1. Introduction
The same properties that make plastics into incredibly versatile materials—malleability and tuneability—also challenge the transition toward a circular economy for plastics. Indeed, polymers are often mixed at various scales, a wide diversity in compounds exists, and polymers are subject to degradation. Large efforts are being undertaken in numerous projects to increase the recycling rates for various post-industrial and post-consumer end-of-life plastics, leading to new developments in sorting and refining technologies, as well as mechanical and chemical recycling technologies (see for instance [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12]). Even on a societal level, changes are being made in how post-consumer plastics are collected, either by curbside bags or container collection, deposit systems or centralized collection facilities. Many of these efforts, however, interfere with the ever-increasing complexity of plastic formulations and products. At the front end of their lifecycle, some initiatives have been implemented with the aim of designing plastic products for circularity [
13,
14,
15]. This can range from design-for-disassembly [
16,
17] and design-for-recycling [
18,
19,
20]—both on a molecular level as well as on a product/object level—to assisting in the rapid detection/identification of material types by (chemical) labelling. Nevertheless, disruptive new ideas often fail to be adopted, as they are faced with existing, rigid, large-scale material flow infrastructures.
When assessing the sustainability of various new technological alternatives for plastics circularity, the past decades have seen a shift from using traditional ex post methodologies toward ex ante methodologies. The two major study areas are life cycle assessment (LCA) and techno-economic assessment (TEA), as well as approaches integrating both. To effectively serve as decision supports for research and development trajectories, applying these assessment methods at low technology readiness levels (TRL) is imperative. Various strategies exist, including the assumptions of ideal (thermodynamic) systems, the use of proxy technology transfers, participatory methods and the application of learning curves [
21]. Essentially, the lower the technology readiness level, the more generic the study intrinsically becomes, as detailed information about full-scale processes and commercialized products in society is lacking.
Almost all sustainability assessments used for plastics cycles start by establishing mass and energy balances, which can further be translated into a life cycle inventory or into economic data. However, this approach requires quite advanced knowledge about the potential unit processes involved. A key factor in both LCA and TEA, from which many other indicators are derived, is the energy performance of systems. Indeed, if less energy is required to recycle waste plastics into a product, this option may be chosen over its linear economy alternative; e.g., producing virgin products that are incinerated at their end-of-life or landfilling (not taking resource scarcity or other environmental indicators into account). However, an energy balance and therefore quite detailed knowledge ofs unit processes is needed to get to this point. Transformative materials innovations (based on disruptive technologies) may in particular therefore fail to demonstrate their environmental and/or economic potential, as the background technological system—a collection of the currently employed methods—is usually considered in LCA or TEA.
Despite the large efforts dedicated to making the transition towards a more circular economy, an important challenge remains in assessing the recyclability of plastics (e.g., [
22]). A reduced level of recyclability of plastics can result (i) from the difficulty to separate different plastic types [
23], (ii) from the heterogeneity and mixing of collected plastic fractions, (iii) from degradation processes during the recycling process that impact crucial mechanical properties of recycled plastics [
24,
25], (iv) from the efforts related to the recycling processes (e.g., [
26])) and (v) from the presence of adverse effects; e.g., contaminations and the accumulation of substances in the recyclate that may pose a health risk [
27]. This shows that assessing plastic recyclability is a complex task that requires further research.
Often, users of the term recyclability fail to connect their design-for-recycling aspirations with the current complex reality of post-consumer plastic waste handling, sorting and recycling, or even worse, they are bound to a rigid background system. From an engineering point of view, all materials are 100% recyclable, as long as enough energy is available and the size of the cycle does not matter. This supports the aforementioned focus on energy aspects to investigate the feasibility and sustainability of recycling. Again, when making the claim that a material is “recyclable”, it can/should be supported by an integrated sustainability assessment. However, given the strict requirement of mass and energy balance estimations, as mentioned above, this analysis and therefore such claims can only be made for existing platform materials and objects, for which at least proxy technologies and products exist. The outcome of these studies is subject to several constraints corresponding to the scope of the study; the studied market, applied technologies, waste collection and sorting, access to manual labor or automation. These constraints regarding the applicability of the outcomes relate the specific contributions of waste transportation, sorting and refining to the overall impact/cost and are mostly case-specific. Again, judging the potential merits of transformative materials or disruptive system innovations may be inaccurate given the limited forecasting ability of traditional assessment methods.
In the search for more generic assessment methods, Rechberger and Brunner [
28] developed statistical entropy analysis (SEA), which was initially applied to waste-to-energy (WtE) plants to investigate whether this technology is a concentrating or diluting waste treatment operation. Later, this method was adopted for predominantly inorganic/metallic materials streams, such as the European [
29] and Chinese [
30] copper cycle and the Austrian phosphorus cycle [
31]. Other recent developments of SEA include the construction of a modified statistical entropy function to assess the recyclability of e-waste [
32], linking SEA to exergy analysis applied to a crushed lithium-ion battery lab scale sieving experiment [
33] and the introduction of mass joint entropy as an indicator together with a combination of SEA with substance flow analysis to evaluate phosphorus management in a food-based bioethanol system [
34]. However, for a decade, the method was only applied to individual substances, limiting its applicability. Recently, however, Parchomenko et al., (2020) [
35] extended the method by addressing statistical entropies at the component (consisting of multiple substances) and product (consisting of multiple components) levels, leading towards multilevel statistical entropy analysis. This extension [
35] allows us to investigate such circular economy strategies as reuse, repair, remanufacturing or combinations of these. It is important to stress that SEA is not intended as a sustainability metric, but it has been developed to assess resource effectiveness, meaning the ability of a system to preserve functionality on the highest level possible, measured in terms of the statistical entropy changes performed by a system. As SEA is independent of any background systems (such as energy mixes) that play an important role and influence the results of, e.g., LCA, SEA only assesses if a specific state is achieved (product entropy, or entropy level of components or of pure substances) and not how it is achieved (e.g., by a robot or by manual work). Nonetheless, in assessing recyclability, the latter is equally relevant.
The goal of this paper is to highlight how multilevel SEA can also be used in predicting the recyclability of plastics in very generic background systems, although the method alone has some limitations. To overcome these, we propose to extend the assessment method by coupling it with energy balances from generic transportation, sorting and refining technologies. In doing so, we demonstrate that not all statistical entropies (typically expressed as relative statistical entropies) calculated by this method are equal in meaning, as two different waste streams with a similar relative statistical entropy may still differ significantly in the way these waste streams can be separated or further recycled (or reduced in relative statistical entropy). This paper does not present clear-cut formulations to address these issues, but rather explores options for quantifying aspects playing a role in recyclability. In addition, the authors propose a metric based on relative statistical entropy and relative decomposition energy as a potential avenue to define the recyclability of plastics. This is motivated by the fact that the energy required to reduce the relative statistical entropy of two different waste streams with a similar relative statistical entropy may differ significantly. Quantifying this generically, although still in its infancy, may resolve this and yield a powerful predictive and generic tool to predict plastics’ circularity. In this respect, this work can be considered as an addition to the work of Roithner and Rechberger (2020) [
36] in which a recyclability effectiveness metric was defined based on classic SEA to quantitatively and qualitatively assess how effectively a studied recycling process can separate and concentrate an input waste stream.
This paper starts by recapping the multilevel SEA method by Parchomenko et al. (2020) [
35] and applies this to a fictitious example of a collection bag for post-consumer recyclable plastics. Subsequently, an extension of the multilevel SEA method with energy consumption aspects is proposed together with a possible recyclability metric. Furthermore, a more realistic example of a collection bag with multiple different bottles, as a proxy for source-separated curbside collection systems such as the P + MD system—i.e., the waste collection bag for plastic packaging, metal packaging and drinking cartons in Belgium [
37,
38]—is elaborated, highlighting how objects with similar relative statistical entropy values may clearly require a different energy input for relative statistical entropy reduction.
2. Methods
In this section, the extended multilevel statistical entropy analysis (SEA) method is described for the assessment of the recyclability of plastics. Firstly, the multilevel statistical entropy analysis method of Parchomenko et al., (2020) [
35] is introduced with its mathematical description. Subsequently, this methodology is applied to the conceptual example of a fictitious plastic bag. Limitations of the multilevel statistical entropy analysis method for a direct application as a recyclability metric are illustrated by this conceptual example. To conclude this section, an extension of the multilevel SEA method is presented in which energy consumption aspects are taken into account. Note that the statistical entropy considered in this contribution is not the same as thermodynamic entropy. To stress the difference with thermodynamic entropy (typically denoted by
S in scientific literature), the statistical entropy is denoted by
H in this contribution (as is typically done in information theory).
2.1. Description of the Multilevel Statistical Entropy Analysis (SEA) Method
Statistical entropy analysis (SEA) is a method based on Shannon’s statistical entropy function [
39,
40,
41] to quantify changes in the substance distribution pattern throughout a system in which materials undergo transitions in different steps [
28,
29,
42]. Among different process steps in a system, the substances can be diluted (increase in statistical entropy), concentrated (decrease in statistical entropy) or remain unchanged (unchanged statistical entropy). The focus of the initial SEA that was applied to the results of material flow systems was on the analysis of individual substances and did not consider components or products. Consequently, SEA cannot be directly applied to the evaluation of circular economy strategies such as reuse, repair, remanufacturing or combinations of these. To evaluate such circular economy strategies, the multilevel SEA method has been developed by Parchomenko et al., (2020) [
35] in which, next to substance level entropies, component and product level statistical entropies are considered. The multilevel SEA method [
35] is described in this subsection by making a distinction between the three different levels: substance level, component level and product level.
2.1.1. Substance Level Entropy
Consider a flow rate
of a material flow
f (which can be a component or a good flow, in mass per time) and the (dimensionless) mass fraction
of a substance
i in the material flow
f. Thus, the substance flow rate
(in mass per time) can be calculated as
In addition, standardized (dimensionless) mass fractions
can be computed by dividing the material flow rate
by the total flow of a substance
i, over the
F material flows
as indicated in Equation (
2) [
33].
The statistical entropy
of a substance
i (which is dimensionless) can be defined as follows [
42]:
In the SEA method, a dimensionless relative statistical entropy
is used, which is the ratio of the statistical entropy value
and the maximum statistical entropy value
The relative statistical entropy is as indicated in Equation (
4).
where
is the minimum natural or geogenic concentration of substance
i in the atmosphere and the hydrosphere, which corresponds with the concentration of the substance
i in a compartment where it is maximally diluted [
28,
35].
Note that, in a closed system, the maximum statistical entropy of a substance i is reached when the substance is equally distributed among the material flows and the concentrations in all material flows are the same for substance i. The minimum statistical entropy is reached if a material flow consists of a single pure substance i (as in that situation, ).
In SEA, only the relative substance level statistical entropy is considered. For a more detailed description of the SEA method, the reader is referred to [
28,
29,
42]. If the research question concerns one single substance and its use in material flow analysis, the calculation of substance-level entropy can help in addressing this research question. If, however, the research question concerns higher levels (e.g., multiple substances, components or products), then an extended SEA approach should be applied in which the component or product level is addressed.
2.1.2. Component Level Entropy
In multilevel SEA, the second step is to calculate component-level entropy values. These component-level entropy values can subsequently be used for the calculation of the product-level entropies. A similar expression to Equation (
3) can be used to describe the statistical entropy of a component
n,
:
where
is the normalized component mass (i.e., the mass fraction of component
n compared with all components at a stage in the system),
is the concentration of substance
i in component
n and
I is the number of substances.
The main differences between Equations (
3) and (
6) are the normalization at the component level and the summation over the different substances.
The relative statistical entropy of a component
n can be expressed as the ratio between the component-level statistical entropy
and the maximum component-level entropy
. Both the relative component-level statistical entropy and maximum component-level entropy are described in Equations (
7) and (8).
where
is the concentration of substance
i in the total mixture/product/system stage.
The maximum component-level entropy corresponds with either the case in which all substances are present in one material flow or equally distributed. Note that when dilution takes place in compartments outside the system boundary, the maximum statistical entropy is calculated as in Equation (5) for open systems.
2.1.3. Product Level Entropy
The final level that is considered in this paper is the product level. The product-level statistical entropy is defined in Equation (
9).
where
is the component concentration of component
n in the product
p,
is the number of entities of component
n and
is the total number of components.
From Equation (
9), it can be seen that the product-level entropy depends on the distribution of components (via the component concentration
) and the distribution of substances over the components (i.e., the relative statistical entropy values of the components
). Consequently, an increase in product-level entropy can be caused by either an increase in the number of distinct components in a product or through a higher dilution or a more equal distribution of the substances over the product’s components.
In multilevel SEA, relative statistical entropies are considered. Therefore, the product-level statistical entropy needs to be divided by the maximum product-level statistical entropy to calculate the relative product-level statistical entropy. The maximum product-level entropy corresponds with the situation in which every substance is uniformly distributed over the product’s components. The maximum degree of substance dilution is in that case determined by the total number of components
N. Thus, the relative product level statistical entropy and maximum product-level entropy can be expressed as in Equations (
11) and (12).
Note that the minimum of 0 is reached in the situation where the product consists of only one component or one type of component (i.e., , thus ) and the situation in which all components consist of a single substance (i.e., the component entropies equal zero).
2.2. Conceptual Example of Multilevel SEA: A Fictitious Plastic Bag
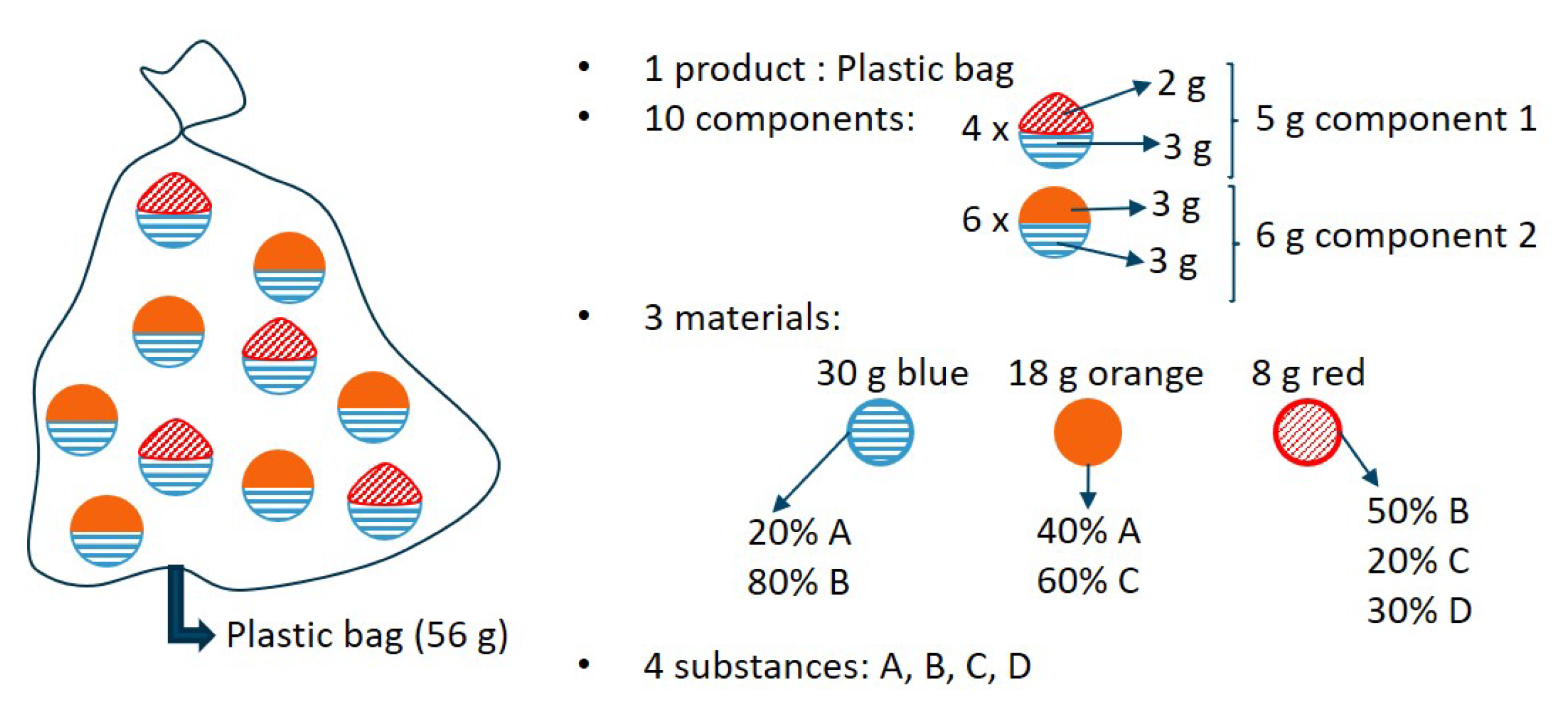
The multilevel SEA method is illustrated based on the conceptual example of a fictitious plastic bag (product) which is filled with 10 components (four units of component 1 and six units of component 2). A unit of component 1 consists of one entity of red material (2 g) and one entity of blue material (3 g). A unit of component 2 consists of one entity of orange material (3 g) and one entity of blue material (3 g). In terms of mass fraction, the blue material consists of 20% substance A and 80% substance B. The orange material consists of 40% substance A and 60% substance C. The red material consists of 50% substance B, 20% substance C and 30% substance D. An illustration of this conceptual example is presented in
Figure 1. In the next paragraphs, the substance-level, component-level and product-level entropies are calculated for this example.
2.2.1. Substance Level Entropy Analysis
To study the transformation or distribution of substance
A over the components by any given operation, the substance-level entropy should be calculated. The substance-level entropy could be of particular interest when studying sorting or refining processes, targeted at a certain substance purity and yield. The statistical entropy for substance
A can be computed by filling in the entries for substance
A in Equation (
3). This results in the following:
The maximum substance-level statistical entropy can be calculated via Equation (5) for closed systems. This results in the following:
The relative substance-level statistical entropy can subsequently be calculated via Equation (
4):
The exercise above could also be done for the other substances. Therefore, the entries and results for the (relative) substance-level statistical entropies for the different substances are summarized in
Table 1. Substance D has the lowest substance-level relative statistical entropy as it is only present in the first component. Substance B has the highest substance-level relative statistical entropy as it is the most diluted substance. Substance A is more diluted than substance C, reflecting the higher substance-level relative statistical entropy of A compared to substance C.
2.2.2. Component-Level Entropy Analysis for the Fictitious Bag Example
If the use and the fate of several related substances in the different components of the system are considered, component-level entropies can be calculated. This could be applicable in research questions regarding the distinct characteristics and composition of various components in a product or system. The component-level entropies are calculated for the two components in this example, component 1 and component 2, using Equation (
6). To illustrate the use of the equations, this is elaborated for the first component. In order to complete Equation (
6), the component mass fraction
and and the substance concentrations in component 1 are needed. These can be computed as indicated in Equations (
16) and (17).
Inputting the values for the different substances in Equation (17) results in following substance concentrations in component 1:
,
,
and
. Thus, the statistical entropy of component 1 can be formulated as
The maximum component-level entropy can be calculated with Equation (8):
where
is the total mass (equal to 56 g for this case study). Consequently, the relative statistical entropy for component 1 can be calculated via Equation (
7):
The entries and results for the (relative) component-level statistical entropies for the different components are summarized in
Table 2. The last column of
Table 2 indicates that the first component has a lower component-level relative statistical entropy than the second component. This can be explained by the higher degree of dilution (40% B, and 30% A and 30% C) of component 2 in its substances compared to component 1 (68% B, 12% A, 12% D and 8% C), as depicted in the second column of
Table 2.
2.2.3. Product-Level Entropy Analysis for the Fictitious Bag Example
For this example, the final hierarchical level in the multilevel SEA method is the product level, in which the use of components and several related substances in the product are studied. The product-level entropy can be calculated with Equation (
9). For the fictitious bag example, this results in the following expression:
The maximum statistical entropy can be calculated via Equation and
. Consequently, the relative statistical entropy of the fictitious bag can be written as (Equation (
11)).
2.3. Limitation of Multilevel SEA if Separation Energy Matters: A Counter Example
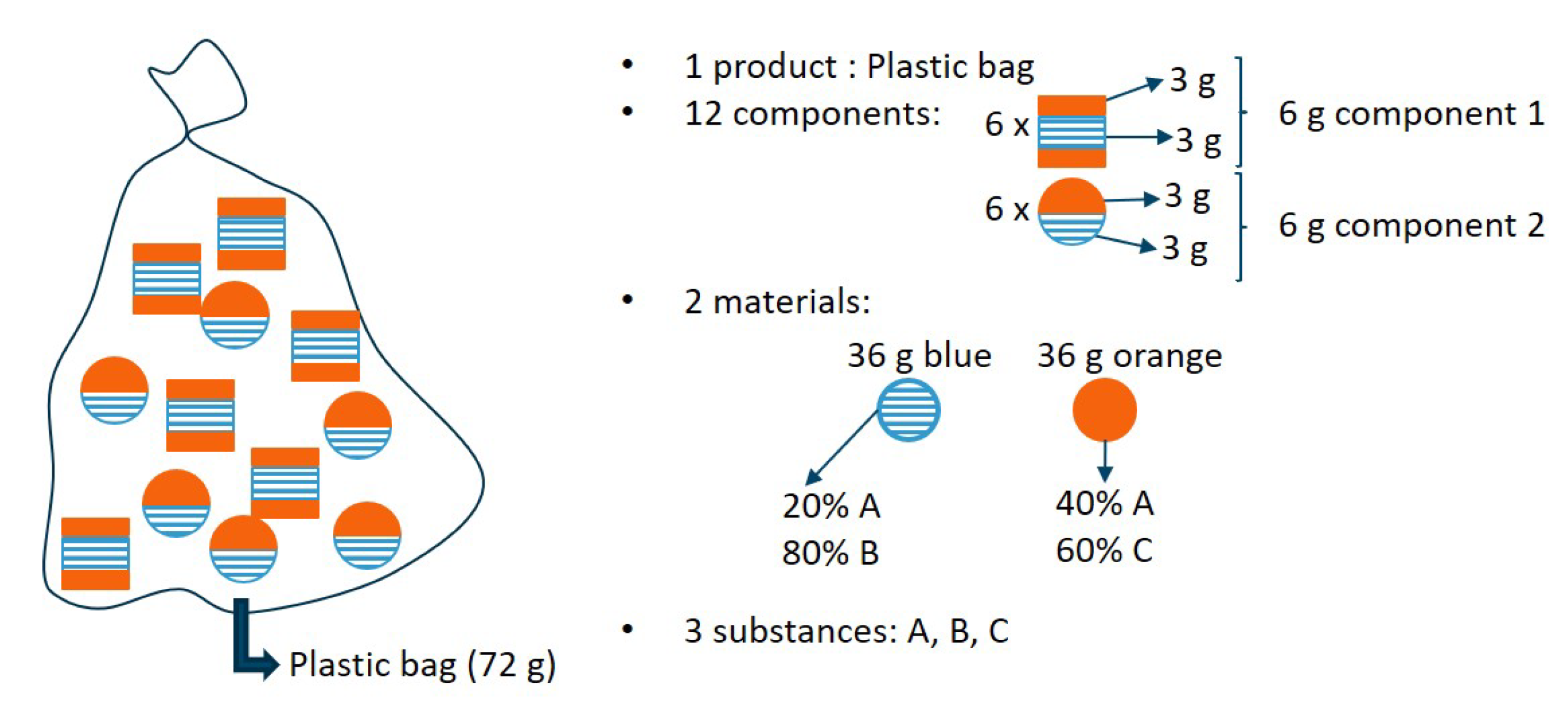
Consider a variation on the fictitious bag example, illustrated in
Figure 2. In this example, two components (which could be any plastic packaging objects, ranging from packaging trays to bottles with caps) are present in the product mixture. Both components have the same substance concentrations (with three substances, A, B and C, and two materials, blue and orange). However, both components have a different structure and are linked differently to one another. One component could, for instance, be glued, whereas the other could be screwed. A comparable situation would be a bag filled with multilayer plastics and monolayer plastics, both with the same substance composition.
In this counter example, it is clear that the relative component statistical entropies will be the same; i.e., 0.500 (see
Table 3 for details on the calculation of the component level entropies). However, both components are not necessarily as easily separated. Multilevel statistical entropy analysis does not account for the energy that is needed to separate these components in its constituent building blocks. Therefore, the efforts to reduce the relative statistical entropy could strongly differ for the various components, although they have the same relative component statistical entropy. In order to use the concept of multilevel SEA for the evaluation of recyclability, an extension is required which addresses the effort needed to separate the components into their building blocks. Therefore, the authors present such an extension using energy consumption aspects in the next subsection.
2.4. Extension of the Multilevel SEA Method with Energy Consumption Aspects to Assess Recyclability
As indicated in the counter example of the previous subsection, an extension of the multilevel SEA method is needed. The authors propose to extend the multilevel SEA method by including energy consumption aspects to assess the recyclability of a product or its components. Firstly, the extension with energy consumption aspects is illustrated for the component level. Subsequently, it is extended to the product level.
Consider a component
n that consists of
M different materials that are linked together to form the component
n. Such a material
m consists of
I different substances that are mixed or blended in a certain way (note that a substance is indicated by
i). As indicated in the counter example, the relative statistical entropy concept does not account for the often distinct way in which materials and/or substances are linked. Depending on the linking of the materials and/or the substances, different processes exist to decompose the component
n in its constituting materials
m and/or its constituting substances
i. The energies required to decompose a component into its materials
or decompose a component into its substances
can be defined per unit mass of component (units: J/kg). A conceptual representation of how these decomposition energies are linked to the recycling of the components is indicated by the full blue arrows in
Figure 3. Note that these energies depend on the process
that is used, which in turn depends on the required decomposition efficiency to either the materials
or the substances
. All decomposition efficiencies defined in this contribution have a value between 0 and 1, corresponding with no decomposition and full decomposition into materials or substances, respectively. In addition, it should be stated that, in practice, processes can exist that do both; i.e., decomposing the components partially into their materials and partially into their substances. Therefore, a generic decomposition energy of a component
n can be defined as
(in J/kg).
Next, a product
p can be considered which consists of
N different components
n. The energy required to decompose the product into its components can be defined as
, where
is the decomposition efficiency of the product to its components, per unit mass of product (units: J/kg). A product
p could also be decomposed directly by a process in its constituent materials using a decomposition energy from a product to its materials described by
, where
is the decomposition efficiency of the product to its components. Similarly, a product could be decomposed in its constituent substances with decomposition energy from a product to its substances
, where
is the decomposition efficiency of the product to its components. These decomposition energies are presented conceptually by the red dotted arrows in
Figure 3. The generic energy of decomposition of a product
p, decomposing the product partially in its components, materials and substances, can denoted by
(in J/kg).
In order to use these concepts of decomposition energy to evaluate the recyclability of different components or products, a relative metric is needed. Therefore, a maximum decomposition
(in J/kg) is defined. This maximum decomposition energy corresponds with the energy that is needed to produce the amount of virgin substances that are present at the highest hierarchical level of the system that is studied, per kg (units: J/kg). In our contribution, this is the product level. This allows us to compare the relative decomposition energies of components with one another, as the same maximum decomposition energy value is used. In the case that the energy required to produce all virgin substances that are present at a lower hierarchical level is used (such as for a component), the relative decomposition energies of different components could not be compared to one another. Therefore, the energy required to produce all virgin substances that are present at the highest hierarchical level—in this contribution, the product level—has been chosen to define the maximum decomposition energy. A mathematical formulation for the maximum decomposition energy is presented in Equation (
24).
where
is the total mass of the studied system (i.e., the mass of the highest hierarchical level that is considered),
is the number of components
n in the product (dimensionless),
is the mass fraction of substance
i in component
n (dimensionless),
is the mass of component
n (in kg) and
is the energy that is needed to produce 1 kg of virgin substance
i (expressed in J/kg).
The maximum decomposition energy defined in Equation (
24) can be used to define the relative decomposition energy of a component
and of a product
in Equations (
25) and (26), respectively. In practice decomposition to the materials is often the goal as these materials (e.g., blends) have been designed with a specific purpose in mind.
Note that the relative decomposition energy (either at component or product level) typically has a value between 0 and 1. However, situations exist in which the relative decomposition energy is greater than 1. In such a situation, the considered decomposition requires more energy per kg than producing the virgin substances that are present per kg. From an energetic point of view, such a recycling route is undesirable. However, such a situation could on the other hand be justifiable in the case of the absence (or an extreme scarcity) of sources to produce the virgin substances.
A first possible recyclability metric for a component based on relative statistical entropy and relative decomposition energies for a component
or product
can be formulated in Equations (
27) and (28), respectively.
Note that the recyclability metrics and typically have a value between 0 and 1 (assuming that the relative decomposition energies have a value between 0 and 1). The minimum recyclability should correspond with the situation in which the relative statistical entropy is either maximal and/or the relative decomposition energy is maximal. The maximum recyclability corresponds with the situation in which the relative statistical entropy equals zero and the relative decomposition energy equals zero. However, negative values of the recyclability metric are possible in which the relative decomposition energy is greater than 1. As mentioned earlier, in such a situation, more energy is required to achieve the preset decomposition than the energy that is needed to produce the virgin substances that are present in the product. In the case of a negative recyclability metric value ( or ), recycling can only be justifiable if sources to produce the virgin substances are not available or extremely scarce.
The potential negative value of
or
could be considered to be less elegant. Therefore, the following alternative recyclability metrics
and
are proposed at the component and product level:
The minimum value of the alternative recyclability metrics and equals zero and corresponds with the maximum component and product-level relative statistical entropy, respectively. The maximum value of the alternative recyclability metric equals infinity and corresponds with the situation in which the relative decomposition energy equals zero (i.e., no energy is needed to decompose the component or product). Note that negative values of the recyclability metrics and are impossible as the relative statistical entropy values cannot exceed 1 and the relative decomposition energy values cannot be smaller than 0. In addition, a situation in which the recyclability metrics and are smaller than the contributions coming from the relative statistical entropies at the component and product level , respectively, correspond with the situation of a negative recyclability metric value of and .
In the case studies presented in this contribution, we focus on the recyclability of the components. The two recyclability metrics that have been presented at the component level, and , are evaluated in the next section. The maximum recyclability corresponds with the situation in which a relative statistical entropy value of zero is exhibited for a component (i.e., the component consists of one single substance or one material) and the situation in which no energy is required for further refinement.