A Preliminary Study on the Determination of the Fertilization Tolerance of an Entisol in the Yuanmou Dry-Hot River Valley Based on Soil Qualities in Plot Scale
Abstract
1. Introduction
2. Materials and Methods
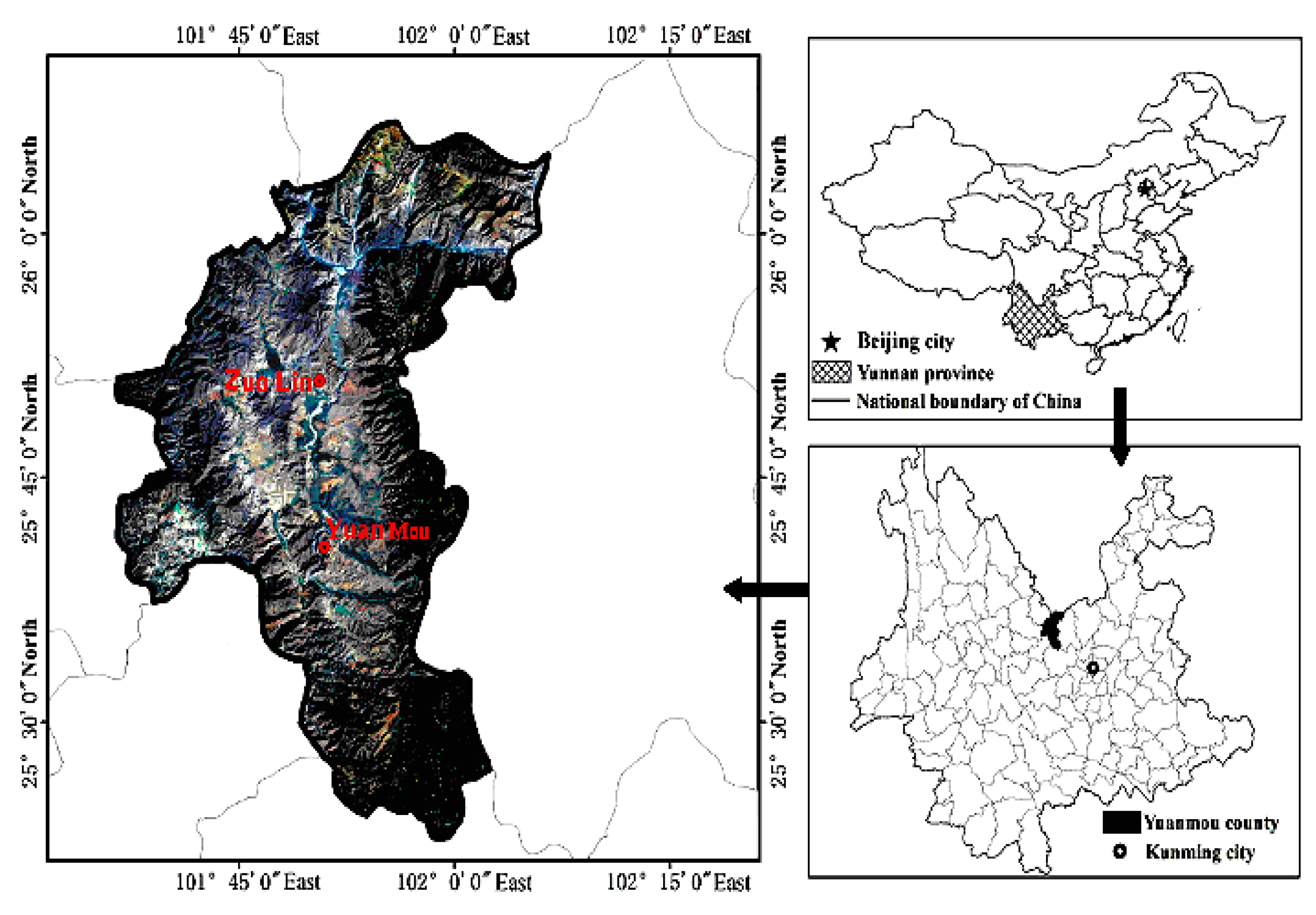
2.1. Study Area
2.2. Field Experiment and Measurements
2.3. Laboratory Measurements
2.4. Soil Quality Index (SQI)
2.5. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Soil Microbial Quantity
3.3. Soil Enzyme Activities
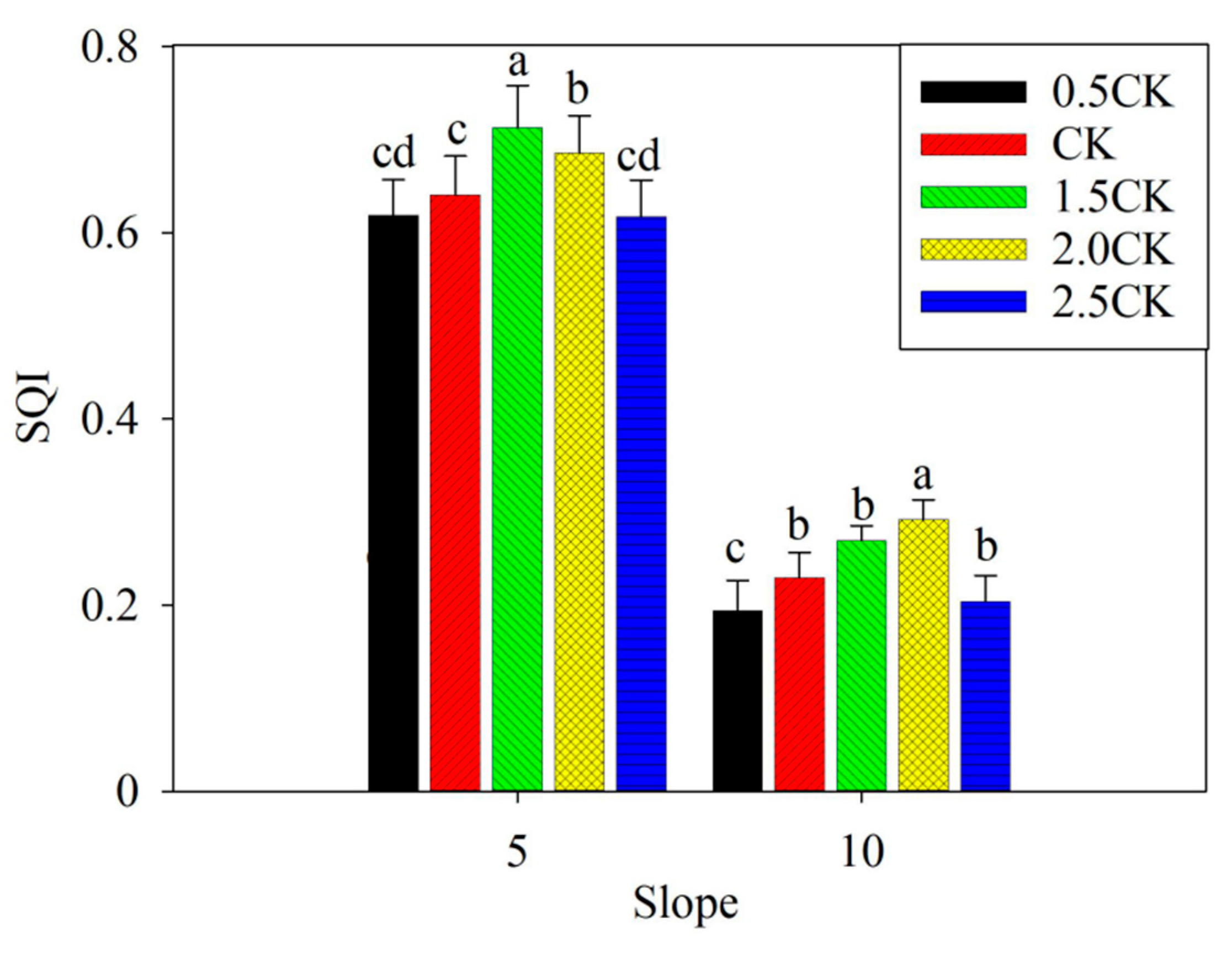
3.4. Soil Quality Index (SQI)
4. Discussion
4.1. Soil Chemical Properties
4.2. Soil Microbial Biomass
4.3. Soil Enzyme Activities
4.4. Soil Quality Index (SQI)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Austin, A.T.; Bustamante, M.M.C.; Nardoto, G.B.; Mitre, S.K.; Pérez, T.; Ometto, J.P.H.B.; Ascarrunz, N.L.; Forti, M.C.; Longo, K.; Gavito, M.E.; et al. Latin America’s nitrogen challenge. Science 2013, 340, 149. [Google Scholar] [CrossRef]
- Kalter, H. Teratology in the 20th century: Environmental causes of congenital malformations in humans and how they were established. Neurotoxicol. Teratol. 2003, 25, 131–282. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Changes in soil biological and biochemical characteristics in a long-term field trial on a sub-tropical inceptisol. Soil Biol. Biochem. 2006, 38, 1577–1582. [Google Scholar] [CrossRef]
- Ge, G.F.; Li, Z.J.; Zhang, J.; Wang, L.G.; Xu, M.G.; Zhang, J.B.; Wang, J.K.; Xie, X.L.; Liang, Y.C. Geographical and climatic differences in long-term effect of organic and inorganic amendments on soil enzymatic activities and respiration in field experimental stations of China. Ecol. Complex. 2009, 6, 421–431. [Google Scholar] [CrossRef]
- Lin, C.W.; Tu, S.H.; Huang, J.J.; Chen, Y.B. The effect of plant hedgerows on the spatial distribution of soil erosion and soil fertility on sloping farmland in the purple-soil area of China. Soil Tillage Res. 2009, 105, 307–312. [Google Scholar] [CrossRef]
- Xiong, M.B.; Shu, F.; Song, G.Y.; Shi, X.J.; Mao, Z.Y. The Effect of Long-term Fix -point Application on the Potassium Forms in Purple Soil. J. Sichuan Agric. Univ. 2001, 19, 44–47. (In Chinese) [Google Scholar]
- Qin, Y.S.; Tu, S.H.; Wang, Z.Y.; Feng, W.Q.; Sun, X.F. Micro-morphological Features of A Purple Soil under Different Long-term Fertilizer Treatments. Ecol. Environ. Sci. 2009, 18, 352–356. (In Chinese) [Google Scholar]
- Liang, H.; Mu, Z.J.; Zhang, J.Z. Impact of Long-term Fertilization on Active Carbon of Purple Soil under Paddy-upland Rotation inWheat Season. Chin. Agric. Sci. Bull. 2001, 27, 221–226. (In Chinese) [Google Scholar]
- Anderson, T.H. Microbial Eco-physiological indicators to asses soil quality. Agric. Ecosyst. Environ. 2003, 98, 285–293. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Winding, A.; Hund-Rinke, K.; Rutgers, M. The use of microorganism in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 230–248. [Google Scholar] [CrossRef]
- Mijangos, I.; Pe’rez, R.; Albizu, I.; Garbisu, C. Effects of fertilization and tillage on soil biological parameters. Enzym. Microb. Technol. 2006, 40, 100–106. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Trasar-Cepeda, C.; Leiro’s, M.C.; Seoane, S.; Gil-Sotres, F. Biochemical properties in managed grassland soils in a temperate humid zone: Modifications of soil quality as a consequence of intensive grassland use. Biol. Fertil. Soils 2009, 45, 711–722. [Google Scholar] [CrossRef]
- Pajares, S.; Gallardo, J.F.; Masciandaro, G.; Ceccanti, B.; Marinari, S.; Etchevers, J.D. Biochemical indicators of carbon dynamics in an Acrisol cultivated under different management practices in the central Mexican highlands. Soil Tillage Res. 2009, 105, 156–163. [Google Scholar] [CrossRef]
- Gomez, E.; Garland, J.; Conti, M. Reproducibility in the response of soil bacterial community-level physiology profiles from a land use intensification gradient. Appl. Soil Ecol. 2004, 26, 21–30. [Google Scholar] [CrossRef]
- Karlen, D.L.; Tomer, M.D.; Neppel, J.; Cambardella, C.A. A preliminary watershed scale soil quality assessment in north central Iowa, USA. Soil Tillage Res. 2008, 99, 291–299. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Alternative soil quality indices for evaluating the effect of intensive cropping, fertilization and manuring for 31 years in the semi-arid soils of India. Environ. Monit. Assess. 2008, 136, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Karlen, D.L.; Wollenhaupt, N.C.; Erbach, D.C.; Berry, E.C.; Swan, J.B.; Eash, N.S.; Jordahl, J.L. Long-term tillage effects on soil quality. Soil Tillage Res. 1994, 32, 313–327. [Google Scholar] [CrossRef]
- Andrews, S.S.; Carroll, C.R. Designing a soil quality assessment tool for sustainable agroecosystem management. Ecol. Appl. 2001, 11, 1573–1585. [Google Scholar] [CrossRef]
- Bastida, F.; Moreno, J.L.; Hernandez, T.; García, C. Microbiological degradation index of soils in a semiarid climate. Soil Biol. Biochem. 2006, 38, 3463–3473. [Google Scholar] [CrossRef]
- Emmerling, C.; Schloter, M.; Hartmann, A.; Kandeler, E. Functional diversity of soil organisms—A review of recent research activities in Germany. J. Plant Nutr.Soil Sci. 2002, 165, 408–420. [Google Scholar] [CrossRef]
- Lentzsch, P.; Wieland, R.; Wirtha, S. Application of multiple regression and neural network approaches for landscape-scale assessment of soil microbial biomass. Soil Biol. Biochem. 2005, 37, 1577–1580. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, S.; Liu, G.B.; Song, Z.L. A comparison of soil qualities of different revegetation types in the Loess Plateau, China. Plant Soil 2011, 347, 163–178. [Google Scholar] [CrossRef]
- Peng, S.L.; Chen, A.Q.; Fang, H.D.; Wu, J.L.; Liu, G.C. Effects of vegetation restoration types on soil quality in Yuanmou dry-hot valley, China. Soil Sci. Plant Nutr. 2013, 59, 347–360. [Google Scholar] [CrossRef]
- Liu, G.C.; Li, L.S.; Wu, G.X.; Zhou, Z.H.; Du, S.H. Determination of Soil Loss Tolerance of a Regosols in Hilly Area of Southwest China. Soil Sci. Soc. Am. J. 2009, 73, 412–417. [Google Scholar] [CrossRef]
- Lin, X.G. Study on the Principle and Method of Soil Microorganism; Higher Education Press: Beijing, China, 2010; Volume 3, pp. 250–253. (In Chinese) [Google Scholar]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of soil quality indexing methods for vegetable production systems in Northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Jimenez, M.P.; Horra, A.M.; Pruzzo, L.; Palma, R.M. Soil quality: A new index based on microbiological and biochemical parameter. Biol. Fertil. Soils 2002, 35, 302–306. [Google Scholar] [CrossRef]
- Bastida, F.; Kandeler, E.; Hernández, T.; García, C. Long-term effect of municipal solid waste amendment on microbial abundance and humus-associated enzyme activities under semiarid conditions. Microb. Ecol. 2007, 55, 651–661. [Google Scholar] [CrossRef]
- Vieira, F.C.B.; Bayer, C.; Mielniczuk, J.; Zanatta, J.; Bissani, C.A. Long-term acidification of a Brazilian Acrisol as affected by no till cropping systems and nitrogen fertiliser. Aust. J. Soil Res. 2008, 46, 17–26. [Google Scholar] [CrossRef]
- Covaleda, S.; Pajares, S.; Gallardo, J.F.; Padilla, J.; Baez, A.; Etchevers, J.D. Effect of different agricultural management systems on chemical fertility in cultivated tepetates of the Mexican transvolcanic belt. Agric. Ecosyst. Environ. 2009, 129, 422–427. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, W.; Yu, X.; Huang, Q. Effects of long-term fertilization on corn productivity and its sustainability in an Ultisol of southern China. Agric. Ecosyst. Environ. 2010, 138, 44–50. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Wei, X.R.; Hao, M.D.; Shao, M.G.; William, J.G. Changes in soil properties and the availability of soil micronutrients after 18 years of cropping and fertilization. Soil Tillage Res. 2006, 91, 120–130. [Google Scholar] [CrossRef]
- Tian, X.H.; Saigusam, A.; Kikawa, N. Effects of controlled-release fertilizers and their application methods on germination and seedling growth of dent and sweet corns. Agric. Sci. China 2005, 4, 455–462. [Google Scholar]
- Xia, L.; Wang, D.Q.; Wang, D.H.; Li, Y.; Gao, X.Z.; Yang, S.J.; Xing, Y.X.; Gao, C.J.; Du, C.Y. Relationship between Soil pH Value and Soil Nutrients in Weifang Tobacco Growing Area. J. Anhui Agric. Sci. 2016, 44, 172–175. [Google Scholar]
- Xia, D.; Wang, J.A.; Liu, G.S.; Ya, P.; Qu, P.Z.; Peng, K.; Wang, P. Correlation between pH value distribution and soil nutrients in Yongde tobacco-growing area. J. Henan Agric. Univ. 2012, 46, 121–126. [Google Scholar]
- López-Bellido, L.; López-Garrido, F.J.; Fuentes, M.; Castillo, J.E.; Fernández, E.J. Influence of tillage, crop rotation and nitrogen fertilization on soil organic matter and nitrogen under rain-fed Mediterranean conditions. Soil Tillage Res. 1997, 43, 277–293. [Google Scholar] [CrossRef]
- Conde, E.; Cardenas, M.; Ponce-Mendoza, A.; Luna-Guido, M.L.; Cruz-Mondragon, C.; Dendooven, L. The impacts of inorganic nitrogen application on mineralization of 14C-labelled maize and glucose, and on priming effect in saline alkaline soil. Soil Biol. Biochem. 2005, 37, 681–691. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The myth of nitrogen fertilization for soil carbon sequestration. J. Environ. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.B.; Hoeftm, R.G.; Mulvaney, R.L. Fate of Nitrogen-15 in a long-term nitrogen rate study: I. Interactions with soil nitrogen. Agron. J. 2005, 97, 1037–1045. [Google Scholar] [CrossRef]
- Rangeh, K.W.; Dwipendra, T.; Christy, B.S.; Vishram, R.; Pradip, K.B. Influence of hill slope on biological pools of carbon, nitrogen, and phosphorus in acidic alfisols of citrus orchard. Catena 2013, 111, 1–8. [Google Scholar]
- Li, X.W.; Hu, Y.F.; Deng, L.J.; Zhang, S.R.; Lin, Z.Y.; Huang, C.; Yang, M.Z. Spatial Variability of Soil Organic Matter in Microtopography of Mid-Sichuan Hilly Region. Chin. J. Soil Sci. 2009, 40, 552–554. (In Chinese) [Google Scholar]
- Sharifi, M.; Zebath, B.J.; Burton, D.L.; Grant, C.A.; Cooper, J.M. Evaluation of some indices of potentially mineralizable nitrogen in soil. Soil Sci. Soc. Am. J. 2007, 71, 1233–1239. [Google Scholar] [CrossRef]
- Fabrizzi, K.P.; Moro’n, A.; García, F.O. Soil carbon and nitrogen organic fractions in degraded vs. non-degraded mollisols in Argentina. Soil Sci. Soc.Am. J. 2003, 67, 1831–1841. [Google Scholar] [CrossRef]
- Bending, G.D.; Turner, M.K.; Rayns, F.; Marx, M.C.; Wood, M. Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol. Biochem. 2004, 36, 1785–1792. [Google Scholar] [CrossRef]
- Crecchio, C.; Curci, M.; Mininni, R.; Ricciuti, P.; Ruggiero, P. Short-term effects of municipal solidwaste compost amendments on soil carbon and nitrogen content, some enzyme activities and genetic diversity. Biol. Fertil. Soils 2001, 34, 311–318. [Google Scholar] [CrossRef]
- Okano, Y.; Hristova, K.R.; Leutenegger, C.M.; Jackson, L.E.; Denison, R.F.; Gebreyesus, B.; Lebauer, D.; Scow, K.M. Application of real-time PCR to study effects of ammonium on population size of ammonia-oxidizing bacteria in soil. Appl. Environ. Microbiol. 2004, 70, 1008–1016. [Google Scholar] [CrossRef]
- Tan, Z.J.; Zhou, W.J.; Zhang, Y.Z.; Zeng, X.B.; Xiao, N.Q.; Liu, Q. Effect of fertilization systems on microbes in the paddy soil. Plant Nutr. Fertil. Sci. 2007, 13, 430–435. (In Chinese) [Google Scholar]
- Li, D.P.; Wu, Z.j.; Chen, L.J.; Zhu, P.; Ren, J. Dynamics of invertase activity of black soil treated by a long term located fertilization and its influence. Chin. J. Eco-Agric. 2005, 13, 102–105. (In Chinese) [Google Scholar]
- Luo, L.F.; Zheng, S.X.; Liao, Y.L.; Xie, J.; Xiang, Y.W.; Nie, J. Effects of Controlled Release Nitrogen Fertilizer on Soil Microbial Properties and Soil Fertility of Paddy Fields; Agricultural University of Hunan (Natural Science Edition): Changsha, China, 2007. (In Chinese) [Google Scholar]
- Hou, Y.L.; Yan, X.Y.; Ren, J.; Wang, X.M. Establishment method and application of regional ecological balanced fertilization models. Chin. J. Soil Sci. 2003, 34, 34–35. (In Chinese) [Google Scholar]
- Zhong, S.; Zeng, H.C.; Jin, Z.Q. Soil microbiological and biochemical properties as affffected by difffferent long-term banana-based rotations in the tropics. Soil Sci. Soc. China 2015, 25, 868–877. [Google Scholar]
- Babujia, L.C.; Hungria, M.; Franchini, J.C.; Brookes, P.C. Microbial biomass and activity at various soil depths in a Brazilian oxisol after two decades of no-tillage and conventional tillage. Soil Biol. Biochem. 2010, 42, 2174–2181. [Google Scholar] [CrossRef]
- Hu, J.L.; Lin, X.G.; Wang, J.H.; Chu, H.Y.; Rui, Y.; Zhang, J.B. Population size and specific potential of P-mineralizing and solubilizing bacteria under long-term P-deficiency fertilization in a sandy loam soil. Pedobiologia 2009, 53, 49–58. [Google Scholar] [CrossRef]
- Luo, M.; Wen, Q.K.; Mu, Y.J.; Xue, L.D.; Shan, N.N. Effects of different fertilization measures on amount of soil phosphobacteria and phosphorus inversion intensity in cotton field. Soil Environ. Sci. 2001, 10, 316–318. (In Chinese) [Google Scholar]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Ge, T.D.; Chen, X.J.; Yuan, H.Z.; Li, B.Z.; Zhu, H.H.; Peng, P.Q.; Li, K.L.; Davey, L.J.; Wu, J.H. Microbial biomass, activity, and community structure in horticultural soils under conventional and organic management strategies. Eur. J. Soil Biol. 2013, 5, 122–128. [Google Scholar] [CrossRef]
- Alwyn, W.; Gunnar, B.; Katarina, H. The effects of 55 years of different inorganic fertiliser regimes on soil properties and microbial community composition. Soil Biol. Biochem. 2013, 67, 41–46. [Google Scholar]
- Baum, C.; Leinweber, P.; Schlichting, A. Effects of chemical conditions in re-wetted peats on temporal variation in microbial biomass and acid phosphatase activity within the growing season. Appl. Soil Ecol. 2003, 22, 167–174. [Google Scholar] [CrossRef]
- Xu, H.W.; Qu, Q.; Chen, Y.H.; Liu, G.B.; Xue, S. Responses of soil enzyme activity and soil organic carbon stability over time after cropland abandonment in different vegetation zones of the Loess Plateau of China. Catena 2021, 196, 1–13. [Google Scholar] [CrossRef]
- Bowles, T.; Acosta-Martínez, V.; Calderón, F.; Jackson, L. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Graham, M.H.; Haynes, R.J. Catabolic diversity of soil microbial communities under sugarcane and other land uses estimated by Biolog and substrate-induced respiration methods. Appl. Soil Ecol. 2005, 29, 155–164. [Google Scholar] [CrossRef]
- Allison, S.D.; Nielsen, C.; Hughes, R.F. Elevated enzyme activities in soils under the invasive nitrogen-fixing tree Falcataria moluccana. Soil Biol. Biochem. 2006, 38, 1537–1544. [Google Scholar] [CrossRef]
- Cheng, D.J.; Liu, S.Q.; Wang, D.W.; Ren, Z.J.; Xue, B.M.; Zhang, X.G. The effect of long-term experiment improving soil fertility on the dynamical changes of soil nutrient and soil enzyme activities. J. Agric. Univ. Hebei 2003, 26, 33–45. (In Chinese) [Google Scholar]
- Qiu, X.K.; Dong, Y.J.; Wan, Y.S. Effects of different fertilizing treatments on contents of soil nutrients and soil enzyme activity. Soils 2010, 42, 249–255. (In Chinese) [Google Scholar]
- Hao, X.H.; Xu, J.R.; Zhang, J.Z.; Li, H.F.; Zhou, T.L.; Zhang, H. Effects of long-term fertilizer on soil fertility and soil enzyme activities in upland red soils. Ecol. Environ. Sci. 2011, 20, 266–269. (In Chinese) [Google Scholar]
- Marcote, I.; Hernández, T.; García, C.; Polo, A. Influence of one or two successive annual applications of organic fertilisers on the enzyme activity of a soil under barley cultivation. Bioresour. Technol. 2001, 79, 147–154. [Google Scholar] [CrossRef]
- Wei, Y.L.; Zhou, Z.H.; Liu, G.C. Physico-chemical properties and enzyme activities of the arable soils in Lhasa, Tibet, China. J. Mt. Sci. 2012, 9, 558–569. [Google Scholar] [CrossRef]
- Jiao, X.G.; Gao, C.S.; Lv, G.H.; Sui, Y.Y. Effect of long-term fertilization on soil enzyme activities under different hydrothermal conditions in northeast china. Agric. Sci. China 2011, 10, 412–422. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 915–1919. [Google Scholar] [CrossRef]
- Niemi, R.M.; Vepsalainen, M. Stability of the fluorogenic enzyme substrates and pH optima of enzyme activities in different Finnish soils. J. Microbiol. Methods 2005, 60, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, B.; Gao, M.R.; Xu, T.P.; Kuang, F.H. Nitrate pollution of groundwater in a typical small watershed in the central Sichuan hilly region. J. Ecol. Rural Environ. 2006, 22, 84–87. (In Chinese) [Google Scholar]
- Wang, Q.K.; Wang, S.L.; Liu, Y.X. Responses to N and P fertilization in a young Eucalyptus dunnii plantation: Microbial properties, enzyme activities and dissolved organic matter. Appl. Soil Ecol. 2008, 40, 484–490. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.Q.; Gong, Y.S.; Yang, H.F.; Fan, M.S.; Yakov, K. Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a North China Plain soil. Eur. J. Soil Biol. 2013, 60, 112–119. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhao, B.Q.; Li, X.H.; Li, Y.T.; Sun, R.L.; Zhu, L.S.; Xu, J.; Wang, L.X.; Li, X.P.; Zhang, F.D. Effects of different fertilization systems on soil nicrobe and its relation to soil fertility. Sci. Agric. Sin. 2005, 38, 1591–1599. (In Chinese) [Google Scholar]
- Pei, H.K. Effect of different fertilizer on enzymatic activity of grassland. Chin. Qinghai J. Anim. Vet. Sci. 2001, 31, 15–16. (In Chinese) [Google Scholar]
- Deng, C.J. Effects of Long-Term Fertilization on Nitrogen Transformation and Activity of Enzymes in Paddy Soil; Central China Agricultural University: Wuhan, China, 2008. (In Chinese) [Google Scholar]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities to two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Bi, S.Q.; Zhang, L.J.; Xue, B.M.; Zhang, J.Z.; Li, X. Primary Research on Soil Enzymes Runoff Rules from Sloping Field. Chin. Agric. Sci. Bull. 2006, 22, 500–503. (In Chinese) [Google Scholar]
- Li, G.L.; Wu, Q.F. The Loss Rules of Soil Enzymes in Sloping Fields. J. Northwest For. Coll. 1997, 12, 42–45. (In Chinese) [Google Scholar]
- Gerzabek, M.H.; Haberhauer, G.; Kandeler, E.; Sessitsch, A.; Kirchmann, H. Response of organic matter pools and enzyme activities in particle size fractions to organic amendments in a long-term field experiment. Dev. Soil Sci. 2002, 28, 329–344. [Google Scholar]
- García-Ruiz, R.; Ochoa, V.; Vinegla, B.; Hinojosa, M.B.; Pena-Santiago, R.; Lie’banas, G.; Linares, J.C.; Carreira, J.A. Soil enzymes, nematode community and selected physico-chemical properties as soil quality indicators in organic and conventional olive oil farming:Influence of seasonality and site features. Appl. Soil Ecol. 2009, 41, 305–314. [Google Scholar] [CrossRef]






| Soil Properties | Value | Soil Properties | Value |
|---|---|---|---|
| Total N (g∙kg−1) | 0.47 ± 0.02 | Ammonifying bacteria (105 cfu ∙ g−1) | 10.13 ± 3.18 |
| Total P (g∙kg−1) | 0.30 ± 0.01 | Nitrifying bacteria (104 cfu ∙ g−1) | 6.80 ± 1.35 |
| Available N (mg∙ kg−1) | 31.50 ± 2.12 | Organic phosphorus bacteria (103 cfu ∙ g−1) | 33.95 ± 10.01 |
| Available P (mg∙ kg−1) | 1.66 ± 0.02 | Inorganic phosphorus bacteria (103 cfu ∙ g−1) | 22.47 ± 8.33 |
| Organic matter (g∙ kg−1) | 9.45 ± 0.99 | Denitrifying bacteria (104 cfu ∙ g−1) | 30.70 ± 5.23 |
| Bulk density (g∙cm−3) | 1.46 ± 0.22 | Invertase (mg Glucose ∙ g−1) | 2.03 ± 0.10 |
| pH value | 8.12 ± 0.03 | Alkaline phosphatase (mg phenol ∙ g−1) | 0.23 ± 0.01 |
| Urease (mg NH4+-N ∙ g−1) | 1.85 ± 0.26 |
| Slope | Treatments | Total N (g∙kg−1) | Total P (g∙kg−1) | Available N (mg∙kg−1) | Available P (mg∙kg−1) | pH | Organic Matter (g∙kg−1) |
|---|---|---|---|---|---|---|---|
| 5° | 0.5 CK(T1) | 0.64 ± 0.02 cd | 0.34 ± 0.02 c | 104.74 ± 4.41 c | 17.21 ± 2.65 d | 7.75 ± 0.01 a | 10.78 ± 0.12 c |
| 1.0 CK(T2) | 0.73 ± 0.06 c | 0.46 ± 0.02 bc | 135.55 ± 5.26 b | 26.54 ± 5.57 c | 7.33 ± 0.03 ab | 12.49 ± 0.43 bc | |
| 1.5 CK(T3) | 0.87 ± 0.01 a | 0.64 ± 0.01 a | 164.07 ± 4.25 a | 33.22 ± 4.09 a | 7.19 ± 0.03 ab | 12.78 ± 0.25 b | |
| 2.0 CK(T4) | 0.81 ± 0.02 ab | 0.51 ± 0.02 b | 143.67 ± 10.50 b | 29.56 ± 6.89 b | 7.06 ± 0.05 ab | 14.06 ± 1.17 ab | |
| 2.5 CK(T5) | 0.76 ± 0.02 bc | 0.45 ± 0.01 bc | 110.25 ± 4.03 c | 19.03 ± 3.94 d | 7.01 ± 0.05 ab | 15.11 ± 0.17 a | |
| 10° | 0.5 CK(T1) | 0.41 ± 0.01 c | 0.45 ± 0.01 c | 97.33 ± 2.38 b | 26.03 ± 0.29 c | 7.95 ± 0.05 a | 9.75 ± 0.60 c |
| 1.0 CK(T2) | 0.49 ± 0.04 b | 0.73 ± 0.02 b | 128.84 ± 5.26 b | 47.80 ± 0.58 b | 7.43 ± 0.03 ab | 10.46 ± 0.34 c | |
| 1.5 CK(T3) | 0.55 ± 0.05 a | 0.74 ± 0.01 b | 133.67 ± 16.34 ab | 49.21 ± 0.70 b | 7.33 ± 0.03 ab | 11.99 ± 1.53 c | |
| 2.0 CK(T4) | 0.55 ± 0.02 a | 0.82 ± 0.03 a | 149.24 ± 3.34 a | 57.39 ± 0.38 a | 7.25 ± 0.20 ab | 13.39 ± 0.40 b | |
| 2.5 CK(T5) | 0.44 ± 0.01 c | 0.72 ± 0.03 b | 107.67 ± 2.75 b | 37.70 ± 0.39 c | 7.14 ± 0.01 ab | 14.75 ± 0.23 a |
| 5° | 10° | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Component | PC1 | PC2 | PC3 | PC4 | PC5 | Component | PC1 | PC2 | PC3 |
| Eigenvalue | 4.061 | 2.777 | 1.962 | 1.559 | 1.157 | Eigenvalue | 4.872 | 3.525 | 2.269 |
| Variance (%) | 29.006 | 19.835 | 14.016 | 11.134 | 8.267 | Variance (%) | 34.802 | 25.177 | 16.210 |
| Cumulative (%) | 29.006 | 48.841 | 62.857 | 73.991 | 82.285 | Cumulative (%) | 34.802 | 59.979 | 76.188 |
| Eigenvectors a | |||||||||
| TN | 0.846 | 0.256 | 0.084 | 0.106 | 0.197 | TN | 0.880 | 0.322 | −0.242 |
| TP | −0.243 | 0.674 | 0.513 | −0.027 | −0.011 | TP | −0.061 | 0.391 | 0.811 |
| AN | 0.952 | 0.078 | −0.108 | −0.004 | 0.133 | AN | 0.803 | 0.378 | −0.226 |
| AP | −0.056 | 0.614 | 0.423 | 0.141 | 0.468 | AP | 0.135 | 0.362 | 0.801 |
| pH | 0.075 | −0.671 | 0.392 | −0.451 | 0.084 | pH | 0.506 | −0.631 | −0.253 |
| OM | −0.389 | −0.096 | 0.090 | 0.802 | 0.317 | OM | 0.911 | 0.283 | −0.099 |
| AB | −0.456 | 0.299 | 0.404 | −0.531 | −0.274 | AB | 0.051 | −0.969 | 0.074 |
| NB | −0.271 | −0.784 | 0.306 | 0.198 | 0.082 | NB | 0.217 | 0.196 | −0.466 |
| DB | 0.745 | −0.035 | −0.259 | −0.116 | 0.154 | DB | −0.171 | 0.917 | 0.098 |
| OPB | −0.395 | 0.696 | −0.284 | 0.120 | −0.278 | OPB | −0.026 | 0.629 | −0.411 |
| IPB | −0.147 | −0.027 | −0.791 | 0.105 | −0.247 | IOPB | 0.134 | 0.438 | −0.339 |
| Urease | 0.846 | −0.099 | 0.243 | 0.052 | −0.288 | Urease | 0.883 | −0.289 | 0.135 |
| Invertase | 0.034 | 0.349 | −0.384 | −0.490 | 0.523 | Invertase | 0.850 | −0.037 | 0.437 |
| Alkaline P | 0.699 | 0.305 | 0.321 | 0.278 | −0.417 | Alkaline P | 0.863 | −0.164 | 0.247 |
| 5° | |||||
|---|---|---|---|---|---|
| Parameters | Available N (AN) | Nitrifying Bacteria (NB) | Inorganic Phosphorus Bacteria (IPB) | Organic Matter (OM) | Invertase (INTS) |
| Average (x0) | 110.54 | 76.67 | 71.73 | 8.29 | 40.24 |
| Curve Type * | + | - | - | + | + |
| Normalization Equation | Y = 1/(1 + (x/110.54)−2.5) | Y = 1/(1 + (x/76.67)2.5) | Y = 1/(1 + (x/71.73)2.5) | Y = 1/(1 + (x/8.29)−2.5) | Y = 1/(1 + (x/40.24)−2.5) |
| 10° | |||||
| Parameters | Organic matter (OM) | Ammonifying bacteria (AB) | Total P (TP) | ||
| Average (x0) | 12.97 | 108.42 | 0.49 | ||
| Curve Type * | + | - | + | ||
| Normalization Equation | Y = 1/(1 + (x/12.97)−2.5) | Y = 1/(1 + (x/108.42)−2.5) | Y = 1/(1 + (x/0.49)−2.5) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Fan, M.; Song, J.; Peng, S.; He, Y.; Wei, Y.; Dai, Y.; Liu, G. A Preliminary Study on the Determination of the Fertilization Tolerance of an Entisol in the Yuanmou Dry-Hot River Valley Based on Soil Qualities in Plot Scale. Sustainability 2021, 13, 3626. https://doi.org/10.3390/su13073626
Zhao L, Fan M, Song J, Peng S, He Y, Wei Y, Dai Y, Liu G. A Preliminary Study on the Determination of the Fertilization Tolerance of an Entisol in the Yuanmou Dry-Hot River Valley Based on Soil Qualities in Plot Scale. Sustainability. 2021; 13(7):3626. https://doi.org/10.3390/su13073626
Chicago/Turabian StyleZhao, Li, Min Fan, Jie Song, Sili Peng, Yuxiao He, Yali Wei, Yi Dai, and Gangcai Liu. 2021. "A Preliminary Study on the Determination of the Fertilization Tolerance of an Entisol in the Yuanmou Dry-Hot River Valley Based on Soil Qualities in Plot Scale" Sustainability 13, no. 7: 3626. https://doi.org/10.3390/su13073626
APA StyleZhao, L., Fan, M., Song, J., Peng, S., He, Y., Wei, Y., Dai, Y., & Liu, G. (2021). A Preliminary Study on the Determination of the Fertilization Tolerance of an Entisol in the Yuanmou Dry-Hot River Valley Based on Soil Qualities in Plot Scale. Sustainability, 13(7), 3626. https://doi.org/10.3390/su13073626







