31 | 0.34 | 0.0006 | ** |
| 27.86 | Germacrene D | 26.40 | 2.82 | 10.18 | 1.53 | 0.0009 | ** |
| 27.98 | Sesquiterpene_11 | 2.81 | 0.36 | 1.18 | 0.31 | 0.0042 | ** |
| 28.06 | Sesquiterpene_12 | 1.09 | 0.41 | 0.91 | 0.14 | 0.5165 | ns |
| 28.13 | Sesquiterpene_13 | 1.85 | 0.33 | 0.40 | 0.16 | 0.0023 | ** |
| 28.21 | Sesquiterpene_14 | 1.39 | 0.35 | 0.75 | 0.17 | 0.0464 | ** |
| 28.33 | Unknown_13 | 1.30 | 0.13 | 2.14 | 0.37 | 0.0205 | * |
| 28.85 | δ-cadinene | 13.13 | 1.10 | 4.40 | 1.10 | 0.0006 | ** |
| 29.09 | β-Sesquiphellandrene | 0.20 | 0.03 | 0.33 | 0.05 | 0.0165 | * |
| 29.28 | Sesquiterpene_15 | 0.52 | 0.02 | 0.19 | 0.04 | 0.0003 | ** |
| 29.48 | Sesquiterpene_16 | 1.40 | 0.28 | 2.26 | 2.73 | 0.6146 | ns |
| 29.66 | Benzenic ring | 0.68 | 0.09 | 0.24 | 0.04 | 0.0014 | ** |
| 30.16 | Calamenene | 3.38 | 1.09 | 3.47 | 0.74 | 0.9112 | ns |
| 30.33 | Caproic acid | 1.67 | 0.24 | 2.15 | 0.21 | 0.0623 | ns |
| 30.87 | Unknown_14 | 2.12 | 0.25 | 1.34 | 0.48 | 0.0668 | ns |
| 32.15 | Unknown_15 | 1.24 | 0.14 | 0.68 | 0.12 | 0.0068 | ** |
| 32.31 | Caryophyllene epoxide | 0.58 | 0.06 | 0.86 | 0.19 | 0.0706 | ns |
| 34.13 | Unknown_16 (phenolic compound) | 0.26 | 0.04 | 0.63 | 0.09 | 0.0028 | ** |
| 34.31 | Phenol, 5-methyl-2-(1-methylethyl) | 0.20 | 0.05 | 0.51 | 0.10 | 0.0083 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leoni, V.; Borgonovo, G.; Giupponi, L.; Bassoli, A.; Pedrali, D.; Zuccolo, M.; Rodari, A.; Giorgi, A. Comparing Wild and Cultivated Arnica montana L. from the Italian Alps to Explore the Possibility of Sustainable Production Using Local Seeds. Sustainability 2021, 13, 3382. https://doi.org/10.3390/su13063382
Leoni V, Borgonovo G, Giupponi L, Bassoli A, Pedrali D, Zuccolo M, Rodari A, Giorgi A. Comparing Wild and Cultivated Arnica montana L. from the Italian Alps to Explore the Possibility of Sustainable Production Using Local Seeds. Sustainability. 2021; 13(6):3382. https://doi.org/10.3390/su13063382
Chicago/Turabian StyleLeoni, Valeria, Gigliola Borgonovo, Luca Giupponi, Angela Bassoli, Davide Pedrali, Marco Zuccolo, Alessia Rodari, and Annamaria Giorgi. 2021. "Comparing Wild and Cultivated Arnica montana L. from the Italian Alps to Explore the Possibility of Sustainable Production Using Local Seeds" Sustainability 13, no. 6: 3382. https://doi.org/10.3390/su13063382
APA StyleLeoni, V., Borgonovo, G., Giupponi, L., Bassoli, A., Pedrali, D., Zuccolo, M., Rodari, A., & Giorgi, A. (2021). Comparing Wild and Cultivated Arnica montana L. from the Italian Alps to Explore the Possibility of Sustainable Production Using Local Seeds. Sustainability, 13(6), 3382. https://doi.org/10.3390/su13063382










